Abstract
Free full text

Impact of Longevity Interventions on a Validated Mouse Clinical Frailty Index
Abstract
This article investigates the effect on the mouse frailty index (FI), of factors known to influence lifespan and healthspan in mice: strain (short-lived DBA/2J mice vs long-lived C57BL/6J mice), calorie restriction (CR), and resveratrol treatment. The mouse FI, based on deficit accumulation, was recently validated in C57BL/6J mice by Whitehead JC, Hildebrand BA, Sun M, et al. (A clinical frailty index in aging mice: comparisons with frailty index data in humans. J Gerontol A Biol Sci Med Sci. 2014;69:621–632) and shares many characteristics of the human FI. FI scores were measured in male and female aged (18 months) ad-libitum fed and CR DBA/2J and C57BL/6J mice, as well as male aged (24 months) C57BL/6J mice ad-libitum fed with or without resveratrol (100mg/kg/day) in the diet for 6 months. Mean scores of two raters were used, and the raters had excellent inter-rater reliability (ICC = 0.88, 95% CI [0.80, 0.92]). Furthermore, the interventions of CR and resveratrol were associated with a significant reduction in FI scores in C57BL/6J mice, compared to age-matched controls. The short-lived DBA/2J mice also had slightly higher FI scores than the C57BL/6J mice, for the male calorie-restricted groups (DBA/2J FI = 0.16±0.03, C57BL/6J FI = 0.11±0.03, p = .01). This study uses the mouse FI developed by Whitehead JC, Hildebrand BA, Sun M, et al. (A clinical frailty index in aging mice: comparisons with frailty index data in humans. J Gerontol A Biol Sci Med Sci. 2014;69:621–632) in a different mouse colony and shows that this tool can be applied to quantify the effect of dietary and pharmaceutical interventions on frailty.
Frailty is a state of high vulnerability for adverse health outcomes. The prevalence of frailty increases with increasing age, resulting in a higher risk of disability, falls, hospitalization, and mortality (1). In humans, frailty can be assessed using a number of scales with varying degrees of difficulty and clinical applicability (2). Two of the mostly commonly used and cited definitions are the phenotype model and the frailty index (FI). The phenotype model defines frailty as the presence of three or more criteria including unintentional weight loss, self-reported exhaustion, weakness measured by grip strength, slow walking speed, and low physical activity (3). The FI (4) measures the proportion of accumulated deficits in a patient, and focuses on the number of deficits rather than the precise nature of the deficits.
Validated animal models to study frailty are lacking. Interleukin (IL)-10 knock-out mice were developed as a colitis model, but show elements of a frailty phenotype including inflammation and reduced muscle strength (5). Their use as a frailty model is limited, however as they are not specifically a model of naturally occurring frailty in old age, and cannot be used to assess frailty as an outcome. Liu and colleagues (6) developed a mouse FI based on the clinical phenotype model which assessed a mouse as frail based on its grip strength, walking speed, physical activity, and endurance. They used this phenotype-based model to show that exercise reversed frailty in old mice (7). Recently, a mouse FI which utilizes 31 simply assessed clinical measures to assess frailty in C57BL/6J mice was developed and validated (8,9). The validity of this scale was correlated against human FI data from the Survey of Health, Ageing, and Retirement in Europe (SHARE) and was found to have good agreement. This mouse FI (8) is an important tool that could be used to assess the effect of any intervention on the important clinical outcome of frailty in an animal model.
The main factors known to affect the lifespan and healthspan of mice are strain, diet, and pharmaceutical interventions. Of the inbred mouse strains, the DBA/2J substrain is considered to be short-lived, with studies showing median lifespan for males to be 23–25 months, compared to 26–30 months for male C57BL/6J mice (10–12). DBA/2J mice have also been shown to be unresponsive to interventions that extend lifespan, such as calorie restriction (CR) (11). C57BL/6J mice are the classical inbred mouse strains commonly used for aging research due to the wealth of phenotypic and genotypic information available (source: http://phenome.jax.org/). Furthermore, they are responsive to interventions that extend lifespan such as CR and pharmaceuticals (11,12). CR, the daily reduction in calories without malnutrition, has long been known to increase the lifespan and healthspan of mice (13). Pharmaceutical agents that mimic the beneficial lifespan and/or healthspan effects of CR have also been investigated. Rapamycin and metformin have shown lifespan extension in mice on standard diets (SD) (14,15), but there are some concerns about side effects. Resveratrol (RSV) has shown healthspan improvements in SD-fed mice (16), and lifespan extension in high-fat diet mice (17). Recently, there has been an increasing focus on improving healthspan and quality of life, rather than lifespan, and assessment of frailty would provide a valuable measure of this in animal studies.
