Abstract
Background
Pancreatic stellate cells (PSCs, which produce the stroma of pancreatic cancer (PC)) interact with cancer cells to facilitate PC growth. A candidate growth factor pathway that may mediate this interaction is the HGF-c-MET pathway.Methods
Effects of HGF inhibition (using a neutralising antibody AMG102) alone or in combination with gemcitabine were assessed (i) in vivo using an orthotopic model of PC, and (ii) in vitro using cultured PC cells (AsPC-1) and human PSCs.Results
We have shown that human PSCs (hPSCs) secrete HGF but do not express the receptor c-MET, which is present predominantly on cancer cells. HGF inhibition was as effective as standard chemotherapy in inhibiting local tumour growth but was significantly more effective than gemcitabine in reducing tumour angiogenesis and metastasis. HGF inhibition has resulted in reduced metastasis; however, interestingly this antimetastatic effect was lost when combined with gemcitabine. This suggests that gemcitabine treatment selects out a subpopulation of cancer cells with increased epithelial-mesenchymal transition (EMT) and stem-cell characteristics, as supported by our findings of increased expression of EMT and stem-cell markers in tumour sections from our animal model. In vitro studies showed that hPSC secretions induced proliferation and migration, but inhibited apoptosis, of cancer cells. These effects were countered by pretreatment of hPSC secretions with a HGF-neutralising antibody but not by gemcitabine, indicating a key role for HGF in PSC-PC interactions.Conclusions
Our studies suggest that targeted therapy to inhibit stromal-tumour interactions mediated by the HGF-c-MET pathway may represent a novel therapeutic approach in PC that will require careful modelling for optimal integration with existing treatment modalities.Free full text

Hepatocyte growth factor inhibition: a novel therapeutic approach in pancreatic cancer
Abstract
Background:
Pancreatic stellate cells (PSCs, which produce the stroma of pancreatic cancer (PC)) interact with cancer cells to facilitate PC growth. A candidate growth factor pathway that may mediate this interaction is the HGF–c-MET pathway.
Methods:
Effects of HGF inhibition (using a neutralising antibody AMG102) alone or in combination with gemcitabine were assessed (i) in vivo using an orthotopic model of PC, and (ii) in vitro using cultured PC cells (AsPC-1) and human PSCs.
Results:
We have shown that human PSCs (hPSCs) secrete HGF but do not express the receptor c-MET, which is present predominantly on cancer cells. HGF inhibition was as effective as standard chemotherapy in inhibiting local tumour growth but was significantly more effective than gemcitabine in reducing tumour angiogenesis and metastasis. HGF inhibition has resulted in reduced metastasis; however, interestingly this antimetastatic effect was lost when combined with gemcitabine. This suggests that gemcitabine treatment selects out a subpopulation of cancer cells with increased epithelial–mesenchymal transition (EMT) and stem-cell characteristics, as supported by our findings of increased expression of EMT and stem-cell markers in tumour sections from our animal model. In vitro studies showed that hPSC secretions induced proliferation and migration, but inhibited apoptosis, of cancer cells. These effects were countered by pretreatment of hPSC secretions with a HGF-neutralising antibody but not by gemcitabine, indicating a key role for HGF in PSC–PC interactions.
Conclusions:
Our studies suggest that targeted therapy to inhibit stromal–tumour interactions mediated by the HGF–c-MET pathway may represent a novel therapeutic approach in PC that will require careful modelling for optimal integration with existing treatment modalities.
Pancreatic ductal adenocarcinoma (PDAC) or pancreatic cancer (PC) is the fourth leading cause of cancer-related death in western societies, with a 5-year survival rate of <4% (Siegel et al, 2013; Yadav and Lowenfels, 2013) and is predicted to become the second leading cause of cancer-related deaths in the United States of America by 2020 (Rahib et al, 2014). Despite aggressive treatments with surgery, chemotherapy and radiotherapy, the clinical outcome of this disease has remained poor. Histologically, PDAC is characterised by a prominent desmoplastic/stromal reaction consisting of extracellular matrix proteins such as collagen, fibronectin, proteoglycans, hyaluronan and stromal cells, including pancreatic stellate cells (PSCs), immune cells, endothelial cells and neuronal cells. Accumulating evidence indicates that the stromal reaction may have an important role in the progression of PDAC (Vonlaufen et al, 2008b; Xu et al, 2014; Pothula et al, 2015). Inhibition of the stromal–tumour interactions should be considered in designing therapeutic strategies to improve PC outcomes (Oettle, 2014).
Our group was the first to establish that PSCs (the effector cells in pancreatic fibrogenesis) are the predominant source of the abundant collagen in the stromal reaction around PC cells in the stroma (Apte et al, 2004). Importantly, we and others have shown that there is a close interaction between PSCs and PC cells that facilitates the growth of tumours within the pancreas and also increases regional as well as distant metastasis (Apte et al, 2004; Bachem et al, 2005; Hwang et al, 2008; Vonlaufen et al, 2008a; Vonlaufen et al, 2008b; Erkan et al, 2009). In vitro studies have established that PSC secretions stimulate proliferation but inhibit apoptosis of PC cells, thereby facilitating cancer cell survival. In turn, PC cell secretions induce PSC activation as assessed by increased proliferation, migration and production of extracellular matrix proteins (Bachem et al, 2005; Hwang et al, 2008; Erkan et al, 2009; Habisch et al, 2010). These in vitro observations have been well supported by findings in orthotopic models of PC, which have shown that injection of a mixture of PC cells and PSCs into the pancreas of immunocompromised mice leads to larger tumours and higher rates of metastasis when compared with mice injected with cancer cells alone (Vonlaufen et al, 2008a; Xu et al, 2010). Importantly, these tumours exhibit a strong stromal reaction surrounding cancer cells, thus resembling the histological features of human PC (Vonlaufen et al, 2008a; Xu et al, 2010).
Factors/pathways mediating the observed interactions between PSCs and cancer cells are yet to be fully elucidated, although in vitro studies have suggested that growth factors such as platelet-derived growth factor, vascular endothelial growth factor and transforming growth factor beta may have a role in the cross-talk between the two cell types (Lohr et al, 2001; Vonlaufen et al, 2008a). Recently, another growth factor, the hepatocyte growth factor (HGF) has been receiving some attention with regard to its possible role in the pathogenesis of PC (Matsushita et al, 2007; Watanabe et al, 2011; Gholamin et al, 2014). Upregulation of HGF and/or its receptor c-MET have been reported in several malignancies and are often associated with poor prognosis (Birchmeier et al, 2003; Canadas et al, 2010; Yang et al, 2011). In PC, increased serum HGF levels or increased pancreatic expression of c-MET have been reported to be associated with poor prognosis (Ebert et al, 1994; Ide et al, 2007; Kemik et al, 2009; Nones et al, 2014; Zhou et al, 2014). However, the HGF–c-MET pathway has not been fully characterised, particularly with regard to stromal–tumour interactions in PDAC.
HGF (also known as scatter factor) is a 90-kDa glycoprotein, secreted by mesenchymal cells (Jiang et al, 2005). Its transmembrane cell surface receptor, MET, a disulphide-linked heterodimer, is a product of the proto-oncogene c-MET and is usually expressed on epithelial cells. HGF–c-MET signalling between mesenchymal and epithelial cells occurs during embryonic development of numerous organs, including kidney, mammary gland, liver, muscle and neural tissues. Binding of HGF to its receptor leads to dimerisation and phosphorylation of c-MET and subsequent activation of several signalling pathways, including MAPK and PI3K, that regulate proliferation, invasion and migration of cancer cells (Birchmeier et al, 2003).
We hypothesise that the HGF-c-MET pathway mediates the facilitatory influence of PSCs on PDAC progression and that targeting this pathway will inhibit local tumour growth and metastasis. In this study, we specifically aimed to determine the effect of inhibition of the ligand HGF on PDAC progression in vivo and to determine the role of HGF in the interactions between PSCs and cancer cells in vitro.
Materials and methods
Orthotopic model of PC
The orthotopic model has been described in detail previously (Vonlaufen et al, 2008b; Xu et al, 2010). Briefly, 6–8-week-old female athymic nude mice (BALBc nu/nu) were anaesthetised and an incision was made in the left flank followed by exteriorisation of the spleen and tail of the pancreas. A mixture of human PC cells (AsPC-1) 1 × 106+human PSCs (hPSCs) 1 × 106 in 50 μl PBS was injected into the tail of pancreas. After the intrapancreatic injection, the organs were replaced and the abdomen was closed. One batch of mice (n=12) was killed 1 week (day 7) postsurgery to assess tumour size so as to ensure similar pretreatment tumour volumes. Other mice, which were to receive treatment, were randomised into subgroups (n=9 mice per group) on postsurgical day 7. These mice were then treated as detailed below for a further 6 weeks (treatment time limited by UNSW ethics approval ACEC 12/102B; all treatments were administered as twice weekly i.p. injections):
μl PBS was injected into the tail of pancreas. After the intrapancreatic injection, the organs were replaced and the abdomen was closed. One batch of mice (n=12) was killed 1 week (day 7) postsurgery to assess tumour size so as to ensure similar pretreatment tumour volumes. Other mice, which were to receive treatment, were randomised into subgroups (n=9 mice per group) on postsurgical day 7. These mice were then treated as detailed below for a further 6 weeks (treatment time limited by UNSW ethics approval ACEC 12/102B; all treatments were administered as twice weekly i.p. injections):
HGF-neutralising antibody (AMG102, 300
 μg per mouse);
μg per mouse);IgG control;
Gemcitabine (75
 mg per Kg BW); and
mg per Kg BW); andCombination of AMG102 and gemcitabine.
The doses of AMG102 and gemcitabine used in this study were based on our preliminary dose–response studies (Arun et al, 2011) involving mice that received injections into the pancreas of AsPC-1 alone or a mixture of AsPC-1+hPSCs. Notably, AsPC-1 alone tumours did not respond to AMG102 (details in Results section). Thus doses selected for use in this study represent those which yielded optimal inhibitory effects on the growth of tumours produced by injection of AsPC-1+hPSCs into the pancreas. AsPC-1 cells were selected as, in our hands, they have a take rate of 100% and exhibit high reproducibility of tumour growth rates and metastasis.
Pancreatic tumour growth was monitored by palpation. Mice were killed at 6 weeks from the first i.p. injection. Tumours were resected and assessed for size and volume by two separate observers (tumour volume was calculated to two decimal points, according to an established formula (1/2(length × breadth × width) using digital Vernier calipers (Intech tools, Thomas town, VIC, Australia); Xu et al, 2010). Tumour tissue was then divided for further processing (histological and biochemical analysis). The abdominal cavity, mesentery, spleen, liver and lungs were examined and scored according to the presence or absence of visible metastatic nodules. Tumours were compared with respect to size and weight, stellate cell activation (alpha-smooth muscle actin (α-SMA)) and expression of angiogenic factors (CD-31), tumour cell markers (cytokeratin), stem-cell marker (CD-133) and epithelial–mesenchymal transition (EMT) marker (TWIST). Tumours were characterised by H&E stain for morphology, while collagen deposition was assessed by Sirius Red staining as described previously (Vonlaufen et al, 2008a; Xu et al, 2010).
Expression of α-SMA, cytokeratin, CD-31, CD-133 and TWIST in primary tumours
Immunostaining of tumour sections for α-SMA (1 :
: 800) and cytokeratin (1
800) and cytokeratin (1 :
: 75) were performed using mouse anti-human α-SMA and mouse anti-human cytokeratin as primary antibodies. Morphometric analysis of stained sections was performed as described previously (Vonlaufen et al, 2008a). Endothelial cells in primary tumours were identified by immunostaining for the endothelial cell marker CD-31. Cells expressing stem cells and EMT characteristics were assessed by immunostaining the tumour sections for stem-cell marker CD-133 and transcription factor TWIST, respectively. For morphometric analysis, random fields of × 40 magnification (n=10 per slide) were selected and examined by an assessor blinded to the study groups. Results were analysed as the total number of positive cells in all 10 selected fields and expressed as the percentage of control (i.e., tumour sections from IgG-treated mice).
