Abstract
Background
Chronic lung allograft dysfunction (CLAD) is a major cause of allograft loss post-lung transplantation. Prior studies have examined the association between respiratory virus infection (RVI) and CLAD were limited by older diagnostic techniques, study design, and case numbers. We examined the association between symptomatic RVI and CLAD using modern diagnostic techniques in a large contemporary cohort of lung transplant recipients (LTRs).Methods
We retrospectively assessed clinical variables including acute rejection, cytomegalovirus pneumonia, upper and lower RVI, and the primary endpoint of CLAD (determined by 2 independent reviewers) in 250 LTRs in a single university transplantation program. Univariate and multivariate Cox models were used to analyze the relationship between RVI and CLAD in a time-dependent manner, incorporating different periods of risk following RVI diagnosis.Results
Fifty patients (20%) were diagnosed with CLAD at a median of 95 weeks post-transplantation, and 79 (32%) had 114 episodes of RVI. In multivariate analysis, rejection and RVI were independently associated with CLAD (adjusted hazard ratio [95% confidence interval]) 2.2 (1.2-3.9), P = .01 and 1.9 (1.1-3.5), P = .03, respectively. The association of RVI with CLAD was stronger the more proximate the RVI episode: 4.8 (1.9-11.6), P < .01; 3.4 (1.5-7.5), P < .01; and 2.4 (1.2-5.0), P = .02 in multivariate analysis for 3, 6, and 12 months following RVI, respectively.Conclusions
Symptomatic RVI is independently associated with development of CLAD, with increased risk at shorter time periods following RVI. Prospective studies to characterize the virologic determinants of CLAD and define the underlying mechanisms are warranted.Free full text

Symptomatic Respiratory Virus Infection and Chronic Lung Allograft Dysfunction
Associated Data
Abstract
Background. Chronic lung allograft dysfunction (CLAD) is a major cause of allograft loss post-lung transplantation. Prior studies have examined the association between respiratory virus infection (RVI) and CLAD were limited by older diagnostic techniques, study design, and case numbers. We examined the association between symptomatic RVI and CLAD using modern diagnostic techniques in a large contemporary cohort of lung transplant recipients (LTRs).
Chronic lung allograft dysfunction (CLAD) is a major cause of allograft loss post-lung transplantation. Prior studies have examined the association between respiratory virus infection (RVI) and CLAD were limited by older diagnostic techniques, study design, and case numbers. We examined the association between symptomatic RVI and CLAD using modern diagnostic techniques in a large contemporary cohort of lung transplant recipients (LTRs).
Methods. We retrospectively assessed clinical variables including acute rejection, cytomegalovirus pneumonia, upper and lower RVI, and the primary endpoint of CLAD (determined by 2 independent reviewers) in 250 LTRs in a single university transplantation program. Univariate and multivariate Cox models were used to analyze the relationship between RVI and CLAD in a time-dependent manner, incorporating different periods of risk following RVI diagnosis.
We retrospectively assessed clinical variables including acute rejection, cytomegalovirus pneumonia, upper and lower RVI, and the primary endpoint of CLAD (determined by 2 independent reviewers) in 250 LTRs in a single university transplantation program. Univariate and multivariate Cox models were used to analyze the relationship between RVI and CLAD in a time-dependent manner, incorporating different periods of risk following RVI diagnosis.
Results. Fifty patients (20%) were diagnosed with CLAD at a median of 95 weeks post-transplantation, and 79 (32%) had 114 episodes of RVI. In multivariate analysis, rejection and RVI were independently associated with CLAD (adjusted hazard ratio [95% confidence interval]) 2.2 (1.2–3.9), P = .01 and 1.9 (1.1–3.5), P = .03, respectively. The association of RVI with CLAD was stronger the more proximate the RVI episode: 4.8 (1.9–11.6), P < .01; 3.4 (1.5–7.5), P < .01; and 2.4 (1.2–5.0), P = .02 in multivariate analysis for 3, 6, and 12 months following RVI, respectively.