Our primary aim was to investigate the effect of an example of each of these effective lifespan and healthspan interventions—DBA/2J (short lived) vs C57BL/6J (long-lived) strain, CR vs ad-libitum (AL) feeding, and RSV vs placebo—on the mouse FI (8) in male and female mice. The secondary aims were to assess the applicability of the FI by using the scale in a different C57BL/6J mouse cohort and to correlate the FI with functional outcomes.
Methods
Animals and Diets
Male and female C57BL/6JJ (n = 18 males, n = 21 females) and DBA/2J (n = 16 males, n = 19 females) mice were obtained from the Jackson Laboratory (Bar Harbor, ME) and bred in house at the National Institute on Aging (Baltimore, MD). Mice were group housed in cages of four with AL access to water. At 6 months of age, mice were randomized to either an AL diet group, or a calorie restricted (CR; 40% of AL) diet group for the remainder of their lives (18). CR mice were fed daily at 7.30 am (± 1h) on the floor of the cage, while AL mice received food in the hopper. Mice were fed 2918 Teklad Global 18% Protein Rodent Diet (Harlan Teklad, Indiannapolis, IN). At 19 months of age, the FI (described below) was scored for each mouse. This time frame was chosen based on a previously reported study of DBA2/J mice (11) to correlate with the time at which we see a drop in survival for calorie restricted DBA2/J mice to approximately 75%.
A second cohort of male C57BL/6J mice were obtained from the NIA Aging Colony (Charles Rivers) at 2 months of age, and then aged in house at the NIA (Baltimore, MD). Mice were fed housechow (2018X 18% Protein Diet) AL until they reach 18 months of age. These mice were then randomized to either a standard AIN-93G diet (SD; Dyets Inc, Bethlehem, PA) (n = 16), or an AIN-93G diet supplemented with RSV at a dose of 100mg/kg (n = 9), both diets fed AL. This time frame was chosen to emulate the conditions of previous studies in which RSV showed beneficial outcomes even when started late in life (16,17). Mice remained on their respective diet until they reached 24 months of age at which time the FI (described below) was scored. This time frame was chosen as Forster and colleagues (11) previously found survival in AL-fed C57BL/6 mice was approximately 75% at 24 months. See Supplementary Table 1.
Body weight and food intake for all mice was monitored biweekly, and all groups had AL access to water. Animal rooms were maintained on a 12-hour light/dark cycle at 20–22 °C, and 30–70% humidity. All animal protocols were approved by the Animal Care and Use Committee of the National Institute on Aging (429-TGB-2017 and 405-TGB-2016).
Frailty Index Assessment
A FI score was calculated for each mouse using the 31-item FI (8) as described in Supplementary Table 1. The severity of each item was assessed on a scale of 0, 0.5, or 1 (8). The temperature and weight scores were scored based on the number of standard deviations the tested mouse was from the mean temperature or weight of a young mouse, as outlined in the article of Whitehead and colleagues (7). Young (12 week) C57BL/6J and DBA/2J weight and temperature means and standard deviations were obtained from the Jacksons Laboratory (http://jaxmice.jax.org/support/weight/000664.html). The sum of the scores for 31 items was then divided by 31 to give a FI.
For the first mouse cohort, 19-month old male DBA/2J and C57BL/6J mice were assessed by two raters. Each mouse was brought to a quiet assessment room and allowed to acclimatize for 30 minutes prior to the assessment. The mouse was weighed, subcutaneous body temperature was obtained (average of two readings) using a subcutaneous implanted transponder (BDMS, Seaford, DE), and then each mouse was scored for each item in the FI by each rater. The mice were scored over 2 days. The raters were blinded to the diet group of the mice and to the scores assigned by the other rater.