75) were performed using mouse anti-human α-SMA and mouse anti-human cytokeratin as primary antibodies. Morphometric analysis of stained sections was performed as described previously (Vonlaufen et al, 2008a). Endothelial cells in primary tumours were identified by immunostaining for the endothelial cell marker CD-31. Cells expressing stem cells and EMT characteristics were assessed by immunostaining the tumour sections for stem-cell marker CD-133 and transcription factor TWIST, respectively. For morphometric analysis, random fields of × 40 magnification (n=10 per slide) were selected and examined by an assessor blinded to the study groups. Results were analysed as the total number of positive cells in all 10 selected fields and expressed as the percentage of control (i.e., tumour sections from IgG-treated mice).
In vitro studies
Cell culture
Cancer cells: The human PC cell line AsPC-1 (American Type Culture Collection, Manassas, VA, USA) was cultured according to supplier's instructions and published protocols.
Human PSCs: hPSCs were isolated as described previously, by the outgrowth method from resected pancreatic tissues obtained from five different patients with PC (Bachem et al, 1998). Normal hPSCs were isolated using a density gradient isolation method from the histologically normal portion of resected pancreatic tissue from patients with benign conditions of the pancreas (Vonlaufen et al, 2010). The purity of hPSC yield was assessed by morphology and immunostaining for glial fibrillary acidic protein (a PSC selective marker) and α-SMA (a PSC activation marker) and the absence of staining for the cancer cell marker cytokeratin, using previously established methods (Apte et al, 1998; Vonlaufen et al, 2010).
Expression of HGF by hPSCs and c-MET by AsPC-1
Quantitative real-time PCR for HGF and c-MET: RNA was isolated from hPSCs using the RNA Plus Mini Kit (Qiagen, Chadstone, VIC, Australia) according to the manufacturer's instructions. Cellular RNA was reverse transcribed and real-time PCR performed in the Roche Light Cycler 480 (Castle Hill, NSW, Australia) using the Quantifast SYBR Green PCR Kit (Qiagen) and predesigned primer sets (Quantitect primers, Qiagen) for human, HGF and c-MET and human18S ribosomal RNA. Data were analysed as described previously (Vonlaufen et al, 2010).
Immunoblotting for HGF and c-MET on cell lysates: AsPC-1 and hPSCs were harvested at 70–80% confluence for lysis, and cell lysate protein concentration was estimated using the Pierce Bicinchoninic Acid (BCA) Protein Assay Kit (Thermo Fisher Scientific, Scoresby, VIC, Australia), according to the manufacturer's instructions. Lysates were then subjected to immunoblotting.
Collection of hPSC secretions and measurement of HGF in hPSC secretions
Culture medium was changed twice weekly, and cells were passaged by trypsinisation when 70–80% confluence was reached. Conditioned medium (hPSC secretions) used for experiments was collected from hPSCs between the third and eighth passage. Media (0.1% SFM4MAb for proliferation and migration assays or 0% IMDM for apoptosis assays) were collected, centrifuged at 1000 g for 10
g for 10 min at 4
min at 4 °C, and the supernatant was concentrated with Centricon YM3 filters (Millipore, Bayswater, VIC, Australia). HGF in hPSC secretions was quantified using a human HGF Quantikine ELISA Kit (R&D Systems) according to the manufacturer's instructions as described previously (Patel et al, 2014). The secretions were stored at −80
°C, and the supernatant was concentrated with Centricon YM3 filters (Millipore, Bayswater, VIC, Australia). HGF in hPSC secretions was quantified using a human HGF Quantikine ELISA Kit (R&D Systems) according to the manufacturer's instructions as described previously (Patel et al, 2014). The secretions were stored at −80 °C until used for functional assays. For assays that required pretreated secretions, appropriate dilution of secretions was performed with co-culture medium so as to standardise HGF to a concentration of 2000
°C until used for functional assays. For assays that required pretreated secretions, appropriate dilution of secretions was performed with co-culture medium so as to standardise HGF to a concentration of 2000 pg HGF
pg HGF ml−1. These concentrations were used as they reflected the actual amounts produced by hPSCs over 24
ml−1. These concentrations were used as they reflected the actual amounts produced by hPSCs over 24 h (Patel et al, 2014).
h (Patel et al, 2014).
Effect of hPSC secretions in the presence and absence of HGF inhibitor on AsPC-1 cell functions
Preparation of hPSC secretions: Secretions from hPSCs (n=5) that had been standardised to 2000 pg HGF
pg HGF ml−1 were pretreated for 1
ml−1 were pretreated for 1 h at 37
h at 37 °C with 60
°C with 60 μg
μg ml−1 AMG102 (HGF-neutralising antibody; Amgen, Thousand Oaks, CA, USA), 60
ml−1 AMG102 (HGF-neutralising antibody; Amgen, Thousand Oaks, CA, USA), 60 μg
μg ml−1 IgG (isotype control for AMG102; Amgen), 300
ml−1 IgG (isotype control for AMG102; Amgen), 300 μg
μg ml−1 gemcitabine (Hospira, Mulgrave, VIC, Australia) or combination of gemcitabine and AMG102. These concentrations of treatment compounds were calculated corresponding to in vivo dose used in these studies (see Supplementary Methods).
ml−1 gemcitabine (Hospira, Mulgrave, VIC, Australia) or combination of gemcitabine and AMG102. These concentrations of treatment compounds were calculated corresponding to in vivo dose used in these studies (see Supplementary Methods).
Assessment of AsPC-1 cell proliferation, migration and apoptosis: The effects of HGF inhibition on AsPC-1 proliferation were measured using the Cell Counting Kit-8 (Dojindo Molecular Technologies, Rockville, MD, USA). AsPC-1 cell migration was assessed using a modified Boyden chamber method as published by us previously using cell culture inserts with a porous membrane at the bottom (8-μm sized pores) (Becton-Dickinson, Bedford, MA, USA) placed in 24-well culture plates. Apoptosis was assessed by Annexin V staining. The above methods are described in detail in the Supplementary Methods.
Collection of AsPC-1 lysates and protein quantification for assessment of activated signalling pathways
AsPC-1 at 90% confluence were exposed to one of the following treatments for 15 min: (i) co-culture medium; (ii) hPSC secretions+IgG; or (iii) hPSC secretions+AMG102 (as described above, using secretions from n=5 different hPSCs). After 15-min exposure, secretions were removed, and cells were rinsed with ice cold PBS and then lysed. Lysates were centrifuged at 14
min: (i) co-culture medium; (ii) hPSC secretions+IgG; or (iii) hPSC secretions+AMG102 (as described above, using secretions from n=5 different hPSCs). After 15-min exposure, secretions were removed, and cells were rinsed with ice cold PBS and then lysed. Lysates were centrifuged at 14 000
000 r.p.m. for 10
r.p.m. for 10 min at 4
min at 4 °C, and the resultant supernatants were collected. Cell lysate protein was estimated using the Pierce Bicinchoninic Acid (BCA) Protein Assay Kit (Thermo Fisher Scientific), according to the manufacturer's instructions, Lysates were then subjected to immunoblotting as detailed below for assessment of PI3 kinase and MAPK activation. Similarly, lysates from AsPC-1 cells were collected after treatment for 10
°C, and the resultant supernatants were collected. Cell lysate protein was estimated using the Pierce Bicinchoninic Acid (BCA) Protein Assay Kit (Thermo Fisher Scientific), according to the manufacturer's instructions, Lysates were then subjected to immunoblotting as detailed below for assessment of PI3 kinase and MAPK activation. Similarly, lysates from AsPC-1 cells were collected after treatment for 10 min for assessment of c-MET activation.
min for assessment of c-MET activation.
Briefly, lysed proteins were separated using 10% SDS polyacrylamide gels and transferred onto nitrocellulose membranes. Membranes were blocked for 1 h in 5% skim milk in Tris buffered saline with Tween-20 (TBS-T), followed by overnight incubation at 4
h in 5% skim milk in Tris buffered saline with Tween-20 (TBS-T), followed by overnight incubation at 4 °C with one of the following rabbit mAb diluted 1
°C with one of the following rabbit mAb diluted 1 :
: 1000 in TBS-T with 5% BSA: (i) phospho-Akt (Cell Signaling Technology, Beverly, MA, USA) or (ii) phospho-p44/42 MAPK (ERK1/2) (Cell Signaling Technology). Membranes were washed and incubated for 1
1000 in TBS-T with 5% BSA: (i) phospho-Akt (Cell Signaling Technology, Beverly, MA, USA) or (ii) phospho-p44/42 MAPK (ERK1/2) (Cell Signaling Technology). Membranes were washed and incubated for 1 h at room temperature with an HRP-conjugated goat anti-rabbit secondary antibody, diluted 1
h at room temperature with an HRP-conjugated goat anti-rabbit secondary antibody, diluted 1 :
: 2000 in blocking buffer. Target proteins were detected using the Bio-Rad ECL Kit (Gladesville, NSW, Australia) and quantified using densitometry.
2000 in blocking buffer. Target proteins were detected using the Bio-Rad ECL Kit (Gladesville, NSW, Australia) and quantified using densitometry.
Membranes were then stripped and re-probed for expression of total PI3 kinase and total ERK (used as loading controls) by incubating overnight at 4 °C with the following antibodies at 1
°C with the following antibodies at 1 :
: 1000 dilution: Akt (pan) (Cell Signaling Technology) and p44/42 MAPK (ERK1/2) (Cell Signaling Technology). Bands representing phosphorylated and total ERK1/2 and Akt were quantified by densitometry. Densitometric readings for phosphorylated ERK1/2 and Akt were corrected for their respective loading controls (total ERK1/2 and Akt). Results were expressed as the percentage of control (i.e., expression of phopho-ERK1/2 or Akt in cells treated with co-culture medium as control).
1000 dilution: Akt (pan) (Cell Signaling Technology) and p44/42 MAPK (ERK1/2) (Cell Signaling Technology). Bands representing phosphorylated and total ERK1/2 and Akt were quantified by densitometry. Densitometric readings for phosphorylated ERK1/2 and Akt were corrected for their respective loading controls (total ERK1/2 and Akt). Results were expressed as the percentage of control (i.e., expression of phopho-ERK1/2 or Akt in cells treated with co-culture medium as control).
Western blotting was also used to assess c-MET phosphorylation, total c-MET and glyceraldehyde phosphate dehydrogenase (GAPDH, as loading control). The primary antibodies (overnight incubation at 4 °C) included anti-rabbit phospho-Met (Tyr1234/1235) (D26, Cell Signaling Technology 1
°C) included anti-rabbit phospho-Met (Tyr1234/1235) (D26, Cell Signaling Technology 1 :
: 1000, rabbit total Met (D1C2, Cell Signaling Technology) 1
1000, rabbit total Met (D1C2, Cell Signaling Technology) 1 :
: 1000 and anti-mouse GAPDH (Abcam) 1
1000 and anti-mouse GAPDH (Abcam) 1 :
: 50000. The secondary antibody was HRP-conjugated goat anti-rabbit antibody, 1
50000. The secondary antibody was HRP-conjugated goat anti-rabbit antibody, 1 :
: 2000. Densitometric readings for phosphorylated c-MET and total c-MET were corrected for their respective loading controls (GAPDH).
2000. Densitometric readings for phosphorylated c-MET and total c-MET were corrected for their respective loading controls (GAPDH).
Statistical analysis
Data are expressed as mean±s.e.m. Student's t-test, one-way analysis of variance with Tukey's post hoc test or Fisher's exact test was applied as appropriate. Analyses were performed using GraphPad Prism 6.00 for Mac OS X (GraphPad Software, La Jolla, CA, USA).
Ethics approval
The South Eastern Sydney Local Health District Human Research Ethics Committee (13/023(HREC/13/POWH/65)) and the Animal Care and Ethics Committee (ACEC12/102B) of The University of New South Wales, Sydney, Australia approved these studies.
Results
Orthotopic model for PC
The survival rate assessed to the point of killing in our model was 100%. As the tumours were not palpable in the first week, which was the time of randomisation for the mice to start receiving treatments, a group of mice (n=12) was killed at 1 week after surgery, to ensure that tumour volumes at the start of treatment were uniform across the different groups. As shown in Figure 1A, there was no difference between the volumes of tumours harvested from the 12 mice at 1 week after surgery.