Fifty patients (20%) were diagnosed with CLAD at a median of 95 weeks post-transplantation, and 79 (32%) had 114 episodes of RVI. In multivariate analysis, rejection and RVI were independently associated with CLAD (adjusted hazard ratio [95% confidence interval]) 2.2 (1.2–3.9), P = .01 and 1.9 (1.1–3.5), P = .03, respectively. The association of RVI with CLAD was stronger the more proximate the RVI episode: 4.8 (1.9–11.6), P < .01; 3.4 (1.5–7.5), P < .01; and 2.4 (1.2–5.0), P = .02 in multivariate analysis for 3, 6, and 12 months following RVI, respectively.
Conclusions. Symptomatic RVI is independently associated with development of CLAD, with increased risk at shorter time periods following RVI. Prospective studies to characterize the virologic determinants of CLAD and define the underlying mechanisms are warranted.
Symptomatic RVI is independently associated with development of CLAD, with increased risk at shorter time periods following RVI. Prospective studies to characterize the virologic determinants of CLAD and define the underlying mechanisms are warranted.
(See the Editorial Commentary by Gardiner and Snydman on pages 320–2.)
Chronic lung allograft dysfunction (CLAD), a progressive and irreversible process, is a major cause of long-term allograft failure and death in lung transplant recipients (LTRs) [1]. CLAD is now recognized as consisting of the following 2 major phenotypes: bronchiolitis obliterans syndrome (BOS) and restrictive CLAD (R-CLAD). The etiology of CLAD is poorly understood, but it is considered a form of chronic allograft rejection based on various lines of evidence [2–4]. However, despite the use of more potent immunosuppressive regimens that have decreased acute allograft rejection, the rates of CLAD have not been proportionately affected [5]. Additionally, some patients with CLAD have not had allograft rejection and many with acute allograft rejection do not ultimately develop CLAD, suggesting that other factors are important in CLAD pathogenesis.
Several studies have examined the role of respiratory virus infection (RVI) in the development of CLAD, with an association reported in many, but not all, studies (Table (Table1)1) [2, 6–10]. We only included studies that specifically assessed for an association between RVI and CLAD/BOS using either univariate or multivariate statistical analysis. These studies had important limitations, including small sample size, retrospective design, short follow-up duration, nonblinded assessment of key endpoints (ie, CLAD), use of nonconsensus definitions of CLAD, lack of modern diagnostic virologic techniques, inclusion of only specific viruses, and limited statistical analyses (Table (Table1).1). In a pooled analysis of studies on RVI and BOS, Vu et al were unable to confirm an association, mainly due to the heterogeneity of the studies and major limitations in design, diagnostic techniques, and definitions [11].
Table 1.
Summary of Previous Studies on the Association Between Chronic Lung Allograft Dysfunction/Bronchiolitis Obliterans Syndrome and Respiratory Virus Infection
| Reference (Author and Reference Number) | Study Design | N | No. of Bronchiolitis Obliterans Syndrome Cases (%) | No. of Respiratory Virus Infection Cases | Upper Respiratory Tract Infection and Lower Respiratory Tract Infection | Multivirus Polymerase Chain Reaction Used | Multivariate Analysis | Association/Comments |
|---|---|---|---|---|---|---|---|---|
| Magnusson et al [6] | Retrospective | 38 | Not recorded | 14 | Noa | Yes | No | Yes, odds ratio, 3.02 (1.3–6.8); P = .008 |
| Gottlieb et al [7] | Prospective cohort | 300b | 31 (10); only 6 with RVI | 30 | Yes | Yes | Yes | Yes, only in RVIs with every symptom present; hazard ratio, 4.05; P = .004 in MVA; small sample size |
| Milstone et al [8] | Prospective cohort | 50 | 4 (8); only 1 with RVI | 17 | Yes | Yes | No | No |
| Khalifah et al [2] | Retrospective | 228 | 92 (40) | 21 | Yes | No | Yes | Yes, in MVA; in univariate analysis only BOS 1 in LRTI |
| Billings et al [9] | Retrospective | 219 | 73 (33); BOS grade 2,3 only. | 33 | Yes | No | No | not overall; BOS 3 in LRTI only, 2.3 (1.1–4.9, P = .004) |
| Bridges et al [10] | Prospective case series | 16 | 4 (25) | 16 | Noa | No | No | Adenovirus associated with BOS; P < .001 |
Abbreviations: BOS, bronchiolitis obliterans syndrome; LRTI, lower respiratory tract infection; MVA, multivariate analysis; RVI, respiratory virus infection.
a Lower respiratory tract infection only.
b There were 388 lung transplant recipients; 300 without BOS at onset of study.
