Abstract
Free full text

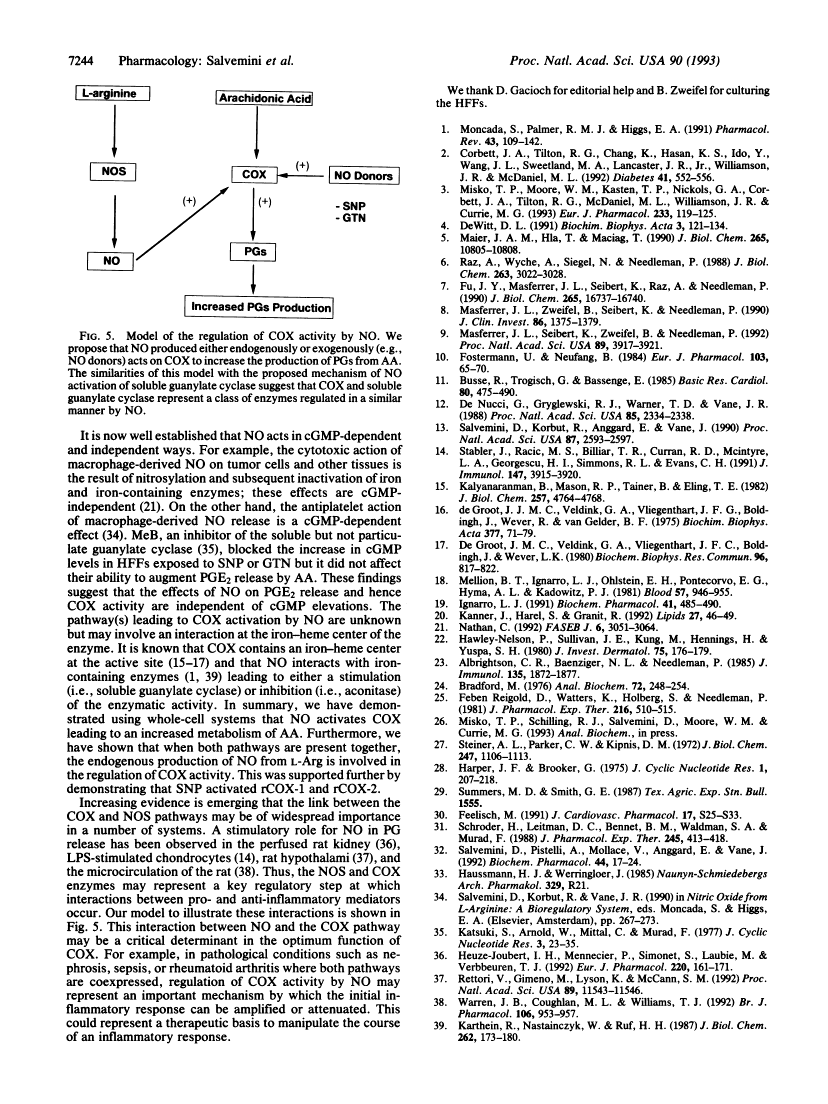
Nitric oxide activates cyclooxygenase enzymes.