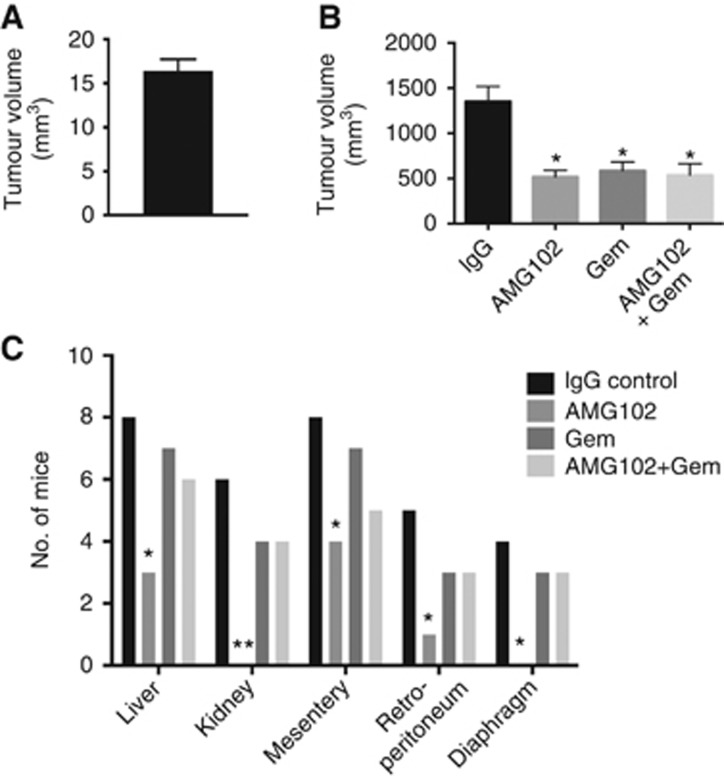
Effect of HGF inhibition and gemcitabine on pancreatic tumours in vivo. (A) Tumour volumes uniform at 1 week postsurgery, before randomisation for treatments: A mixture of AsPC-1 cells and human PSCs was injected into the tail of the pancreas of nude mice (n=12). At 1 week postsurgery (the time of randomisation for treatments), the tumours were harvested and measured. Tumours in all 12 animals were similar in size (16.4±1.2 mm3). (B) Pancreatic tumour volumes in mice treated with AMG102 or gemcitabine (Gem), alone and in combination: Pancreatic tumour volumes were reduced to a similar extent by AMG102 and gemcitabine, whether administered as single agents or in combination, compared with tumours in IgG-treated (control) animals. (*P<0.01 vs IgG n=9 per group). (C) Regional and distant metastasis in mice treated with AMG102 or gemcitabine (Gem), alone and in combination: Mice treated with AMG102 had significantly reduced metastatic spread to the organs depicted in the figure when compared with IgG-treated mice. No decrease in metastasis was observed in mice treated with gemcitabine. The inhibitory effect on metastasis noted with AMG102 as a single agent was lost when the HGF inhibitor was combined with gemcitabine. (*P<0.05, **P<0.005 vs IgG, n=9 per group).
mm3). (B) Pancreatic tumour volumes in mice treated with AMG102 or gemcitabine (Gem), alone and in combination: Pancreatic tumour volumes were reduced to a similar extent by AMG102 and gemcitabine, whether administered as single agents or in combination, compared with tumours in IgG-treated (control) animals. (*P<0.01 vs IgG n=9 per group). (C) Regional and distant metastasis in mice treated with AMG102 or gemcitabine (Gem), alone and in combination: Mice treated with AMG102 had significantly reduced metastatic spread to the organs depicted in the figure when compared with IgG-treated mice. No decrease in metastasis was observed in mice treated with gemcitabine. The inhibitory effect on metastasis noted with AMG102 as a single agent was lost when the HGF inhibitor was combined with gemcitabine. (*P<0.05, **P<0.005 vs IgG, n=9 per group).
Effect of HGF inhibition and gemcitabine on tumour volume
In our initial studies, we assessed the effects of HGF inhibition on tumours formed by AsPC-1 alone and on tumours formed by a mixture of AsPC-1 and hPSCs after 6 weeks postimplantation. In control mice (treated with IgG), AsPC-1+hPSC tumours were significantly larger than those formed by AsPC-1 (tumour volume mm3; mean±s.e.m. – 1312.63±175.27* vs 667.94±93.93, respectively; *P<0.001 vs AsPC-1 alone tumours; n=8 mice per group, confirming our previously published results. Interestingly, AMG102 failed to reduce tumour volumes of AsPC-1 alone tumours, (AMG 102 treatment 517.07±68.95 vs IgG treatment 667.94±93.93) but significantly inhibited tumour growth in AsPC-1+hPSC tumours (AMG102 treatment 532.47±91.83* vs IgG treatment 1312.63±175.27; *P<0.001 vs IgG treatment; n=8 mice per group).
For our main model, as noted in the Methods section, 1 week after surgery, mice were randomised to treatment groups receiving either HGF-neutralising antibody (AMG102) or isotype IgG or gemcitabine (a standard chemotherapeutic agent) or the combination HGF antibody and gemcitabine. After a treatment protocol of twice weekly intraperitoneal injections for 6 weeks, the tumours were harvested and tumour volumes were analysed. AMG102 significantly inhibited tumour growth when compared with IgG-treated animals (Figure 1B). Importantly, HGF inhibition was as effective as gemcitabine in reducing tumour volumes in our model (Figure 1B), although there was no additive or synergistic effect on tumour volume reduction when both these agents were used in combination (Figure 1B).
Effect of HGF inhibition and gemcitabine on tumour metastasis
Mice treated with AMG102 had significantly reduced metastasis when compared with the mice receiving IgG as control (Figure 1C). However, gemcitabine treatment did not have any inhibitory effect on the metastatic spread. More intriguingly, when combined with gemcitabine, AMG102 lost its inhibitory effect on metastasis (Figure 1C).
The lack of an antimetastatic effect with gemcitabine and the loss of the protective effect of AMG102 in the presence of gemcitabine suggested that gemcitabine treatment selects out a population of highly aggressive cancer cells (e.g., stem cells) with higher migratory potential (via increased EMT). This hypothesis was investigated by assessing primary tumours histologically for the presence of relevant markers.
Histological characterisation of primary tumours
H&E-stained sections of the tumours in untreated mice exhibited cancer cells embedded in a strong desmoplastic reaction, thus resembling human PC. Morphometric analysis of tumour sections immunostained for cytokeratin allowed calculation of cancer cell density in tumours from each treatment group (expressed as the number of cytokeratin-positive cells mm−2). AMG102 alone (91.3±2.4 expressed as the percentage of IgG treatment as control) resulted in a modest but significant reduction of cytokeratin expression compared with IgG-treated tumours (Figure 2A). Gemcitabine alone (58.2±4.2) or in combination with AMG102 (57±2.9) also reduced cytokeratin expression but to a significantly greater extent than AMG102 alone (Figure 2A). However, as the tumour volumes in all the three treatments were similar (Figure 1B), it is possible that, in gemcitabine-treated tumours with large reduction in cancer cell density, other components such as fibrosis may have made up the tumour volume.
mm−2). AMG102 alone (91.3±2.4 expressed as the percentage of IgG treatment as control) resulted in a modest but significant reduction of cytokeratin expression compared with IgG-treated tumours (Figure 2A). Gemcitabine alone (58.2±4.2) or in combination with AMG102 (57±2.9) also reduced cytokeratin expression but to a significantly greater extent than AMG102 alone (Figure 2A). However, as the tumour volumes in all the three treatments were similar (Figure 1B), it is possible that, in gemcitabine-treated tumours with large reduction in cancer cell density, other components such as fibrosis may have made up the tumour volume.
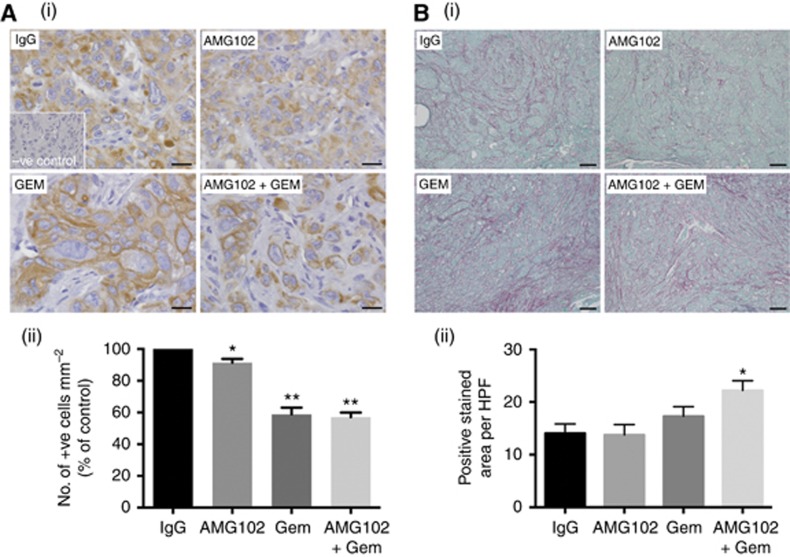
Effect of treatments on tumour morphology (n=9 mice per treatment group). (A) Effect of HGF inhibition and gemcitabine on cancer cell density: The figure includes: (i) representative images of tumour sections immunostained for the cancer cell marker cytokeratin (scale bar: 20 μm; negative control in the inset); and (ii) a bar graph depicting morphometric analysis for cancer cell density (calculated as the number of brown-stained cells per unit area of tumour and expressed as the percentage of control (IgG-treated tumours)). Compared with the IgG-treated group, cancer cell density was significantly reduced by AMG102 (*P<0.05 vs IgG). The effect was greater in animals treated with gemcitabine, both as single agent (GEM) and in combination with AMG102 (AMG102+GEM) (**P<0.0001 vs IgG and AMG102). (B) Effect of HGF inhibition and gemcitabine on fibrosis: The figure includes (i) representative images of tumour sections stained for Sirius Red to assess collagen deposition. (scale bar: 100
μm; negative control in the inset); and (ii) a bar graph depicting morphometric analysis for cancer cell density (calculated as the number of brown-stained cells per unit area of tumour and expressed as the percentage of control (IgG-treated tumours)). Compared with the IgG-treated group, cancer cell density was significantly reduced by AMG102 (*P<0.05 vs IgG). The effect was greater in animals treated with gemcitabine, both as single agent (GEM) and in combination with AMG102 (AMG102+GEM) (**P<0.0001 vs IgG and AMG102). (B) Effect of HGF inhibition and gemcitabine on fibrosis: The figure includes (i) representative images of tumour sections stained for Sirius Red to assess collagen deposition. (scale bar: 100 μm); and (ii) a bar graph depicting morphometric analysis for collagen deposition (calculated by assessing ratio of red-staining area to total tumour area and expressed as positive stained area per HPF). Compared with the IgG- or AMG102-treated animals, fibrillar collagen deposition was increased in animals treated with gemcitabine in combination with HGF inhibition (*P<0.05 vs IgG and AMG102).
μm); and (ii) a bar graph depicting morphometric analysis for collagen deposition (calculated by assessing ratio of red-staining area to total tumour area and expressed as positive stained area per HPF). Compared with the IgG- or AMG102-treated animals, fibrillar collagen deposition was increased in animals treated with gemcitabine in combination with HGF inhibition (*P<0.05 vs IgG and AMG102).
The effect of treatments on fibrosis was assessed by software-assisted morphometric analysis on sections stained for Sirius Red (which stains fibrillar collagen) (Figure 2B). Significantly higher Sirius Red expression was noted in tumours from mice receiving combination treatment (Gem+AMG102; 22.2±1.8; Figure 2B(ii)). Tumours in mice treated with gemcitabine alone (17.3±1.8) also showed higher Sirius Red expression than controls (14.05±1.8) or AMG102-treated mice (13.77±2) although this increase did not reach statistical significance. Immunostaining for PSC activation marker, α-SMA was also performed on these tumours but no significant difference was observed between treatment groups (percentage of control, IgG-treated mice, mean±s.e.m.; IgG-treated tumours (100), AMG102-reated tumours (117.6±13), Gemcitabine-treated tumours (123±27.9) and tumours treated with combination (111.4±19.4) n=9 per group).