To address some of the limitations in prior studies, we examined the association of RVI and CLAD in a relatively large, contemporaneous cohort of LTRs. We included patients diagnosed with RVI using modern molecular assays; applied systematic, recently published consensus definitions of CLAD; assessed CLAD using 2 blinded reviewers; and used time-dependent models and multivariate analyses that controlled for factors previously associated with CLAD. Based on prior clinical studies, animal models, and other experimental data suggesting that the risk for CLAD may be higher in the initial period after RVI, we also modeled the association of RVI with CLAD assuming different durations of risk following RVI.
METHODS
Cohort
We retrospectively assessed a cohort of 250 consecutive adult patients who received a first lung transplant at the University of Washington Medical Center (UWMC) between January 2007 and May 2012. Clinical variables and endpoints were collected for 1 year after the most recently transplanted patient, allowing a possible maximum duration of follow-up of 5.4 years for the first patient included and a minimum of 1 year for the last patient included. The University of Washington has a comprehensive electronic health record system, and patient demographics and clinical and laboratory information were collected via medical record review using standardized data collection forms by trained personnel who were blinded to the primary endpoint (CLAD). The University of Washington Institutional Review Board approved the study.
Transplantation Protocols
Cytomegalovirus Prevention
Patients who were either recipient or donor seropositive for cytomegalovirus (CMV) received 3 months of intravenous (IV) ganciclovir or oral valganciclovir followed by chronic acyclovir prophylaxis. CMV recipient and donor seronegative patients received chronic acyclovir prophylaxis. Monitoring for CMV viremia was done by blood quantitative polymerase chain reaction (PCR) after discontinuation of prophylaxis, weekly for 1 month, every 2 weeks for 2 months, and then monthly through year 1, and when CMV disease was clinically suspected.
Immunosuppression and Treatment of Rejection
Routine induction therapy was given with basiliximab. Maintenance immunosuppression consisted of prednisone, a calcineurin inhibitor (generally tacrolimus), and an antimetabolite (generally mycophenolate mofetil or mycophenolic acid).
Assessment and Treatment of Rejection
No routine surveillance bronchoscopy to evaluate for rejection was performed during the study period. Patients with clinically suspected rejection underwent bronchoscopy with bronchoalveolar lavage (BAL) and transbronchial biopsy. An episode of acute rejection was defined if a biopsy of lung tissue demonstrated grade A1 or B1R or higher rejection or if the patient received high-dose methylprednisolone. Biopsies were assessed according to The International Society for Heart & Lung Transplantation guidelines [12]. Standard treatment for acute rejection was methylprednisolone 1 g IV × 3 days. For refractory rejection, antithymocyte globulin was generally used, although a second course of methylprednisolone may have been used based on clinician discretion.
Follow-up of Lung Transplant Recipients
The standard protocol for outpatient follow-up after initial hospital discharge was weekly for 4 weeks, every 2 weeks for 1 month, every 2–3 months until 12 months post-transplantation, and then every 3–12 months depending on distance from transplantation center and involvement of local pulmonologist. Formal spirometry was performed at all clinic visits and if clinically indicated. All patients were instructed to perform home spirometry and to notify the team if there was a ≥10% decrease in forced expiratory volume in 1 second (FEV1).
Respiratory Virus Testing
RVI testing was done only for evaluation of compatible clinical signs and symptoms or radiographic abnormalities; no surveillance testing in asymptomatic patients was performed. Nasal swabs, washes, sputum, or BAL specimens were tested for respiratory virus RNA using either direct fluorescent antibody (FA) and culture or a laboratory-developed PCR assay that tests for the following 12 viruses: respiratory syncytial virus (RSV), parainfluenza 1–4, influenza A and B, adenovirus, coronavirus, rhinovirus, metapneumovirus, and bocavirus, as previously described [13–18]. The PCR platform was the same throughout the entire study period, and routine testing of all respiratory samples included PCR.
Determination of Exposures and Outcomes
A respiratory viral episode was defined as upper (nasal swab or nasal wash) or lower (sputum, BAL) respiratory specimen positive for a respiratory virus by culture, FA, or PCR. Patients with both upper and lower respiratory tract specimens that were positive were categorized as having a lower respiratory tract infection (LRTI). In patients who tested positive for subsequent RVI after their initial infection, a new viral episode was defined if at least 1 month had passed after the initial positive specimen for a specific respiratory virus pathogen or if there was at least 1 test negative for the initial respiratory virus followed by a newly positive test for the same virus.