Abstract
We have evaluated the role of nitric oxide (NO) on the activity of the constitutive and induced forms of cyclooxygenase (COX; COX-1 and COX-2, respectively). Induction of NO synthase (NOS) and COX (COX-2) in the mouse macrophage cell line RAW264.7 by Escherichia coli lipopolysaccharide (1 microgram/ml, 18 h) caused an increase in the release of nitrite (NO2-) and prostaglandin E2 (PGE2), products of NOS and COX, respectively. Production of both NO2- and PGE2 was blocked by the NOS inhibitors NG-monomethyl-L-arginine or aminoguanidine. The effects of NG-monomethyl-L-arginine or aminoguanidine were reversed by coincubation with L-Arg, the precursor for NO synthesis, but not by D-Arg. RAW264.7 cells stimulated for 18 h with lipopolysaccharide in L-Arg-free medium (to reduce NO generation by the endogenous NOS pathway) failed to release NO2- and accumulated at least 4-fold less PGE2 when compared to cells in the presence of L-Arg. PGE2 production elicited by a 15-min arachidonic acid treatment of lipopolysaccharide-induced RAW264.7 cells in L-Arg-deficient medium was decreased 3-fold when compared to the release obtained with cells induced in medium containing L-Arg. To examine the NO activation of the induced form of COX in the absence of an endogenous L-Arg, human fetal fibroblasts were first stimulated for 18 h with interleukin 1 beta. These cells released PGE2 but not NO2-, consistent with the induction of COX but not NOS in the fibroblast. Exogenous NO either as a gaseous solution or released by a NO donor, sodium nitroprusside or glyceryl trinitrate, increased COX activity in the interleukin 1 beta-stimulated fibroblasts by 5-fold; these effects were abolished by coincubation with hemoglobin (10 microM), which binds and inactivates NO, but not by methylene blue, an inhibitor of the soluble guanylate cyclase. Furthermore, sodium nitroprusside (0.25-1 mM) increased arachidonic acid-stimulated PGE2 production by murine recombinant COX-1 and COX-2. These results demonstrate that NO enhances COX activity through a mechanism independent of cGMP and suggest that, in conditions in which both the NOS and COX systems are present, there is an NO-mediated increase in the production of proinflammatory prostaglandins that may result in an exacerbated inflammatory response. The data suggest that NO directly interacts with COX to cause an increase in the enzymatic activity.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.1M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [Abstract] [Google Scholar]
- Corbett JA, Tilton RG, Chang K, Hasan KS, Ido Y, Wang JL, Sweetland MA, Lancaster JR, Jr, Williamson JR, McDaniel ML. Aminoguanidine, a novel inhibitor of nitric oxide formation, prevents diabetic vascular dysfunction. Diabetes. 1992 Apr;41(4):552–556. [Abstract] [Google Scholar]
- Misko TP, Moore WM, Kasten TP, Nickols GA, Corbett JA, Tilton RG, McDaniel ML, Williamson JR, Currie MG. Selective inhibition of the inducible nitric oxide synthase by aminoguanidine. Eur J Pharmacol. 1993 Mar 16;233(1):119–125. [Abstract] [Google Scholar]
- DeWitt DL. Prostaglandin endoperoxide synthase: regulation of enzyme expression. Biochim Biophys Acta. 1991 May 8;1083(2):121–134. [Abstract] [Google Scholar]
- Maier JA, Hla T, Maciag T. Cyclooxygenase is an immediate-early gene induced by interleukin-1 in human endothelial cells. J Biol Chem. 1990 Jul 5;265(19):10805–10808. [Abstract] [Google Scholar]
- Raz A, Wyche A, Siegel N, Needleman P. Regulation of fibroblast cyclooxygenase synthesis by interleukin-1. J Biol Chem. 1988 Feb 25;263(6):3022–3028. [Abstract] [Google Scholar]
- Fu JY, Masferrer JL, Seibert K, Raz A, Needleman P. The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes. J Biol Chem. 1990 Oct 5;265(28):16737–16740. [Abstract] [Google Scholar]