Effect of treatments on cancer stem-cell and EMT markers in vivo
The paradoxical increase in metastasis with gemcitabine treatment both as single agent and in combination (Figure 1C) suggested that gemcitabine treatment may be selecting out a subpopulation of cancer cells, possible stem-like cells with an aggressive phenotype and increased migratory potential owing to an increase in EMT. Immunohistochemistry of tumours sections for the stem-cell marker (CD-133) and the transcription factor TWIST (marker for EMT) demonstrated that tumours from mice treated with gemcitabine alone or in combination with AMG102 exhibited significantly higher expression of CD-133 (2617±351, 2554±360, respectively, percentage of IgG control) and TWIST (207±12, 207±10, respectively, percentage of IgG control) compared with tumours from IgG-treated controls or mice treated with AMG102 alone (115±9.5% IgG control) (Figures 3A and B). These results support the notion that gemcitabine treatment selects out an aggressive subpopulation of tumour cells with increased stemness and EMT.
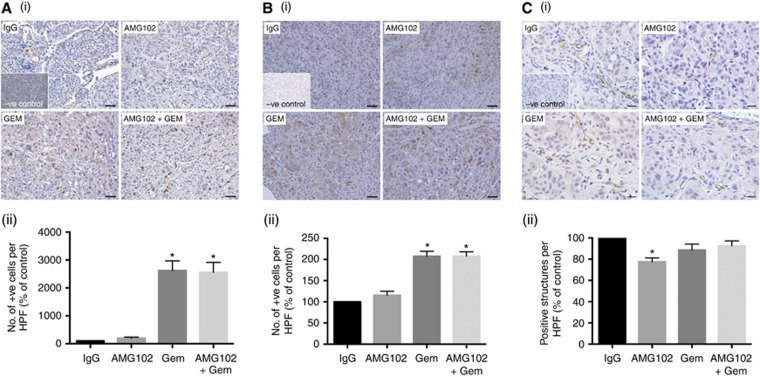
Effect of AMG102 and gemcitabine treatments, alone and in combination on stem-cell and EMT markers, and neo-angiogenesis in vivo: (n=9 mice per treatment group). (A) Effect of HGF inhibition and gemcitabine on CD-133 (stem-cell marker) expression: The figure includes: (i) representative images of tumour sections immunostained for the stem-cell marker CD-133 (scale bar: 50 μm; negative control in the inset); and (ii) a bar graph depicting morphometric analysis for stem-cell density (calculated as the number of brown-stained cells per unit area of tumour and expressed as the percentage of control (IgG-treated tumours)). Compared with IgG- and AMG102-treated groups, stem-cell density was significantly increased by gemcitabine treatment both as a single agent (GEM) and in combination with AMG102 (AMG102+GEM) (*P<0.001 vs IgG and AMG102). (B) Effect of HGF inhibition and gemcitabine on TWIST (epithelial–mesenchymal transition (EMT) marker): The figure includes: (i) representative images of tumour sections immunostained for the EMT marker TWIST (scale bar: 50
μm; negative control in the inset); and (ii) a bar graph depicting morphometric analysis for stem-cell density (calculated as the number of brown-stained cells per unit area of tumour and expressed as the percentage of control (IgG-treated tumours)). Compared with IgG- and AMG102-treated groups, stem-cell density was significantly increased by gemcitabine treatment both as a single agent (GEM) and in combination with AMG102 (AMG102+GEM) (*P<0.001 vs IgG and AMG102). (B) Effect of HGF inhibition and gemcitabine on TWIST (epithelial–mesenchymal transition (EMT) marker): The figure includes: (i) representative images of tumour sections immunostained for the EMT marker TWIST (scale bar: 50 μm; negative control in the inset); and (ii) a bar graph depicting morphometric analysis for brown positive cells undergoing EMT (calculated as the number of brown positive cells per unit area of tumour and expressed as the percentage of control (IgG-treated tumours)). Compared with IgG- and AMG102-treated groups, the number of cells undergoing EMT was significantly increased by gemcitabine treatment both as a single agent (GEM) and in combination with AMG102 (AMG102 and GEM) (*P<0.0001 vs IgG and AMG102). (C) Effect of HGF inhibition and gemcitabine on endothelial cell marker expression: The figure includes: (i) representative images of tumour sections immunostained for the endothelial cell marker CD-31. (scale bar: 20
μm; negative control in the inset); and (ii) a bar graph depicting morphometric analysis for brown positive cells undergoing EMT (calculated as the number of brown positive cells per unit area of tumour and expressed as the percentage of control (IgG-treated tumours)). Compared with IgG- and AMG102-treated groups, the number of cells undergoing EMT was significantly increased by gemcitabine treatment both as a single agent (GEM) and in combination with AMG102 (AMG102 and GEM) (*P<0.0001 vs IgG and AMG102). (C) Effect of HGF inhibition and gemcitabine on endothelial cell marker expression: The figure includes: (i) representative images of tumour sections immunostained for the endothelial cell marker CD-31. (scale bar: 20 μm; negative control in the inset); and (ii) a bar graph depicting morphometric analysis for CD-31-positive cells (represented by brown positive structures per HPF, expressed as the percentage of control (IgG-treated tumours)). Compared with the IgG-treated group, there was a significant reduction in CD-31 expression in AMG102-treated mice (*P<0.005 vs IgG). However, with gemcitabine treatment either as a single agent (GEM) or in combination with HGF inhibition (AMG102 and GEM) there was no effect on the expression of CD-31.
μm; negative control in the inset); and (ii) a bar graph depicting morphometric analysis for CD-31-positive cells (represented by brown positive structures per HPF, expressed as the percentage of control (IgG-treated tumours)). Compared with the IgG-treated group, there was a significant reduction in CD-31 expression in AMG102-treated mice (*P<0.005 vs IgG). However, with gemcitabine treatment either as a single agent (GEM) or in combination with HGF inhibition (AMG102 and GEM) there was no effect on the expression of CD-31.
Role of HGF inhibition in tumour angiogenesis in PC
As HGF is known to have an angiogenic effect in several other cancers (Bussolino et al, 1992; Grant et al, 1993), tumour sections were assessed for the endothelial cell marker CD-31. Morphometric analysis of stained sections indicated significantly decreased CD-31 expression in tumour from mice treated with AMG102 (77.4±3.7) compared with tumours in IgG (percentage of control) or gemcitabine-treated mice (88.5±5), suggesting that HGF inhibition reduces neo-angiogenesis in PC (Figure 3C). These results concur with the proposed angiogenic role of HGF in cancers and also well support our recently published in vitro results showing that HGF inhibition directly reduces proliferation and migration of human microvascular endothelial cells, HMEC-1 (Patel et al, 2014).
Role of HGF-c-MET in PSC–PC cell interactions in vitro
HGF secretion by PSCs and expression of its receptor c-MET by PC cells
To determine the role of HGF-c-MET in stellate cell and cancer cell interactions, in vitro experiments assessing HGF production by PSCs and the presence of its receptor c-MET on cancer cells were conducted. mRNA for HGF was found to be expressed by both normal and cancer-associated PSCs but not by cancer cells (AsPC-1) (Figure 4A). In contrast, the receptor c-MET was expressed by AsPC-1 but not by normal or cancer-associated hPSCs (Figure 4A). These results were also reflected at the protein level for HGF and c-MET. PSC lysates from three different patients exhibited the presence of all the isoforms of HGF, similar to the positive control, rhHGF (Figure 4B), while c-MET expression was observed in AsPC-1 (as confirmed by the positive control human pancreatic ductal epithelial cells but not in PSC lysates from three different patients (Figure 4B)).
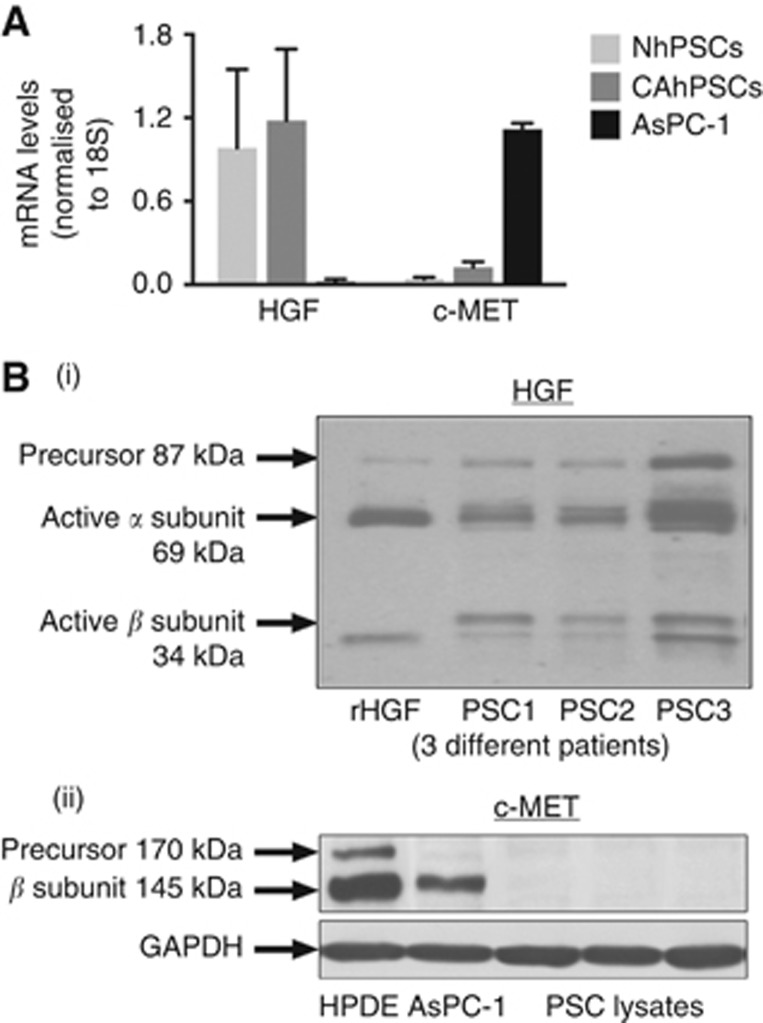
Expression of HGF and c-MET in human PSCs and PC cells (AsPC-1). (A) Expression of HGF mRNA in human PSCs and c-MET mRNA in AsPC-1 cells: The graph depicts mRNA expression for HGF by both normal and cancer-associated PSCs with no expression in AsPC-1. On the other hand, c-MET mRNA was expressed by AsPC-1 with PSCs exhibiting negligible expression in PSCs. (B) Expression of HGF protein in human PSCs and c-MET in PC cells: (i) Immunoblot for HGF showing expression of known isoforms of HGF protein in PSC lysates, corresponding to HGF isoforms found in the control recombinant human HGF. (ii) Immunoblot for c-MET expression in AsPC-1 lysates demonstrating c-MET expression in AsPC-1 and in the positive control human pancreatic ductal epithelial (HPDE) cells but absence of c-MET expression in PSC lysates.
Cancer cell proliferation
AsPC-1 cells were exposed to co-culture medium with increasing concentrations of recombinant human HGF from 500 to 5000 pg
pg ml−1 to establish a dose response to HGF concentrations that induced proliferation. In all, 2000
ml−1 to establish a dose response to HGF concentrations that induced proliferation. In all, 2000 pg
pg ml−1 rhHGF induced maximal proliferation in cancer cells, which was inhibited by AMG102 (Figure 5A(i)). hPSC secretions with known amounts of HGF (2000
ml−1 rhHGF induced maximal proliferation in cancer cells, which was inhibited by AMG102 (Figure 5A(i)). hPSC secretions with known amounts of HGF (2000 pg
pg ml−1) induced AsPC-1 proliferation compared with controls, that is, cancer cells incubated with co-culture medium alone, concurring with our previous reports of hPSC-induced cancer cell proliferation (Vonlaufen et al, 2008a). This hPSC-induced proliferation was inhibited in the presence of HGF-neutralising antibody and gemcitabine (Figure 5A(ii); data are expressed as the percentage of control (mean±s.e.m.): hPSC secretions+IgG 186±8*, hPSC secretions+AMG102 150±8.8#, hPSC secretions+gemcitabine, 153±1** hPSC secretions+combination 160±5.5); *P<0.0001 vs control; #P<0.05 vs hPSC+IgG; **P<0.05 vs hPSC+IgG; n=5 separate hPSC preparations).
ml−1) induced AsPC-1 proliferation compared with controls, that is, cancer cells incubated with co-culture medium alone, concurring with our previous reports of hPSC-induced cancer cell proliferation (Vonlaufen et al, 2008a). This hPSC-induced proliferation was inhibited in the presence of HGF-neutralising antibody and gemcitabine (Figure 5A(ii); data are expressed as the percentage of control (mean±s.e.m.): hPSC secretions+IgG 186±8*, hPSC secretions+AMG102 150±8.8#, hPSC secretions+gemcitabine, 153±1** hPSC secretions+combination 160±5.5); *P<0.0001 vs control; #P<0.05 vs hPSC+IgG; **P<0.05 vs hPSC+IgG; n=5 separate hPSC preparations).