CLAD was diagnosed based on routine serial spirometry monitoring. BOS was defined as having an FEV1 ≤ 80% of the patient's prior baseline best FEV1 (average of two best FEV1 values) for at least 3 weeks, and R-CLAD was defined as having a forced vital capacity (FVC) ≤ 80% of the patient's baseline best FVC in addition to meeting the criteria for BOS, both persisting for at least 3 weeks and with no improvement at any measured time in the future [19–21]. Two transplant pulmonologists independently evaluated all patients for CLAD. In the case of differing assessments, they reviewed the primary data and reached a consensus diagnosis. Alternate explanations for the decrease in spirometry, such as airway stenosis, were investigated through chart review. If present at the time of decreased spirometric values, the patient was not considered as having CLAD at that time.
CMV pneumonia was diagnosed based on positive shell vial and/or positive viral culture from a BAL in the setting of compatible symptoms and radiographic findings.
Statistical Analyses
We examined the association between symptomatic RVI and CLAD in both univariate and multivariate models using Cox proportional hazards models. Covariates included age, type of transplantation (bilateral or single lung), acute rejection, and CMV pneumonia. We analyzed CMV pneumonia and acute rejection as time-dependent indicator variables that remained “on” after the first episode of CMV pneumonia or acute rejection for the duration of the person's follow-up. RVI was analyzed as a time-dependent step function for the entire duration of follow-up as well as for specific risk periods (3 months, 6 months, and 12 months) following the diagnosis of RVI. We hypothesized that the relationship of RVI with CLAD would be greater with shorter time periods based on the waning of the immune response to RVI over time. The primary outcome was development of CLAD. A secondary outcome was the composite endpoint of CLAD or death. The Kaplan–Meier method was used to estimate and graph the probability of CLAD and its phenotypes BOS and R-CLAD. The proportional hazards assumption was tested using plots of Schoenfeld residuals. All analyses were performed using Stata software, version 12.1 (Stata Corporation, College Station, Texas).
RESULTS
Cohort
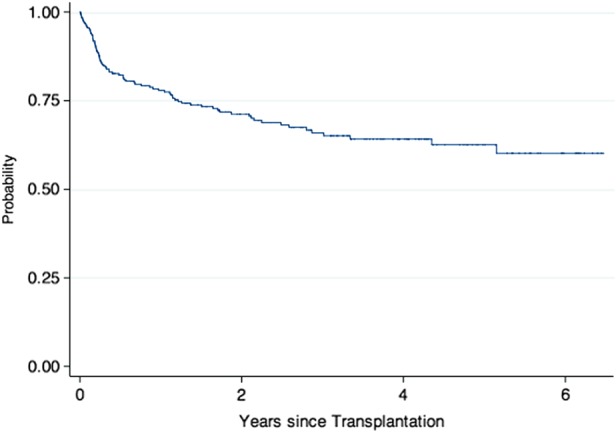
A total of 250 consecutive patients who received a first lung transplant at UWMC between January 2007 and May 2012 were included. Table Table22 shows patient characteristics. There were no significant differences in demographics between patients with RVI and those without (data not shown). CLAD was diagnosed at a median time of 95 weeks (interquartile range [IQR], 53–157 weeks) post-transplantation. Supplementary Figure 1 shows the Kaplan–Meier curve for overall survival. Overall survival of the cohort was at or above that reported among lung transplantation centers in the United States. During the follow-up period, 85 patients (34%) died. One-year survival was 88%, and median time to death was 89 weeks (IQR, 22–144 weeks). At last follow up, mortality was higher for patients with CLAD: 22 (44%) vs 63 (31.5%) without CLAD. Figure Figure11 shows the Kaplan–Meier curve of the development of CLAD and of BOS and R-CLAD separately. The definition of CLAD requires documentation of a decrease in FEV1 of at least 20% for at least 3 weeks. However, to confirm that the functional decline was permanent, we also assessed later time points. Among the 50 patients diagnosed with CLAD, 31 (62%) had pulmonary function test (PFT) information for at least 6 months following CLAD diagnosis. In all of these patients, the drop in FEV1 values was sustained and continued to meet criteria for CLAD. Similarly, PFT values for those patients with <6 months of follow-up from CLAD diagnosis also had persistent spirometry findings consistent with CLAD.