- Masferrer JL, Zweifel BS, Seibert K, Needleman P. Selective regulation of cellular cyclooxygenase by dexamethasone and endotoxin in mice. J Clin Invest. 1990 Oct;86(4):1375–1379. [Europe PMC free article] [Abstract] [Google Scholar]
- Masferrer JL, Seibert K, Zweifel B, Needleman P. Endogenous glucocorticoids regulate an inducible cyclooxygenase enzyme. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3917–3921. [Europe PMC free article] [Abstract] [Google Scholar]
- Förstermann U, Neufang B. The endothelium-dependent vasodilator effect of acetylcholine: characterization of the endothelial relaxing factor with inhibitors of arachidonic acid metabolism. Eur J Pharmacol. 1984 Aug 3;103(1-2):65–70. [Abstract] [Google Scholar]
- Busse R, Trogisch G, Bassenge E. The role of endothelium in the control of vascular tone. Basic Res Cardiol. 1985 Sep-Oct;80(5):475–490. [Abstract] [Google Scholar]
- de Nucci G, Gryglewski RJ, Warner TD, Vane JR. Receptor-mediated release of endothelium-derived relaxing factor and prostacyclin from bovine aortic endothelial cells is coupled. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2334–2338. [Europe PMC free article] [Abstract] [Google Scholar]
- Salvemini D, Korbut R, Anggård E, Vane J. Immediate release of a nitric oxide-like factor from bovine aortic endothelial cells by Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2593–2597. [Europe PMC free article] [Abstract] [Google Scholar]
- Stadler J, Stefanovic-Racic M, Billiar TR, Curran RD, McIntyre LA, Georgescu HI, Simmons RL, Evans CH. Articular chondrocytes synthesize nitric oxide in response to cytokines and lipopolysaccharide. J Immunol. 1991 Dec 1;147(11):3915–3920. [Abstract] [Google Scholar]
- Kalyanaraman B, Mason RP, Tainer B, Eling TE. The free radical formed during the hydroperoxide-mediated deactivation of ram seminal vesicles is hemoprotein-derived. J Biol Chem. 1982 May 10;257(9):4764–4768. [Abstract] [Google Scholar]
- de Groot JJ, Veldink GA, Vliegenthart JF, Boldingh J, Wever R, van Gelder BF. Demonstration by EPR spectroscopy of the functional role of iron in soybean lipoxygenase-1. Biochim Biophys Acta. 1975 Jan 23;377(1):71–79. [Abstract] [Google Scholar]
- Greenwald JE, Alexander MS, Fertel RH, Beach CA, Wong LK, Bianchine JR. Role of ferric iron in platelet lipoxygenase activity. Biochem Biophys Res Commun. 1980 Sep 30;96(2):817–822. [Abstract] [Google Scholar]
- Mellion BT, Ignarro LJ, Ohlstein EH, Pontecorvo EG, Hyman AL, Kadowitz PJ. Evidence for the inhibitory role of guanosine 3', 5'-monophosphate in ADP-induced human platelet aggregation in the presence of nitric oxide and related vasodilators. Blood. 1981 May;57(5):946–955. [Abstract] [Google Scholar]
- Ignarro LJ. Signal transduction mechanisms involving nitric oxide. Biochem Pharmacol. 1991 Feb 15;41(4):485–490. [Abstract] [Google Scholar]
- Kanner J, Harel S, Granit R. Nitric oxide, an inhibitor of lipid oxidation by lipoxygenase, cyclooxygenase and hemoglobin. Lipids. 1992 Jan;27(1):46–49. [Abstract] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [Abstract] [Google Scholar]
- Hawley-Nelson P, Sullivan JE, Kung M, Hennings H, Yuspa SH. Optimized conditions for the growth of human epidermal cells in culture. J Invest Dermatol. 1980 Aug;75(2):176–182. [Abstract] [Google Scholar]
- Albrightson CR, Baenziger NL, Needleman P. Exaggerated human vascular cell prostaglandin biosynthesis mediated by monocytes: role of monokines and interleukin 1. J Immunol. 1985 Sep;135(3):1872–1877. [Abstract] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. [Abstract] [Google Scholar]
- Reingold DF, Watters K, Holmberg S, Needleman P. Differential biosynthesis of prostaglandins by hydronephrotic rabbit and cat kidneys. J Pharmacol Exp Ther. 1981 Mar;216(3):510–515. [Abstract] [Google Scholar]
- Steiner AL, Parker CW, Kipnis DM. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [Abstract] [Google Scholar]