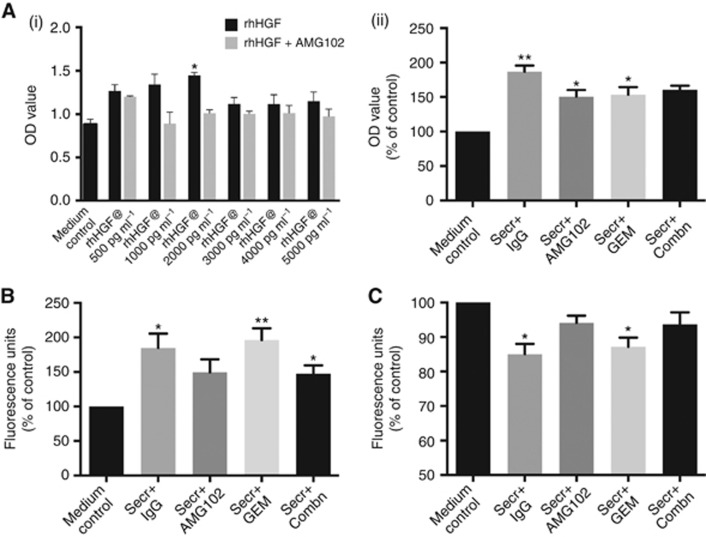
Effect of hPSC secretions and treatments on cancer cell functions in vitro: (n=5 separate hPSC preparations). For proliferation and migration studies, 0.1% SFM4MAb was used as the medium control and the results are expressed as the percentage of control. (A) Cancer cell proliferation: (i) AsPC-1 cells were incubated with medium control or medium treated with increasing concentrations (500–5000 pg
pg ml−1) of recombinant human HGF±AMG102. Compared with controls, rhHGF at 2000
ml−1) of recombinant human HGF±AMG102. Compared with controls, rhHGF at 2000 pg
pg ml−1 induced maximal AsPC-1 proliferation (*P<0.005 vs medium control), and this effect was inhibited by AMG102. This experiment was performed as a ‘proof of concept' and to establish optimal concentrations of HGF to induce AsPC-1 cell functions (proliferation). (ii) AsPC-1 cells were incubated with medium control or untreated hPSC secretions or hPSC secretions pretreated either with AMG102 or gemcitabine or a combination of both. Compared with controls, hPSC secretions (Secr+IgG group) significantly induced AsPC-1 proliferation (**P<0.0001 vs control). This hPSC-induced AsPC-1 proliferation was significantly inhibited with AMG102 and gemcitabine as single agents (*P<0.05 vs Secr+IgG). However, the combination of both (Secr+Combn) did not have an inhibitory effect on AsPC-1 proliferation. (B) Cancer cell migration: hPSC secretions significantly induced AsPC-1 migration (Secr+IgG) when compared with medium control (*P<0.05 vs medium control). This hPSC-induced migration persisted in the presence of gemcitabine treatment (**P<0.01, *P<0.05 vs medium control) but was prevented with AMG102-treated secretions. (C) Cancer cell apoptosis: For apoptosis studies, AsPC-1 cells were serum starved in IMDM (Iscove's Modified Dulbecco's Medium) medium (medium control) to induce apoptosis or incubated with untreated hPSC secretions or hPSC secretions pretreated with AMG102, gemcitabine or AMG102+gemcitabine. Results were expressed as the percentage of control (medium control). hPSC secretions significantly inhibited AsPC-1 apoptosis (Secr+IgG) when compared with medium control (*P<0.01 vs medium control). The hPSC-induced antiapoptotic effect on AsPC-1 persisted in the presence of gemcitabine (Secr+Gem; *P<0.01 vs medium control) but not with AMG102 either as single agent (Secr+AMG102) or in combination with gemcitabine (Secr+Combn).
ml−1 induced maximal AsPC-1 proliferation (*P<0.005 vs medium control), and this effect was inhibited by AMG102. This experiment was performed as a ‘proof of concept' and to establish optimal concentrations of HGF to induce AsPC-1 cell functions (proliferation). (ii) AsPC-1 cells were incubated with medium control or untreated hPSC secretions or hPSC secretions pretreated either with AMG102 or gemcitabine or a combination of both. Compared with controls, hPSC secretions (Secr+IgG group) significantly induced AsPC-1 proliferation (**P<0.0001 vs control). This hPSC-induced AsPC-1 proliferation was significantly inhibited with AMG102 and gemcitabine as single agents (*P<0.05 vs Secr+IgG). However, the combination of both (Secr+Combn) did not have an inhibitory effect on AsPC-1 proliferation. (B) Cancer cell migration: hPSC secretions significantly induced AsPC-1 migration (Secr+IgG) when compared with medium control (*P<0.05 vs medium control). This hPSC-induced migration persisted in the presence of gemcitabine treatment (**P<0.01, *P<0.05 vs medium control) but was prevented with AMG102-treated secretions. (C) Cancer cell apoptosis: For apoptosis studies, AsPC-1 cells were serum starved in IMDM (Iscove's Modified Dulbecco's Medium) medium (medium control) to induce apoptosis or incubated with untreated hPSC secretions or hPSC secretions pretreated with AMG102, gemcitabine or AMG102+gemcitabine. Results were expressed as the percentage of control (medium control). hPSC secretions significantly inhibited AsPC-1 apoptosis (Secr+IgG) when compared with medium control (*P<0.01 vs medium control). The hPSC-induced antiapoptotic effect on AsPC-1 persisted in the presence of gemcitabine (Secr+Gem; *P<0.01 vs medium control) but not with AMG102 either as single agent (Secr+AMG102) or in combination with gemcitabine (Secr+Combn).
Cancer cell migration
AsPC-1 cell migration was induced upon exposure to hPSC secretions, in agreement with our previously published report (Vonlaufen et al, 2008a). Interestingly, this induction of AsPC-1 migration persisted in the presence of gemcitabine but was prevented in the presence of the HGF-neutralising antibody as a single agent (Figure 5B; data are expressed as the percentage of control (mean±s.e.m.): hPSC secretions+IgG 184.6±20.9*, hPSC secretions+AMG102 149.5±18.8, hPSC secretions+gemcitabine, 196.1±17#; hPSC secretions+combination 147.5±11.8* #P<0.01 *P<0.05 vs control; n=5 separate hPSC preparations).
Cancer cell apoptosis
AsPC-1 cells were subjected to serum starvation to induce apoptosis. This induced apoptosis was significantly inhibited in the presence of untreated hPSC secretions (thus possibly facilitating cancer cell survival), an effect that was reversed in the presence of HGF-neutralising antibody. However, the antiapoptotic effect of hPSC secretions persisted in the presence of gemcitabine. (Figure 5C; data are expressed as the percentage of control (mean±s.e.m.): hPSC secretions+IgG 85±3*, hPSC secretions+AMG102 94±2, hPSC secretions+gemcitabine, 87.2±2.6*, hPSC secretions+combination 93.7±3.4); *P<0.01 vs control; n=5 separate hPSC preparations).
Signalling pathways mediating the effects of HGF inhibition on cancer cells
To establish whether the effects of HGF inhibition are mediated by ERK and/or PI3 kinase, AsPC-1 cells were exposed to pretreated hPSC secretions in the presence and absence of HGF antibody. Immunoblotting revealed increased phosphorylation of ERK in cancer cells exposed to hPSC secretion for 15 min, and this effect was reversed in the presence of HGF-neutralising antibody (Figure 6A). With regard to the PI3 kinase pathway, no statistically significant change in phosphorylation of Akt was observed.
min, and this effect was reversed in the presence of HGF-neutralising antibody (Figure 6A). With regard to the PI3 kinase pathway, no statistically significant change in phosphorylation of Akt was observed.
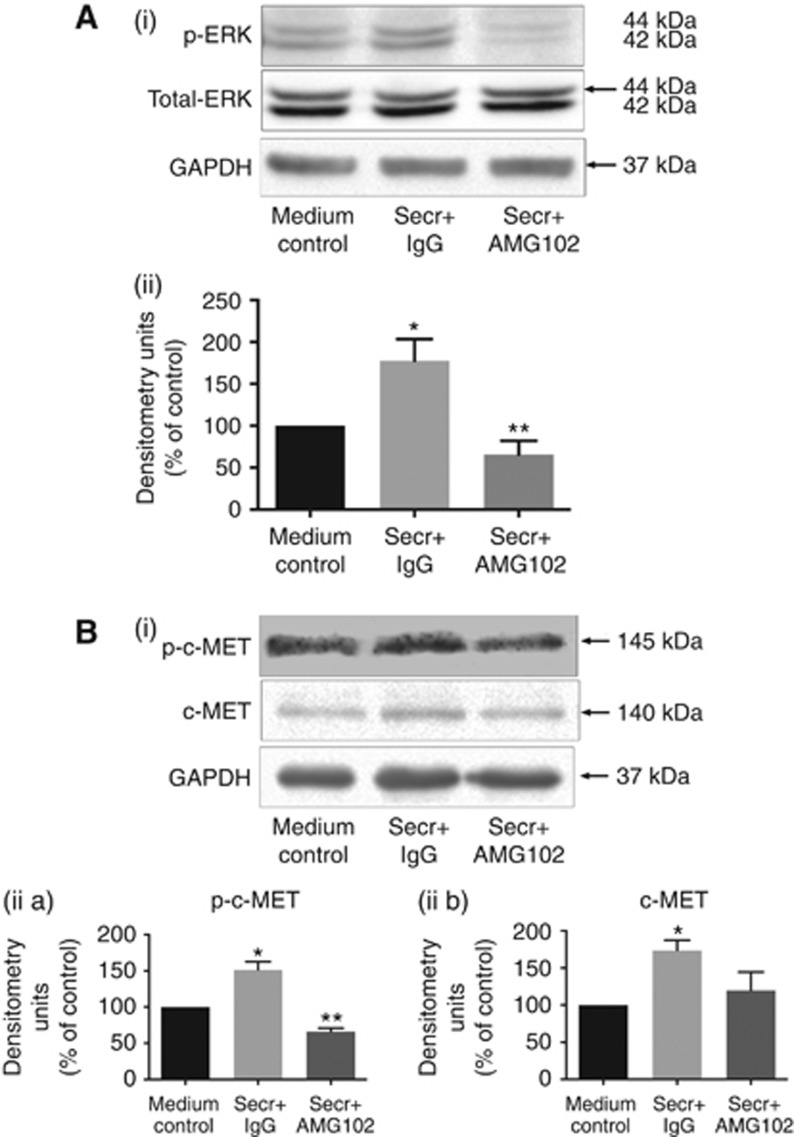
Effect of HGF Inhibition on signaling pathways in AsPC-1 cells. (A) ERK1/2 pathway: (i) Representative immunoblots for phosphorylated and total ERK1/2 in AsPC-1 cell lysates collected after exposure of cells to hPSC secretions pretreated with IgG or AMG102 for 15 min. The top panel shows phosphorylated ERK1/2 while the lower panel indicates total ERK1/2, used as a loading control. GAPDH blots are provided to additionally show equal loading. (ii) Graph showing densitometry results (calculated by correction for respective loading controls and expressed as the percentage of control (co-culture medium)). Compared with medium control, hPSC secretions significantly increased ERK1/2 phosphorylation (Secr+IgG; *P<0.05 vs medium control). This hPSC-induced phosphorylation of ERK1/2 was inhibited with HGF inhibition (Secr+AMG102; **P<0.01 vs Secr+IgG, n=5 separate PSC preparations). (B) c-MET pathway: (i) Representative immunoblots for phosphorylated (top panel) and total c-MET (middle panel) in AsPC-1 cell lysates collected after exposure of cells to hPSC secretions pretreated with IgG or AMG102 for 10
min. The top panel shows phosphorylated ERK1/2 while the lower panel indicates total ERK1/2, used as a loading control. GAPDH blots are provided to additionally show equal loading. (ii) Graph showing densitometry results (calculated by correction for respective loading controls and expressed as the percentage of control (co-culture medium)). Compared with medium control, hPSC secretions significantly increased ERK1/2 phosphorylation (Secr+IgG; *P<0.05 vs medium control). This hPSC-induced phosphorylation of ERK1/2 was inhibited with HGF inhibition (Secr+AMG102; **P<0.01 vs Secr+IgG, n=5 separate PSC preparations). (B) c-MET pathway: (i) Representative immunoblots for phosphorylated (top panel) and total c-MET (middle panel) in AsPC-1 cell lysates collected after exposure of cells to hPSC secretions pretreated with IgG or AMG102 for 10 min. The lowest panel shows GAPDH used as a loading control. (ii a and ii b) Graphs showing densitometry results (corrected for respective loading controls and expressed as the percentage of control (co-culture medium)). Compared with medium control, hPSC secretions increased phospho-c-MET (ii a) and total c-MET expression (ii b) (Secr+IgG; *P<0.05 vs medium control, n=3 separate hPSC preparations). This hPSC-induced phosphorylation of c-MET was inhibited with HGF inhibition (Secr+AMG102; **P<0.002 vs Secr+IgG, n=3 separate hPSC preparations); however, reduction in total c-MET expression with AMG102 did not reach statistical significance.