Table 2.
Characteristics of Lung Transplant Recipients
| Characteristic | All (n = 250) n (%) |
|---|---|
| Age, median (range), y | 57 (18–71) |
| Female | 106 (42.4) |
| Underlying diseasea | |
 Idiopathic pulmonary fibrosis Idiopathic pulmonary fibrosis | 78 (31.2) |
 Chronic obstructive pulmonary disease Chronic obstructive pulmonary disease | 67 (26.8) |
 Cystic fibrosis Cystic fibrosis | 52 (20.8) |
 Alpha-1-antitrypsin Alpha-1-antitrypsin | 12 (4.8) |
 Bronchiectasis Bronchiectasis | 6 (2.4) |
| Cytomegalovirus Serostatus | |
 R+ R+ | 132 (52.8) |
 D+/R− D+/R− | 87 (34.8) |
 D−/R− D−/R− | 25 (10) |
 Unknown Unknown | 6 (2.4) |
| Type of Transplantation | |
 Bilateral Bilateral | 207 (82.8) |
| Year of Transplantation | |
 2007–2009 2007–2009 | 139 (55.6) |
 2010–2012 2010–2012 | 111 (44.4) |
| Duration of Follow-up | |
 Median (range), weeks Median (range), weeks | 143 (0.3–338) |
| Developed chronic lung allograft dysfunction | 50 (20.0) |
 Bronchiolitis obliterans syndromeb Bronchiolitis obliterans syndromeb | 33 (66.0) |
 Restrictive chronic lung allograft dysfunctionb Restrictive chronic lung allograft dysfunctionb | 17 (34.0) |
| Died during study | 85 (34.0) |
a Frequencies are shown for the 5 most common diseases in the cohort. All other diseases appeared in 5 or fewer patients.
b Percentage is out of total number of chronic lung allograft dysfunction patients.
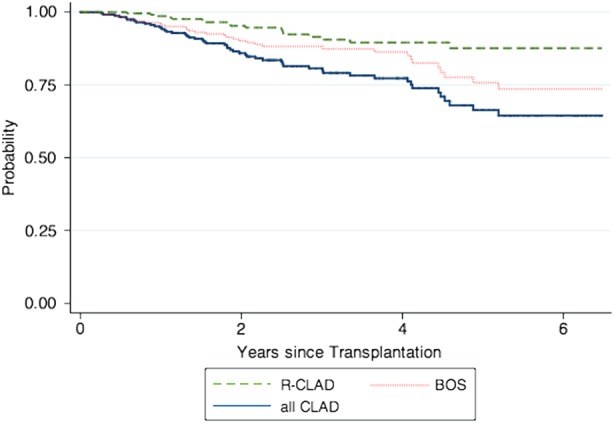
Kaplan–Meier curve showing development of all chronic lung allograft dysfunction (CLAD), bronchiolitis obliterans syndrome (BOS), and restrictive CLAD (R-CLAD).
Respiratory Tract Infection
A total of 114 viral episodes were diagnosed in 79 patients (31.6%). Table Table33 details the characteristics of the RVI episodes. Median time to first RVI was 19 weeks (IQR, 9–63 weeks). Nine viral episodes had more than 1 virus isolated, 8 had 2 viruses (all had rhinovirus as 1 of the viruses), and 1 had 3 viruses for a total of 124 viruses isolated. Figure Figure22 is a Kaplan–Meier curve showing the time to development of first RVI following transplantation. Among the 50 patients with CLAD, 21 (42%) had 1 or more RVIs preceding CLAD diagnosis compared with 56 (28%) patients who remained CLAD-free during follow-up.
Table 3.