For the second mouse cohort, 24-month old male C57BL/6J mice were assessed by two raters. Mice were scored over one day, and the raters were blinded to the treatment group of the mice and to the score assigned by the other rater. Temperatures were not measured for these mice, so their final FI score was calculated for a 30-item scale only.
Functional Assessments
At 16 months of age, a subset of the cohort 1 DBA/2J (male n = 5, female n = 10) and C57BL/6J (male n = 9, female n = 11) mice had functional assessments performed (see timeline in Supplementary Table 1). Motor co-ordination, balance, and endurance were assessed by the rotarod; forelimb and hindlimb strength using the cage top test and forelimb and neuromuscular strength were assessed using the wirehang test. For all assessments, mice were bought to the testing room and allowed to acclimitise for 15 minutes prior to the commencement of testing.
For rotatord measurements, mice were given a habituation trial at a constant speed of 4rpm for 1 minute before the first trial. On the same day, there was a total of three trials given, separated by 30 minutes rest periods, during which the rotarod accelerated from 4 to 40rpm over a period of 5 minutes. Latency to fall was recorded and averaged over all three trials as described previously (19).
For cage top measurements, the ability to hang upside down from a wire screen was tested as previously described and was modified from the Kondziella’s inverted screen test (20,21). The wire screen was modified from the wire lid of a mouse cage. The cage top was turned upside down, with its sides covered in duct tape, and the experimenter placed the mouse on top of the cage top. The cage top was then turned until the mouse was hanging from the cage top with all four limbs grasping the bars. Each mouse was given three trials with a maximum possible latency of 60 seconds. If the mouse fell before 10 seconds, the procedure was repeated two more times.
For wirehang measurements, a 55-cm wide 2-mm-thick metallic wire was secured to two vertical stands approximately 1m above a thick foam pad. Mice were raised by their tail above the wire, slowly lowered until the mouse grasped the center of the wire with both front paws, then slowly lowered until the body was below the wire and released. The latency time that the mouse remained on the wire was recorded, with a maximum of 60 seconds. If the mouse fell before 10 seconds, the test was repeated. Each mouse was given three trials and the overall score was an average of three trials. If on the 3rd trial, the mouse still fell before 10 seconds, the best latency out of three trials was recorded.
Statistics
Data are expressed as mean ± SEM unless otherwise indicated. The primary aim was assessed by taking the mean of the FI scores of the two raters for each mouse. The data were assessed as normal using a D’Agostino & Pearson omnibus normality test, and thus differences between FI scores across groups were calculated with one-way analysis of variance with Tukey’s honest significant difference (HSD) post hoc test or t-tests where appropriate. The proportion of mice scored 0, 0.5, or 1 for an individual FI item, by raters 1 and 2, was compared across mouse groups using a Chi-squared test. To determine whether functional outcomes were correlated with FI scores, the correlation coefficient (r 2) was calculated. Inter-rater reliability was assessed with the two-way random, consistency, average intraclass correlation coefficient (ICC). The inter-rater reliability for specific items of the FI across raters was assessed by calculating the percentage of mice scored in agreement for each item across two raters. The percentage agreement was reported instead of Cohen’s or Fleiss’ kappa coefficient, as the nature of the index (only three possible scores, some scores used very infrequently) resulted in kappa values that severely underestimated the agreement (22). Data analysis was completed using the statistics program SPSS (Version 21.0, SPSS Inc., Chicago, IL) and GraphPad Prism (Version 6.04, GraphPad Software, La Jolla, CA).
Results
Effect of Strain, CR, and RSV on FI Scores
Male CR DBA/2J mice had higher FI scores than male CR C57BL/6J mice (p = .01). There was no change in FI scores in the male and female AL-fed DBA/2J mice, and female CR DBA/2J mice compared to sex-matched C57BL/6J mice (Figure 1A and andBB).