min. The lowest panel shows GAPDH used as a loading control. (ii a and ii b) Graphs showing densitometry results (corrected for respective loading controls and expressed as the percentage of control (co-culture medium)). Compared with medium control, hPSC secretions increased phospho-c-MET (ii a) and total c-MET expression (ii b) (Secr+IgG; *P<0.05 vs medium control, n=3 separate hPSC preparations). This hPSC-induced phosphorylation of c-MET was inhibited with HGF inhibition (Secr+AMG102; **P<0.002 vs Secr+IgG, n=3 separate hPSC preparations); however, reduction in total c-MET expression with AMG102 did not reach statistical significance.
To assess the activation of c-MET in response to HGF inhibition, AsPC-1 cells were treated as described above (hPSC secretions±HGF antibody). Immunoblotting showed increased phosphorylated c-MET as well as total c-MET in cancer cells exposed to hPSC secretions for 10 min, of which phosphorylation of c-MET was reduced by HGF antibody (Figure 6B). GAPDH was used as a loading control.
min, of which phosphorylation of c-MET was reduced by HGF antibody (Figure 6B). GAPDH was used as a loading control.
Discussion
Using both in vivo and in vitro approaches, this study provides novel evidence to indicate that HGF has a key role in stromal–tumour interactions in PC, as evidenced by the finding that inhibition of this ligand significantly reduces local tumour growth and distant metastasis. We have demonstrated that the antitumour effect of HGF inhibition is as effective as the standard chemotherapeutic agent gemcitabine, but importantly, is superior to gemcitabine with regard to reducing metastasis. Strikingly and somewhat unexpectedly, we observed that the antimetastatic effect of HGF inhibition was lost when combined with gemcitabine. This antagonistic effect of gemcitabine could possibly be explained by the fact that this chemotherapeutic agent selects out a population of cancer cells with increased survivability and a propensity for migration (as evidenced by increased expression of stem-cell and EMT markers) and that this stem-cell niche acts as a major source for distant spread. The ability of gemcitabine to increase stemness has been reported previously (Hermann et al, 2007; Simeone, 2008; Quint et al, 2012), However, we believe that our novel findings indicate that caution needs to be exercised when combining this routinely used chemotherapeutic drug with other targeted therapy, because such a combination may not be uniformly beneficial (Moore et al, 2007; Van Cutsem et al, 2009; Kindler et al, 2010).
Our in vitro studies using patient-derived primary cultures of PSCs have demonstrated for the first time that these cells secrete HGF but do not express the receptor c-MET. In contrast, cancer cells express c-MET but do not produce HGF. The above observations suggest that the HGF–c-MET pathway may represent a novel, specific target for therapy in order to improve the outcome of PC. Although some other studies reported the effects of HGF/c-MET inhibition in PC, the preclinical models they used were by orthotopic implantation of cancer cells alone (Tomioka et al, 2001; Brandes et al, 2015), thus not resembling human PC (owing to lack of stroma). In contrast, our study incorporates the stromal component of PC owing to the presence of hPSCs in our model.
The advantages of our orthotopic model over transgenic mouse models of PC are that it is produced using human cells and the resultant pancreatic tumours closely resemble human PC, making our findings translatable to clinical settings. Thus any observed responsiveness of the tumours (in terms of growth and metastasis) to therapeutic interventions may be reasonably expected to be translatable to the human situation. Importantly, we have found that HGF is predominantly produced by human PSCs. Furthermore, the HGF-neutralising antibody AMG102 used in our study is specific for human HGF and does not interact with mouse HGF (Burgess et al, 2010; Gordon et al, 2010). Although transgenic mouse models offer an intact immune system, the cells in the pancreatic tumours are mouse cells and the HGF secreted by these cells would not be neutralised by AMG102. Thus the translatability of any results from the K-ras model would be significantly limited.
Mice in our model were treated with AMG102 (HGF-neutralising antibody) or gemcitabine, alone and in combination while mice treated with IgG/vehicle served as controls. We found that HGF inhibition and gemcitabine whether administered separately or in combination reduced tumour size to a similar extent when compared with tumours in control (IgG treated) mice. Notably, tumours that originated from AsPC-1 cells alone without hPSCs did not respond to HGF inhibition, confirming the specificity of AMG102 to human HGF.
Pancreatic tumour sections from treated and untreated mice were specifically examined for cancer cell density (cytokeratin staining), fibrosis (Sirius Red staining) and angiogenesis (CD-31 staining). We found that HGF inhibition alone caused (i) a modest (albeit statistically significant) decrease in cell density, (ii) no change in fibrosis and (iii) a decrease in angiogenesis. As the volume of tumours in HGF inhibited mice was significantly lower than in untreated mice, the modest reduction in cancer cell density implies that HGF inhibition has a major inhibitory effect on the rate of cancer cell proliferation, when applied early during tumorigenesis (as was carried out in our study with treatments commencing 7 days after implantation of cells into the pancreas). In our model, the lack of an effect on fibrosis implies that HGF inhibition did not influence PSC function, which is not surprising given our observation that PSCs do not express the HGF receptor c-MET. However, angiogenesis, as assessed by CD-31 expression was significantly reduced by HGF inhibition. This was an expected result as endothelial cells are well known to express c-MET. Furthermore, these observations support our recently published in vitro findings of a close interaction between PSCs and human microvascular endothelial cells (HMEC1) that is mediated, at least in part, by HGF secreted by PSCs acting on HMEC1 cells via the receptor c-MET on the latter (Patel et al, 2014).
In contrast to HGF inhibition alone, gemcitabine alone and in combination with the HGF inhibitor, (i) significantly reduced cancer cell density as would be expected of a cytotoxic drug and (ii) significantly increased fibrosis but (iii) had no effect on CD-31 staining. These findings imply that gemcitabine, when administered at the seventh day after cell implantation time point, immediately caused cancer cell death, thus significantly reducing cancer cell numbers at the start of treatment. The increase in fibrosis may reflect increased activation of PSCs in response to factors released by cancer cells during cell death. This increased fibrosis could possibly have compensated for the reduced cancer cell density, so that the final volumes of tumours in mice treated with gemcitabine (with and without HGF inhibitor) were similar to tumour volumes in mice treated with HGF inhibition alone. Interestingly, in contrast to HGF inhibition alone, gemcitabine did not influence angiogenesis within tumours, implying that this drug alone does not influence endothelial cell function as has been reported previously in PC (Bruns et al, 2004).
Metastatic spread of cancer in our model was assessed by careful examination of regional (retroperitoneum, mesentery, spleen) and distant sites (liver, diaphragm, mediastinum). Compared with control mice, HGF inhibition alone significantly reduced regional and distant metastasis. In contrast, gemcitabine failed to decrease metastasis to the sites assessed. Intriguingly, HGF inhibition failed to achieve an antimetastatic effect when combined with gemcitabine. These findings raised the possibility that gemcitabine treatment was selecting out a population of cancer cells that had increased survival properties as well as increased migratory potential, prompting us to further characterise primary tumours for the expression of stem-cell and EMT markers such as CD-133 and TWIST, respectively. Expression of both CD-133 and TWIST was significantly increased in tumours from mice treated with gemcitabine (alone and in combination with the HGF inhibitor), compared with tumours from mice treated with either IgG or the HGF antibody alone. These observations support the concept that gemcitabine treatment leads to the emergence of a subgroup of stem-like cancer cells that may be relatively resistant to HGF inhibition and have increased migratory potential. These data concur with recent studies reporting that gemcitabine-resistant PC cells undergo EMT and acquire stem-cell characteristics (Shah et al, 2007; Wang et al, 2009; Quint et al, 2012; Herreros-Villanueva et al, 2014). Our observations are also supported by findings in other cancers, where chemotherapy-resistant cells acquired increased metastatic and self-renewal characteristics (Holohan et al, 2013).
The observed lack of synergism between inhibition of the ligand HGF and gemcitabine (particularly with reference to metastatic spread) differs from the reports of synergism between inhibition of the HGF receptor c-MET and gemcitabine (Li et al, 2011; Hage et al, 2013; Brandes et al, 2015). Most of the work with c-MET inhibition and gemcitabine has been conducted using transgenic models and murine c-MET inhibitors. Whether such synergism persists in an orthotopic model involving human cells is unclear. We would submit that, in contrast to the use of tyrosine kinase inhibitors (for c-MET inhibition) that likely have multiple targets in addition to the c-MET receptor, our studies using AMG102 have, for the first time, reported a highly specific effect, as this compound only neutralises human HGF and not murine HGF. Nonetheless, a logical next step for our work would be to determine the effects of a combination of human HGF inhibition and c-MET inhibition on PC progression. These studies are currently underway in our laboratory.
The above in vivo findings were supported by the results of our in vitro experiments examining the effects of hPSCs on cancer cell migration, proliferation and apoptosis. In keeping with previously reported studies, we found that cancer cell migration was stimulated by hPSC secretions (Hwang et al, 2008; Masamune et al, 2008; Vonlaufen et al, 2008a). This induction in migration was prevented by HGF inhibition alone but persisted in the presence of secretions containing gemcitabine (alone or in combination with AMG102). These results further support our in vivo observations that any antimigratory influence of HGF inhibition is overcome in the presence of gemcitabine, owing to gemcitabine-induced increase in EMT in cancer cells which would facilitate their migratory potential (Shah et al, 2007; Wang et al, 2009; Quint et al, 2012).
Cancer cell proliferation was induced in presence of hPSC secretions as reported previously and this induced proliferation was inhibited to a modest extent in the presence of the HGF inhibitor. These observations support our in vivo observations, which implied a significantly reduced proliferative rate of cancer cells in mice treated with AMG102. Cancer cell proliferation was also reduced by gemcitabine, as expected (owing to its known cytotoxic effects). Unexpectedly, proliferation remained unchanged in cancer cells treated with gemcitabine+AMG102. The reasons for this and lack of any additive effects on AsPC-1 proliferation and migration are unclear. It has to be acknowledged that overall the effects of HGF inhibition with and without gemcitabine on cancer cell proliferation were modest. This may reflect the fact that hPSC secretions containing several other mitogenic factors could sustain cancer cell proliferation during the short incubation periods used in the study.