Details of Respiratory Virus Infection
| Respiratory Virus Infection Characteristic | n (%) |
|---|---|
| Unique patients with respiratory virus infection | 79 |
| Respiratory viral episodes | 114 |
| Lower tract infectiona | 97 (85.1) |
| Viral episodes per patient | |
 1 1 | 50 |
 2 2 | 25 |
 ≥3b ≥3b | 4 |
| Respiratory virusc | |
 Rhinovirus Rhinovirus | 42 (33.9) |
 Parainfluenza 1–4 Parainfluenza 1–4 | 21 (16.9) |
 Coronavirus Coronavirus | 20 (16.1) |
 Influenza A, B Influenza A, B | 16 (12.9) |
 Adenovirus Adenovirus | 10 (8.1) |
 Respiratory syncytial virus Respiratory syncytial virus | 10 (8.1) |
 Metapneumovirus Metapneumovirus | 4 (3.2) |
 Bocavirus Bocavirus | 1 (0.8) |
| Viral episodes with >1 virusa | 9 (8.7) |
| Diagnosed by polymerase chain reaction | 107 (93.9)d |
a Percent of viral episodes (114).
b Three patients had 3 episodes, and 1 had 5 episodes.
c Percent of viruses isolated (124).
d Other 7 viral episodes diagnosed by fluorescent antibody, culture, or both.
Association of RVI With Outcomes
We analyzed the relationship between several factors and CLAD in both univariate and multivariate analyses in a time-dependent manner, and modeled periods of risk of 3 months, 6 months, 12 months, and “ever” (ie, at last follow-up) for RVI. In both univariate and multivariate analysis, RVI at all modeled risk periods was independently associated with development of CLAD (Table (Table4).4). The association was stronger at shorter modeled periods of risk. We confirmed the previously reported association of acute rejection with development of CLAD (Table (Table4).4). Our secondary endpoint, the composite endpoint of CLAD and death, showed a similar trend as the primary endpoint in both univariate and multivariate analysis, with a higher hazard ratio (HR) of RVI at 3 months and progressively declining HR as time from RVI increased (HR, 4.85; 95% confidence interval [CI], 2.76–8.43; P < .01 vs HR, 2.75; 95% CI, 1.72–4.37; P < .01 at 3 months vs 12 months post-RVI, respectively). We also analyzed the association between gastroesophageal reflux disease (GERD) and CLAD using 2 methods, first requiring a positive pH probe test for GERD diagnosis and second including anyone with a clinical diagnosis of GERD. No statistically significant association was seen in univariate analysis (data not shown) with either definition of GERD; therefore, GERD was not included in the final models. We were also interested in whether 1 CLAD phenotype was more strongly associated with RVI than the other (R-CLAD vs BOS). While the numbers are small, precluding a formal analysis, overall, a higher percentage of R-CLAD patients had at least 1 preceding viral episode as compared with BOS (9/17 [52.9%] vs 12/33 [36.4%], respectively).
Table 4.
Univariate and Multivariate Analyses of the Development of Chronic Lung Allograft Dysfunction Modeled for Differing Risk Periods for Respiratory Virus Infection
| Variable | HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value |
|---|---|---|---|---|
| Respiratory virus infection (“ever”) | ||||
 Age Age | 1.01 (.99–1.03) | .22 | 1.01 (.99–1.03) | .27 |
 Bilateral transplant Bilateral transplant | 0.87 (.39–1.93) | .73 | 1.15 (.49–2.71) | .74 |
 Rejection Rejection | 2.33 (1.30–4.18) | <.01 | 2.16 (1.18–3.93) | .01 |
 RVI RVI | 2.14 (1.20–3.82) | .01 | 1.92 (1.07–3.45) | .03 |
 Cytomegalovirus pneumonia Cytomegalovirus pneumonia | 1.44 (.74–2.80) | .28 | 1.16 (.59–2.29) | .67 |
| Risk period following RVIa | ||||
  3 mo 3 mo | 5.36 (2.20–13.04) | <.01 | 4.77 (1.91–11.64) | <.01 |
  6 mo 6 mo | 3.75 (1.69–8.29) | <.01 | 3.37 (1.50–7.54) | <.01 |
  12 mo 12 mo | 2.70 (1.34–5.41) | <.01 | 2.44 (1.20–4.96) | .02 |
Abbreviations: CI, confidence interval; HR, hazard ratio; RVI, respiratory virus infection.
a HR adjusted for age, bilateral vs single transplant, rejection, and cytomegalovirus pneumonia in multivariate analysis. However, HRs were similar to those for the no-risk period, so data not shown.