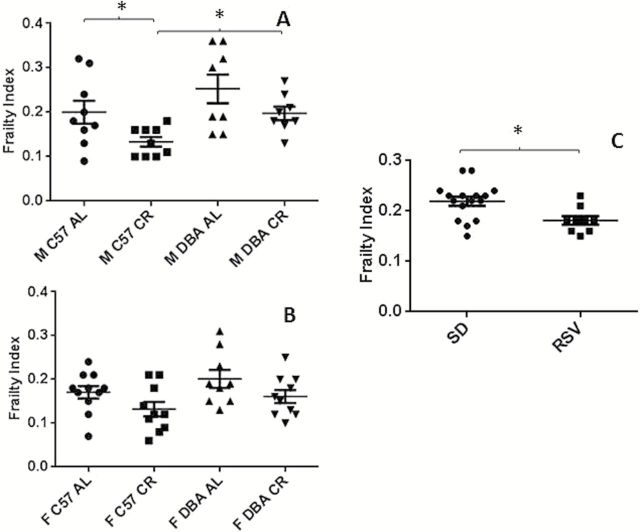
Frailty index scores for (A) male C57BL/6J and DBA/2 mice fed either an AL diet, or a 40% CR diet (19±2 months age, n = 8–9) (B) female C57BL/6J and DBA/2J mice fed either an AL diet, or a 40% CR diet (19±1 months age, n = 9–11) and (C) male C57BL/6J mice fed either a standard AIN-93G diet, or an AIN-93G diet supplemented with resveratrol (100mg/kg mouse/day) (n = 16 SD, n = 9 RSV, 24±0 months age). Data are presented as mean ± SEM. M, male; F, female; C57, C57BL/6JJ; DBA, DBA/2J; AL, ad libitum; CR, calorie restricted; SD, standard diet; RSV, resveratrol diet. *p < .05.
In male C57BL/6J mice, CR significantly reduced the FI score, relative to their AL-fed counterparts (p = .04). There was a trend towards reduced FI in calorie restricted female C57BL/6J, male DBA/2J, and female DBA/2J mice compared to the sex and strain matched AL-fed groups (Figure 1A and andBB).
There was no significant sex effect upon FI with no change in FI observed in all female groups compared to the diet and strain matched male groups (Figure 1A and andBB).
In the second cohort of mice, 6 months of RSV treatment significantly reduced the FI in C57BL/6J mice relative to SD-fed mice (p = .01) (Figure 1C).
The proportion of mice scored 0, 0.5, or 1 was determined for each sex, strain and diet group, for each item (Supplementary Table 2 for males and supplementary Table 3 for females). There were some interesting strain and diet effects on the scoring of specific items. Temperature and body weight scores greater than 0 were more common in AL-fed mice of both strains and sexes, than in the CR groups. AL-fed male mice were also more likely to have rectal prolapse than the male mice on the CR diet. Alopecia was more common in the male and female DBA/2J mice, whilst loss of fur color was more common in the C57BL/6J mice of both sexes. Male and female DBA/2J mice were much more likely to have hearing loss with 100% of male mice from both diet groups scoring 0.5 or 1. Compared to both diet groups of male C57BL/6J mice, male DBA/2J mice were also more likely to have microphthalmia, a change in breathing rate and piloerection (Supplementary Table 2). Female DBA/2J mice were more likely than female C57BL/6J mice to have kyphosis, tremor, and a change in breathing rate. Distended abdomen was more common in female C57BL/6J mice than in female DBA/2J mice. Coat condition was more likely to be scored as normal in female C57BL/6J CR mice, than female AL-fed C57BL/6J mice or either female diet group of DBA/2J mice (Supplementary Table 3).
The only significant effect of RSV on scores of FI items, was an increase in the proportion of mice with an above 0 score for corneal opacity in the SD-fed group (34%) compared to the RSV diet group (0%, p = .005) (Supplementary Table 4).
Correlation between FI Scores and Functional Outcomes
Figure 2 shows the correlation between FI scores and the functional outcome measures of latency to fall for rotor rod, wire hang and cage top, for a small subset of the CR and AL-fed male and female DBA/2J and C57BL/6J mice. Standard correlation analysis showed no significant correlation between FI scores and any functional outcomes measured in this subset of animals.