- Harper JF, Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [Abstract] [Google Scholar]
- Schröder H, Leitman DC, Bennett BM, Waldman SA, Murad F. Glyceryl trinitrate-induced desensitization of guanylate cyclase in cultured rat lung fibroblasts. J Pharmacol Exp Ther. 1988 May;245(2):413–418. [Abstract] [Google Scholar]
- Salvemini D, Pistelli A, Mollace V, Anggård E, Vane J. The metabolism of glyceryl trinitrate to nitric oxide in the macrophage cell line J774 and its induction by Escherichia coli lipopolysaccharide. Biochem Pharmacol. 1992 Jul 7;44(1):17–24. [Abstract] [Google Scholar]
- Katsuki S, Arnold W, Mittal C, Murad F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J Cyclic Nucleotide Res. 1977 Feb;3(1):23–35. [Abstract] [Google Scholar]
- Heuzé-Joubert I, Mennecier P, Simonet S, Laubie M, Verbeuren TJ. Effect of vasodilators, including nitric oxide, on the release of cGMP and cAMP in the isolated perfused rat kidney. Eur J Pharmacol. 1992 Sep 22;220(2-3):161–171. [Abstract] [Google Scholar]
- Rettori V, Gimeno M, Lyson K, McCann SM. Nitric oxide mediates norepinephrine-induced prostaglandin E2 release from the hypothalamus. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11543–11546. [Europe PMC free article] [Abstract] [Google Scholar]
- Warren JB, Coughlan ML, Williams TJ. Endotoxin-induced vasodilatation in anaesthetized rat skin involves nitric oxide and prostaglandin synthesis. Br J Pharmacol. 1992 Aug;106(4):953–957. [Europe PMC free article] [Abstract] [Google Scholar]
- Karthein R, Nastainczyk W, Ruf HH. EPR study of ferric native prostaglandin H synthase and its ferrous NO derivative. Eur J Biochem. 1987 Jul 1;166(1):173–180. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.90.15.7240
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc47112?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.90.15.7240
Article citations
Ethanol extract from Astilbe chinensis inflorescence suppresses inflammation in macrophages and growth of oral pathogenic bacteria.
PLoS One, 19(7):e0306543, 03 Jul 2024
Cited by: 0 articles | PMID: 38959234 | PMCID: PMC11221678
Adjuvant-induced arthritis promotes vascular hyporesponsiveness to phenylephrine through a nitric oxide-related mechanism.
Braz J Med Biol Res, 57:e13304, 17 May 2024
Cited by: 0 articles | PMID: 38775546 | PMCID: PMC11101166
Immunosuppressive effects of the mycotoxin patulin in macrophages.
Arch Microbiol, 206(4):166, 15 Mar 2024
Cited by: 0 articles | PMID: 38485821
Review
Role of Helicobacter pylori Infection in Pathogenesis, Evolution, and Complication of Atherosclerotic Plaque.
Biomedicines, 12(2):400, 08 Feb 2024
Cited by: 1 article | PMID: 38398002 | PMCID: PMC10886498
Review Free full text in Europe PMC
Anti-Inflammatory Properties of Oxygenated Isocoumarins and Xanthone from Thai Mangrove-Associated Endophytic Fungus Setosphaeria rostrata.
Molecules, 29(3):603, 26 Jan 2024
Cited by: 3 articles | PMID: 38338348 | PMCID: PMC10856793
Go to all (790) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Co-induction of nitric oxide synthase and cyclo-oxygenase: interactions between nitric oxide and prostanoids.
Br J Pharmacol, 114(7):1335-1342, 01 Apr 1995
Cited by: 215 articles | PMID: 7541688 | PMCID: PMC1510271
Endogenous nitric oxide enhances prostaglandin production in a model of renal inflammation.
J Clin Invest, 93(5):1940-1947, 01 May 1994
Cited by: 184 articles | PMID: 7514189 | PMCID: PMC294301
The effect of nitric oxide on cytokine-induced release of PGE2 by human cultured astroglial cells.
Br J Pharmacol, 124(4):742-746, 01 Jun 1998
Cited by: 45 articles | PMID: 9690866 | PMCID: PMC1565428
[Analysis of the mechanism for the anti-inflammatory effect of the anti-rheumatic drug auranofin].
Yakugaku Zasshi, 120(3):265-274, 01 Mar 2000
Cited by: 0 articles | PMID: 10723268
Review