DISCUSSION
In a large contemporary cohort of LTRs who underwent symptom-guided testing for RVI using molecular diagnostic methods, we found high rates of RVI and an independent association between symptomatic RVI and CLAD. The association between RVI and CLAD was stronger with shorter modeled periods of risk but was also present when all follow-up time was considered. We demonstrate for the first time that the strength of the association of symptomatic RVI with CLAD is significantly influenced by time (eg, the proximity of the RVI episode with CLAD diagnosis). The stronger association in the first several months after RVI has important implications for future trials of antiviral agents, including powering trials for an endpoint of CLAD. This study also addresses several important limitations of prior studies, significantly extends our understanding of the role of RVI and CLAD, and provides a strong rationale for the conduct of large prospective studies to characterize the specific virologic determinants and mechanistic links between RVI and CLAD.
Several prior studies have assessed the association between RVI and BOS (Table (Table1).1). Magnusson et al found an association between BOS and RVI (odds ratio, 3.0; 95% CI, 1.3–6.8; P = .008) in a retrospective cohort study [6]. However, they only considered LRTIs based largely on surveillance BALs and their cohort was small (38 LTRs, 14 with RVIs). While they followed the LTRs for 10 years for outcomes, RVI episodes were only recorded for the first year post-transplantation. Khalifah et al also reported an association between RVI and BOS in univariate and multivariate analyses in a retrospective cohort study [2], but they assessed only 4 viruses using nonmolecular methods, did not distinguish BOS and R-CLAD or use blinded reviewer assessment of CLAD, and included relatively few cases of RVI [22]. Billings et al found no relationship between RVI and CLAD overall, but a subanalysis demonstrated an association with high-grade BOS only for LRTI and had many of the same limitations as that of Khalifah et al [9]. In contrast to the above studies, Milstone et al reported no association; however, the sample size was small (only 4 BOS cases, 17 RVIs) [8]. Thus, these prior studies were relatively heterogeneous and had 1 or more significant limitations that might explain discrepancies with the present study.
Our study had important strengths. We examined a large cohort of patients (among the largest to assess this issue) and diagnosed RVI using modern molecular diagnostic techniques that detected a large number of respiratory viruses. We used time-dependent multivariate analyses that controlled for factors associated previously with CLAD and also modeled differing periods of risk to provide insight into mechanisms the underlie the relationship between RVI and CLAD. Importantly, the primary endpoint (CLAD) was assigned using recently developed consensus definitions and by 2 blinded transplant pulmonologists. We also acknowledge certain limitations, including the retrospective single-center study design. Despite comprehensive medical records and close follow-up, we cannot exclude missing data. Thus, our rates of RVI should be considered minimal estimates because patients may have been diagnosed outside our medical center. We were not able to assess all the factors that have been linked to CLAD, including pseudomonas pneumonia and/or colonization [22–24] and small conidial forms of Aspergillus [22]. However, there is no consensus that these are proven risk factors for CLAD. Our study size was not large enough to permit assessment of risk of CLAD associated with specific viral organisms, nor was it large enough to assess differences between the 2 CLAD phenotypes, BOS and R-CLAD.
Although we found an independent association of symptomatic RVI with CLAD, it is possible that RVI could simply be a marker for patients who are otherwise predisposed to CLAD, rather than a cause of CLAD. There are several lines of evidence suggesting that RVI might be an underrecognized contributor (rather than marker) in a substantial proportion of CLAD cases. As summarized in Table Table1,1, RVI has been linked to CLAD in several prior studies [2, 6–10, 25–29]. RVI is relatively common in LTRs, and a significant proportion of cases detected by sensitive methods can be asymptomatic. Importantly, both symptomatic and asymptomatic RVI has been linked to CLAD. Additionally, in vitro and animal studies have identified specific biologically plausible mechanisms through which RVI could mediate CLAD [30, 31]. RVI-mediated regulatory T-cell dysfunction leading to augmentation of innate, allo-, and auto-immune pathways are possible mechanistic pathways to explain a causal link between RVI and CLAD. Finally, a small clinical trial of a novel antiviral (siRNA) for a specific RVI (RSV) in LTRs demonstrated reduced risk for CLAD in the group randomized to active RSV therapy [32]. In total, these epidemiologic and experimental data are consistent with a causal association between RVI and CLAD.