HGF has been reported to inhibit cancer cell apoptosis in a number of cancers (Bowers et al, 2000; Grotegut et al, 2010). Several studies have indicated similar protective effects on cancer cell apoptosis by hPSCs (Vaquero et al, 2003; Boehrer et al, 2006; Hwang et al, 2008; Vonlaufen et al, 2008a). In our in vitro experiments, apoptosis was induced by serum-starving cancer cells. We found that this induced apoptosis was prevented in the presence of hPSC secretions, while inhibition of HGF in hPSC secretions reversed the antiapoptotic effect. These findings suggest a key role for HGF in hPSC secretions as an antiapoptotic and pro-survival factor for cancer cells, a postulate that is further supported by our observations that, in hPSC secretions pretreated with gemcitabine alone, the antiapoptotic effects of hPSC secretions were maintained.
In view of the above effects of HGF inhibition in the presence and absence of gemcitabine on cancer cell functions, we examined the activation (phosphorylation) as well as the expression of the HGF receptor c-MET and the downstream signalling pathways ERK1/2 and PI3K/AKT that are known to influence cancer cell survival and migration (Hwang et al, 2008; Menakongka and Suthiphongchai, 2010; Collisson et al, 2012). We have shown for the first time that hPSC secretions significantly induced c-MET activation as well as total c-MET expression in cancer cells. Similar c-MET activation upon treatment with hepatic stellate cell secretions has been reported in hepatocellular carcinoma (Yu et al, 2013). Importantly, our data showed that HGF inhibition significantly reduced c-MET activation (but not total c-MET expression) back to control levels, indicating that HGF has a major role (via its receptor c-MET) in the hPSC-induced proliferation and migration of cancer cells and that these effects are mediated, at least in part, via activation of the ERK1/2 (but not the PI3K) pathway.
A majority of reports in the literature support the concept that the stromal PSCs facilitate tumour growth and metastasis. Controversially, two recent studies (Ozdemir et al, 2014; Rhim et al, 2014) have suggested that PSCs and the stroma produced by them may represent a protective reaction by the host tissue to PC cells. It is possible that the role of the stroma changes as the carcinogenic process proceeds, with the stroma in early stages (PanIns) likely representing an effort to seal off the disease from the surrounding normal parenchyma, while that in later stages representing the successful subversion of stromal cells by cancer cells to their own advantage. As early diagnosis of PC continues to be a challenge, the development of alternative therapies to reprogram the stroma remains an essential step to improve outcome of the disease.
In this study, we have demonstrated that targeting of a specific pathway such as the HGF-c-MET pathway, by inhibiting its active ligand, is as effective as a chemotherapeutic agent in inhibiting pancreatic tumour growth. Importantly, HGF inhibition is clearly superior to gemcitabine with regard to protection against metastasis. Our findings indicate that gemcitabine selects out a population of treatment-resistant and aggressive cancer cells, which may explain the gemcitabine resistance commonly observed in the clinical situation. Integrating targeted therapies with systemic chemotherapy has had limited success despite encouraging results in vivo models (Li et al, 2014). The results of our study reinforce the need to use models optimised to more closely resemble the human setting and demonstrate that simple combination approaches may not always yield the expected results. Our findings also suggest that targeting the stroma as monotherapy may be an effective maintenance or neoadjuvant approach.
We believe that our study provides a very strong rationale for the use of targeted therapy to inhibit key stromal pathways as a component of novel strategies to improve outcomes of PC.
Acknowledgments
This work was supported by a grant from the Cancer Council of New South Wales (RG-13-01). Amgen Inc supplied AMG102 and isotype IgG.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
References
- Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, Korsten MA, Pirola RC, Wilson JS (1998) Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut 43(1): 128–133. [Europe PMC free article] [Abstract] [Google Scholar]
- Apte MV, Park S, Phillips PA, Santucci N, Goldstein D, Kumar RK, Ramm GA, Buchler M, Friess H, McCarroll JA, Keogh G, Merrett N, Pirola R, Wilson JS (2004) Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas 29(3): 179–187. [Abstract] [Google Scholar]
- Arun SJ, Xu Z, Fiala-Beer E, Yang L, Phillips PA, Goldstein D, Biankin A, Pirola R, Wilson JS, Apte MV (2011) Hepatocyte growth factor: a potential therapeutic target in pancreatic cancer. Pancreas 40(8): 1310–1311. [Google Scholar]
- Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, Siech M, Beger H, Grunert A, Adler G (1998) Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology 115(2): 421–432. [Abstract] [Google Scholar]
- Bachem MG, Schunemann M, Ramadani M, Siech M, Beger H, Buck A, Zhou S, Schmid-Kotsas A, Adler G (2005) Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology 128(4): 907–921. [Abstract] [Google Scholar]
- Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF (2003) Met, metastasis, motility and more. Nat Rev Mol Cell Biol 4(12): 915–925. [Abstract] [Google Scholar]
- Boehrer S, Nowak D, Hoelzer D, Mitrou PS, Chow KU (2006) Novel agents aiming at specific molecular targets increase chemosensitivity and overcome chemoresistance in hematopoietic malignancies. Curr Pharm Des 12(1): 111–128. [Abstract] [Google Scholar]
- Bowers DC, Fan S, Walter KA, Abounader R, Williams JA, Rosen EM, Laterra J (2000) Scatter factor/hepatocyte growth factor protects against cytotoxic death in human glioblastoma via phosphatidylinositol 3-kinase- and AKT-dependent pathways. Cancer Res 60(15): 4277–4283. [Abstract] [Google Scholar]
- Brandes F, Schmidt K, Wagner C, Redekopf J, Schlitt HJ, Geissler EK, Lang SA (2015) Targeting cMET with INC280 impairs tumour growth and improves efficacy of gemcitabine in a pancreatic cancer model. BMC Cancer 15: 71. [Europe PMC free article] [Abstract] [Google Scholar]
- Bruns CJ, Koehl GE, Guba M, Yezhelyev M, Steinbauer M, Seeliger H, Schwend A, Hoehn A, Jauch KW, Geissler EK (2004) Rapamycin-induced endothelial cell death and tumor vessel thrombosis potentiate cytotoxic therapy against pancreatic cancer. Clin Cancer Res 10(6): 2109–2119. [Abstract] [Google Scholar]
- Burgess TL, Sun J, Meyer S, Tsuruda TS, Sun J, Elliott G, Chen Q, Haniu M, Barron WF, Juan T, Zhang K, Coxon A, Kendall RL (2010) Biochemical characterization of AMG 102: a neutralizing, fully human monoclonal antibody to human and nonhuman primate hepatocyte growth factor. Mol Cancer Ther 9(2): 400–409. [Abstract] [Google Scholar]
- Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer A, Comoglio PM (1992) Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol 119(3): 629–641. [Europe PMC free article] [Abstract] [Google Scholar]
- Canadas I, Rojo F, Arumi-Uria M, Rovira A, Albanell J, Arriola E (2010) C-MET as a new therapeutic target for the development of novel anticancer drugs. Clin Transl Oncol 12(4): 253–260. [Abstract] [Google Scholar]
- Collisson EA, Trejo CL, Silva JM, Gu S, Korkola JE, Heiser LM, Charles RP, Rabinovich BA, Hann B, Dankort D, Spellman PT, Phillips WA, Gray JW, McMahon M (2012) A central role for RAF—>MEK—>ERK signaling in the genesis of pancreatic ductal adenocarcinoma. Cancer Discov 2(8): 685–693. [Europe PMC free article] [Abstract] [Google Scholar]
- Ebert M, Yokoyama M, Friess H, Buchler MW, Korc M (1994) Coexpression of the c-met proto-oncogene and hepatocyte growth factor in human pancreatic cancer. Cancer Res 54(22): 5775–5778. [Abstract] [Google Scholar]
- Erkan M, Reiser-Erkan C, Michalski CW, Deucker S, Sauliunaite D, Streit S, Esposito I, Friess H, Kleeff J (2009) Cancer-stellate cell interactions perpetuate the hypoxia-fibrosis cycle in pancreatic ductal adenocarcinoma. Neoplasia 11(5): 497–508. [Europe PMC free article] [Abstract] [Google Scholar]
- Gholamin S, Fiuji H, Maftouh M, Mirhafez R, Shandiz FH, Avan A (2014) Targeting c-MET/HGF signaling pathway in upper gastrointestinal cancers: rationale and progress. Curr Drug Targets 15(14): 1302–1311. [Abstract] [Google Scholar]
- Gordon MS, Sweeney CS, Mendelson DS, Eckhardt SG, Anderson A, Beaupre DM, Branstetter D, Burgess TL, Coxon A, Deng H, Kaplan-Lefko P, Leitch IM, Oliner KS, Yan L, Zhu M, Gore L (2010) Safety, pharmacokinetics, and pharmacodynamics of AMG 102, a fully human hepatocyte growth factor-neutralizing monoclonal antibody, in a first-in-human study of patients with advanced solid tumors. Clin Cancer Res 16(2): 699–710. [Abstract] [Google Scholar]
- Grant DS, Kleinman HK, Goldberg ID, Bhargava MM, Nickoloff BJ, Kinsella JL, Polverini P, Rosen EM (1993) Scatter factor induces blood vessel formation in vivo. Proc Natl Acad Sci USA 90(5): 1937–1941. [Europe PMC free article] [Abstract] [Google Scholar]
- Grotegut S, Kappler R, Tarimoradi S, Lehembre F, Christofori G, Von Schweinitz D (2010) Hepatocyte growth factor protects hepatoblastoma cells from chemotherapy-induced apoptosis by AKT activation. Int J Oncol 36(5): 1261–1267. [Abstract] [Google Scholar]
- Habisch H, Zhou S, Siech M, Bachem MG (2010) Interaction of Stellate Cells with Pancreatic Carcinoma Cells. Cancers 2(3): 1661–1682. [Europe PMC free article] [Abstract] [Google Scholar]
- Hage C, Rausch V, Giese N, Giese T, Schonsiegel F, Labsch S, Nwaeburu C, Mattern J, Gladkich J, Herr I (2013) The novel c-Met inhibitor cabozantinib overcomes gemcitabine resistance and stem cell signaling in pancreatic cancer. Cell Death Dis 4: e627. [Europe PMC free article] [Abstract] [Google Scholar]
- Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C (2007) Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 1(3): 313–323. [Abstract] [Google Scholar]
- Herreros-Villanueva M, Bujanda L, Billadeau DD, Zhang JS (2014) Embryonic stem cell factors and pancreatic cancer. World J Gastroenterol 20(9): 2247–2254. [Europe PMC free article] [Abstract] [Google Scholar]
- Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG (2013) Cancer drug resistance: an evolving paradigm. Nat Rev Cancer 13(10): 714–726. [Abstract] [Google Scholar]
- Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, Ji B, Evans DB, Logsdon CD (2008) Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res 68(3): 918–926. [Europe PMC free article] [Abstract] [Google Scholar]
- Ide T, Kitajima Y, Miyoshi A, Ohtsuka T, Mitsuno M, Ohtaka K, Miyazaki K (2007) The hypoxic environment in tumor-stromal cells accelerates pancreatic cancer progression via the activation of paracrine hepatocyte growth factor/c-Met signaling. Ann Surg Oncol 14(9): 2600–2607. [Abstract] [Google Scholar]
- Jiang WG, Martin TA, Parr C, Davies G, Matsumoto K, Nakamura T (2005) Hepatocyte growth factor, its receptor, and their potential value in cancer therapies. Crit Rev Oncol Hematol 53(1): 35–69. [Abstract] [Google Scholar]
- Kemik O, Purisa S, Kemik AS, Tuzun S (2009) Increase in the circulating level of hepatocyte growth factor in pancreatic cancer patients. Bratisl Lek Listy 110(10): 627–629. [Abstract] [Google Scholar]
- Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, Innocenti F, Mulcahy MF, O'Reilly E, Wozniak TF, Picus J, Bhargava P, Mayer RJ, Schilsky RL, Goldberg RM (2010) Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol 28(22): 3617–3622. [Europe PMC free article] [Abstract] [Google Scholar]
- Li C, Wu JJ, Hynes M, Dosch J, Sarkar B, Welling TH, Pasca di Magliano M, Simeone DM (2011) c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology 141(6): 2218–2227 e5. [Abstract] [Google Scholar]
- Li Q, Yuan Z, Yan H, Wen Z, Zhang R, Cao B (2014) Comparison of gemcitabine combined with targeted agent therapy versus gemcitabine monotherapy in the management of advanced pancreatic cancer. Clin Ther 36(7): 1054–1063. [Abstract] [Google Scholar]
- Lohr M, Schmidt C, Ringel J, Kluth M, Muller P, Nizze H, Jesnowski R (2001) Transforming growth factor-beta1 induces desmoplasia in an experimental model of human pancreatic carcinoma. Cancer Res 61(2): 550–555. [Abstract] [Google Scholar]
- Masamune A, Kikuta K, Watanabe T, Satoh K, Hirota M, Shimosegawa T (2008) Hypoxia stimulates pancreatic stellate cells to induce fibrosis and angiogenesis in pancreatic cancer. Am J Physiol Gastrointest Liver Physiol 295(4): G709–G717. [Abstract] [Google Scholar]
- Matsushita A, Gotze T, Korc M (2007) Hepatocyte growth factor-mediated cell invasion in pancreatic cancer cells is dependent on neuropilin-1. Cancer Res 67(21): 10309–10316. [Abstract] [Google Scholar]
- Menakongka A, Suthiphongchai T (2010) Involvement of PI3K and ERK1/2 pathways in hepatocyte growth factor-induced cholangiocarcinoma cell invasion. World J Gastroenterol 16(6): 713–722. [Europe PMC free article] [Abstract] [Google Scholar]
- Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W National Cancer Institute of Canada Clinical Trials G (2007) Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 25(15): 1960–1966. [Abstract] [Google Scholar]
- Nones K, Waddell N, Song S, Patch AM, Miller D, Johns A, Wu J, Kassahn KS, Wood D, Bailey P, Fink L, Manning S, Christ AN, Nourse C, Kazakoff S, Taylor D, Leonard C, Chang DK, Jones MD, Thomas M, Watson C, Pinese M, Cowley M, Rooman I, Pajic M, Apgi, Butturini G, Malpaga A, Corbo V, Crippa S, Falconi M, Zamboni G, Castelli P, Lawlor RT, Gill AJ, Scarpa A, Pearson JV, Biankin AV, Grimmond SM (2014) Genome-wide DNA methylation patterns in pancreatic ductal adenocarcinoma reveal epigenetic deregulation of SLIT-ROBO, ITGA2 and MET signaling. Int J Cancer 135(5): 1110–1118. [Abstract] [Google Scholar]
- Oettle H (2014) Progress in the knowledge and treatment of advanced pancreatic cancer: from benchside to bedside. Cancer Treat Rev 40(9): 1039–1047. [Abstract] [Google Scholar]
- Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV, De Jesus-Acosta A, Sharma P, Heidari P, Mahmood U, Chin L, Moses HL, Weaver VM, Maitra A, Allison JP, LeBleu VS, Kalluri R (2014) Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25(6): 719–734. [Europe PMC free article] [Abstract] [Google Scholar]
- Patel MB, Pothula SP, Xu Z, Lee AK, Goldstein D, Pirola RC, Apte MV, Wilson JS (2014) The role of the hepatocyte growth factor/c-MET pathway in pancreatic stellate cell-endothelial cell interactions: antiangiogenic implications in pancreatic cancer. Carcinogenesis 35(8): 1891–1900. [Abstract] [Google Scholar]
- Pothula SP, Xu Z, Goldstein D, Pirola RC, Wilson JS, Apte MV (2015) Key role of pancreatic stellate cells in pancreatic cancer. Cancer Lett e-pub ahead of print 10 November 2015 10.1016/j.canlet.2015.10.035. [Abstract]
- Quint K, Tonigold M, Di Fazio P, Montalbano R, Lingelbach S, Ruckert F, Alinger B, Ocker M, Neureiter D (2012) Pancreatic cancer cells surviving gemcitabine treatment express markers of stem cell differentiation and epithelial-mesenchymal transition. Int J Oncol 41(6): 2093–2102. [Abstract] [Google Scholar]
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM (2014) Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 74(11): 2913–2921. [Abstract] [Google Scholar]
- Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP, Tattersall IW, Westphalen CB, Kitajewski J, Fernandez-Barrena MG, Fernandez-Zapico ME, Iacobuzio-Donahue C, Olive KP, Stanger BZ (2014) Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 25(6): 735–747. [Europe PMC free article] [Abstract] [Google Scholar]
- Shah AN, Summy JM, Zhang J, Park SI, Parikh NU, Gallick GE (2007) Development and characterization of gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol 14(12): 3629–3637. [Abstract] [Google Scholar]
- Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63(1): 11–30. [Abstract] [Google Scholar]
- Simeone DM (2008) Pancreatic cancer stem cells: implications for the treatment of pancreatic cancer. Clin Cancer Res 14(18): 5646–5648. [Abstract] [Google Scholar]
- Tomioka D, Maehara N, Kuba K, Mizumoto K, Tanaka M, Matsumoto K, Nakamura T (2001) Inhibition of growth, invasion, and metastasis of human pancreatic carcinoma cells by NK4 in an orthotopic mouse model. Cancer Res 61(20): 7518–7524. [Abstract] [Google Scholar]
- Van Cutsem E, Vervenne WL, Bennouna J, Humblet Y, Gill S, Van Laethem JL, Verslype C, Scheithauer W, Shang A, Cosaert J, Moore MJ (2009) Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol 27(13): 2231–2237. [Abstract] [Google Scholar]
- Vaquero EC, Edderkaoui M, Nam KJ, Gukovsky I, Pandol SJ, Gukovskaya AS (2003) Extracellular matrix proteins protect pancreatic cancer cells from death via mitochondrial and nonmitochondrial pathways. Gastroenterology 125(4): 1188–1202. [Abstract] [Google Scholar]
- Vonlaufen A, Joshi S, Qu C, Phillips PA, Xu Z, Parker NR, Toi CS, Pirola RC, Wilson JS, Goldstein D, Apte MV (2008. a) Pancreatic stellate cells: partners in crime with pancreatic cancer cells. Cancer Res 68(7): 2085–2093. [Abstract] [Google Scholar]
- Vonlaufen A, Phillips PA, Xu Z, Goldstein D, Pirola RC, Wilson JS, Apte MV (2008. b) Pancreatic stellate cells and pancreatic cancer cells: an unholy alliance. Cancer Res 68(19): 7707–7710. [Abstract] [Google Scholar]
- Vonlaufen A, Phillips PA, Yang L, Xu Z, Fiala-Beer E, Zhang X, Pirola RC, Wilson JS, Apte MV (2010) Isolation of quiescent human pancreatic stellate cells: a promising in vitro tool for studies of human pancreatic stellate cell biology. Pancreatology 10(4): 434–443. [Abstract] [Google Scholar]
- Wang Z, Li Y, Kong D, Banerjee S, Ahmad A, Azmi AS, Ali S, Abbruzzese JL, Gallick GE, Sarkar FH (2009) Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res 69(6): 2400–2407. [Europe PMC free article] [Abstract] [Google Scholar]
- Watanabe S, Kishimoto T, Yokosuka O (2011) Hepatocyte growth factor inhibits anoikis of pancreatic carcinoma cells through phosphatidylinositol 3-kinase pathway. Pancreas 40(4): 608–614. [Abstract] [Google Scholar]
- Xu Z, Pothula SP, Wilson JS, Apte MV (2014) Pancreatic cancer and its stroma: a conspiracy theory. World J Gastroenterol 20(32): 11216–11229. [Europe PMC free article] [Abstract] [Google Scholar]
- Xu Z, Vonlaufen A, Phillips PA, Fiala-Beer E, Zhang X, Yang L, Biankin AV, Goldstein D, Pirola RC, Wilson JS, Apte MV (2010) Role of pancreatic stellate cells in pancreatic cancer metastasis. Am J Pathol 177(5): 2585–2596. [Europe PMC free article] [Abstract] [Google Scholar]
- Yadav D, Lowenfels AB (2013) The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 144(6): 1252–1261. [Europe PMC free article] [Abstract] [Google Scholar]
- Yang SY, Miah A, Pabari A, Winslet M (2011) Growth Factors and their receptors in cancer metastases. Front Biosci 16: 531–538. [Abstract] [Google Scholar]
- Yu G, Jing Y, Kou X, Ye F, Gao L, Fan Q, Yang Y, Zhao Q, Li R, Wu M, Wei L (2013) Hepatic stellate cells secreted hepatocyte growth factor contributes to the chemoresistance of hepatocellular carcinoma. PLoS One 8(9): e73312. [Europe PMC free article] [Abstract] [Google Scholar]
- Zhou W, Jubb AM, Lyle K, Xiao Q, Ong CC, Desai R, Fu L, Gnad F, Song Q, Haverty PM, Aust D, Grutzmann R, Romero M, Totpal K, Neve RM, Yan Y, Forrest WF, Wang Y, Raja R, Pilarsky C, de Jesus-Acosta A, Belvin M, Friedman LS, Merchant M, Jaffee EM, Zheng L, Koeppen H, Hoeflich KP (2014) PAK1 mediates pancreatic cancer cell migration and resistance to MET inhibition. J Pathol 234(4): 502–513. [Europe PMC free article] [Abstract] [Google Scholar]
Articles from British Journal of Cancer are provided here courtesy of Cancer Research UK
Full text links
Read article at publisher's site: https://doi.org/10.1038/bjc.2015.478
Read article for free, from open access legal sources, via Unpaywall:
https://www.nature.com/articles/bjc2015478.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1038/bjc.2015.478
Article citations
The Therapeutic Role of NPS-1034 in Pancreatic Ductal Adenocarcinoma as Monotherapy and in Combination with Chemotherapy.
Int J Mol Sci, 25(13):6919, 24 Jun 2024
Cited by: 0 articles | PMID: 39000029
Identification of immune- and oxidative stress-related signature genes as potential targets for mRNA vaccines for pancreatic cancer patients.
Medicine (Baltimore), 103(27):e38666, 01 Jul 2024
Cited by: 0 articles | PMID: 38968513 | PMCID: PMC11224846
Circulating microRNAs in association with pancreatic cancer risk within 5 years.
Int J Cancer, 155(3):519-531, 11 Apr 2024
Cited by: 0 articles | PMID: 38602070
Pancreatic Cancer Treatment Targeting the HGF/c-MET Pathway: The MEK Inhibitor Trametinib.
Cancers (Basel), 16(5):1056, 05 Mar 2024
Cited by: 1 article | PMID: 38473413 | PMCID: PMC10930669
Review Free full text in Europe PMC
Recent advances in targeted therapy for pancreatic adenocarcinoma.
World J Gastrointest Oncol, 15(4):571-595, 01 Apr 2023
Cited by: 14 articles | PMID: 37123059 | PMCID: PMC10134207
Review Free full text in Europe PMC
Go to all (55) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Paracrine HGF promotes EMT and mediates the effects of PSC on chemoresistance by activating c-Met/PI3K/Akt signaling in pancreatic cancer in vitro.
Life Sci, 263:118523, 08 Oct 2020
Cited by: 25 articles | PMID: 33039386
Targeting the HGF/c-MET pathway in advanced pancreatic cancer: a key element of treatment that limits primary tumour growth and eliminates metastasis.
Br J Cancer, 122(10):1486-1495, 23 Mar 2020
Cited by: 28 articles | PMID: 32203220 | PMCID: PMC7217847
The role of the hepatocyte growth factor/c-MET pathway in pancreatic stellate cell-endothelial cell interactions: antiangiogenic implications in pancreatic cancer.
Carcinogenesis, 35(8):1891-1900, 29 May 2014
Cited by: 47 articles | PMID: 24876152
Targeting HGF/c-MET Axis in Pancreatic Cancer.
Int J Mol Sci, 21(23):E9170, 01 Dec 2020
Cited by: 32 articles | PMID: 33271944 | PMCID: PMC7730415
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Cancer Research UK (1)
Advancing Genotype Guided Stratified Therapy for Pancreatic Cancer
Professor Andrew Biankin, University of Glasgow
Grant ID: 17263
Chief Scientist Office (1)
Scottish Genomes Partnership Agreement
Professor Timothy Aitman, University of Edinburgh
Grant ID: SGP/1
Medical Research Council (1)
The Scottish Genomes Partnership
Professor Timothy Aitman, University of Edinburgh
Grant ID: MC_PC_15080
Pancreatic Cancer UK (1)
Testing genotype-guided therapies in pancreatic cancer
Professor Andrew Biankin, University of Glasgow
Grant ID: FLF2015_04_GLASGOW