In summary, our study addresses many of the limitations of prior studies and adds significantly to the body of evidence linking RVI to development of CLAD. This association is relevant as RVI could represent a distinct, diagnosable, and potentially treatable major cause of allograft dysfunction and loss in LTRs. If the association were confirmed in future prospective studies, at a minimum, it could identify specific patients at increased risk for development of CLAD who could then be entered into trials of preventive or treatment strategies. With the development of new antivirals for several respiratory viruses [32–35], it will be possible to directly test the hypothesis that RVI causes CLAD by means of interventional controlled trials. Prior to embarking on such studies, it is imperative to carefully characterize the virologic determinants and mechanisms that underlie CLAD in order to aid in the design of such interventional trials. While our study strongly suggests an association between symptomatic RVI and CLAD, we propose a large, multicenter, prospective natural history study with careful virologic and clinical assessments and mechanistic studies as a logical next step. A better understanding of the role of respiratory viruses in the pathogenesis of CLAD through future prospective studies is critical in devising rational strategies to improve long-term patient and graft survival in LTRs.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We gratefully acknowledge Michaela Kusumi and Fatima Ali for their assistance with the human participants application process and data collection.
We gratefully acknowledge Michaela Kusumi and Fatima Ali for their assistance with the human participants application process and data collection.
Financial support. M. B. was supported by National Institutes of Health
K24 HL093294.
M. B. was supported by National Institutes of Health
K24 HL093294.
Potential conflicts of interest. A. P. L. has received research funding through Gilead and Ansun Biopharma. M. B. has served as a consultant for Gilead Sciences and Roche/Genentech. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
A. P. L. has received research funding through Gilead and Ansun Biopharma. M. B. has served as a consultant for Gilead Sciences and Roche/Genentech. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Articles from Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/cid/civ871
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/cid/article-pdf/62/3/313/16789346/civ871.pdf
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1093/cid/civ871
Article citations
Association between Respiratory Virus Infection and Development of <i>De Novo</i> Donor-Specific Antibody in Lung Transplant Recipients.
Viruses, 16(10):1574, 05 Oct 2024
Cited by: 0 articles | PMID: 39459908 | PMCID: PMC11512259
Immune responses of lung transplant recipients against SARS-CoV-2 and common respiratory coronaviruses: Evidence for pre-existing cross-reactive immunity.
Transpl Immunol, 81:101940, 20 Oct 2023
Cited by: 0 articles | PMID: 37866672
Identifying risk factors for cutaneous disease among solid organ transplant recipients: A retrospective review.
JAAD Int, 11:157-164, 23 Feb 2023
Cited by: 0 articles | PMID: 37128267 | PMCID: PMC10148151
Immunocompromised Host Pneumonia: Definitions and Diagnostic Criteria: An Official American Thoracic Society Workshop Report.
Ann Am Thorac Soc, 20(3):341-353, 01 Mar 2023
Cited by: 10 articles | PMID: 36856712 | PMCID: PMC9993146
The effect of COVID-19 on transplant function and development of CLAD in lung transplant patients: A multicenter experience.
J Heart Lung Transplant, 41(9):1237-1247, 19 Jun 2022
Cited by: 7 articles | PMID: 35843852 | PMCID: PMC9212897
Go to all (51) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Association between Respiratory Virus Infection and Development of <i>De Novo</i> Donor-Specific Antibody in Lung Transplant Recipients.
Viruses, 16(10):1574, 05 Oct 2024
Cited by: 0 articles | PMID: 39459908 | PMCID: PMC11512259
Invasive fungal infections after respiratory viral infections in lung transplant recipients are associated with lung allograft failure and chronic lung allograft dysfunction within 1 year.
J Heart Lung Transplant, 42(7):953-963, 19 Feb 2023
Cited by: 0 articles | PMID: 36925381
Community-acquired Respiratory Viruses Are a Risk Factor for Chronic Lung Allograft Dysfunction.
Clin Infect Dis, 69(7):1192-1197, 01 Sep 2019
Cited by: 24 articles | PMID: 30561555 | PMCID: PMC7797743
When tissue is the issue: A histological review of chronic lung allograft dysfunction.
Am J Transplant, 20(10):2644-2651, 15 Apr 2020
Cited by: 15 articles | PMID: 32185874
Review
Funding
Funders who supported this work.
NHLBI NIH HHS (1)
Grant ID: K24 HL093294
National Institutes of Health (1)
Grant ID: K24 HL093294