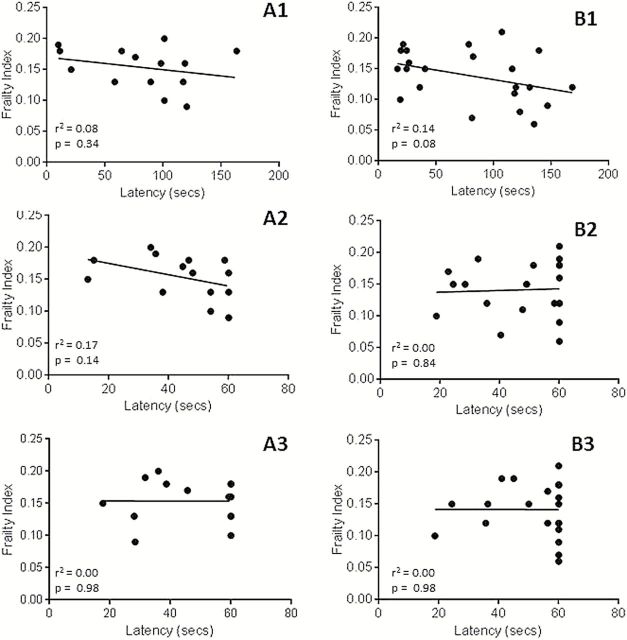
The correlation between frailty index and latency to fall for (A1) rotor rod, (A2) wire hang, and (A3) cage top functional testing in all tested male C57BL/6J and DBA/2J mice and the correlation between frailty index and latency to fall for (B1) rotor rod, (B2) wire hang, and (B3) cage top functional testing in all tested female C57BL/6J and DBA/2J mice. None of the correlations showed a significant association (p > .05).
Inter-Rater Reliability
The inter-rater correlation coefficient across the two raters for all mice was excellent (ICC = 0.88, 95% CI [0.80, 0.92]).
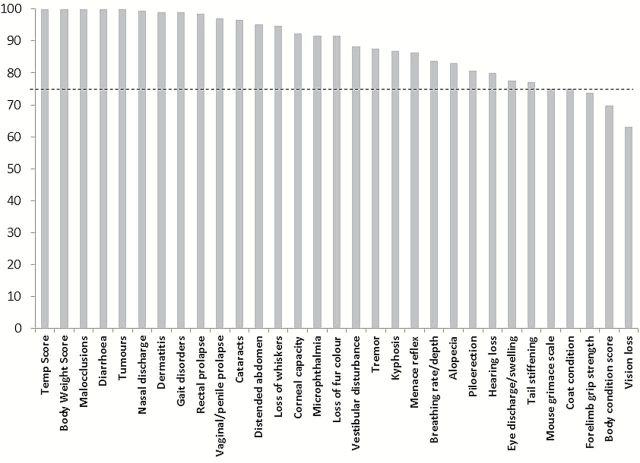
The average percentage agreement was calculated for each item of the frailty scale as explained in the methods section (Figure 3). The items of the scale which had the worst agreement across raters were coat condition, body condition score, vision loss, forelimb grip strength, and menace reflex, with less than 75% agreement.
Discussion
In this study, we report for the first time, the impact of strain, dietary, and pharmaceutical interventions on the mouse clinical FI (8). We found that both CR from age of 6 months, and 6 months of dietary RSV, significantly reduced the FI in old C57BL/6J male mice, compared to those fed control AL diets. Interestingly, FI scores were also higher for male calorie-restricted DBA/2J mice compared to age- and diet-matched C57BL/6J mice.
Twenty-four-month old C57BL/6J mice with chronic RSV treatment had a lower FI than AL-fed mice. Long-term treatment with RSV in mice fed a SD has previously been shown to delay functional decline, and increase healthspan, without increasing lifespan (16). The mouse FI may provide a new and important healthspan measure to be used as an outcome in interventional longevity studies. Consistent with previous studies, we found that the RSV diet group had a decreased proportion of mice with corneal opacity compared to the control diet group (16,17).
Nineteen-month old male C57BL/6J mice with CR from age 6 months had a lower FI than AL-fed male C57BL/6J mice. This is consistent with previous studies demonstrating that CR both delays age-related decline and increases lifespan in mice (13,23). There has, however, been some recent controversy about whether the effects of CR on mice are universally positive (24–26). The age of initiation of CR (27), the mouse strain (24,25), and the composition of the diet (28) are increasingly being recognized as important. It would be interesting in future studies to look at the effect of CR on frailty in a non-inbred mouse strain, and at the effect of dietary composition of CR on clinical mouse FI.
Consistent with a previous study showing DBA/2J mice are not responsive to the lifespan extension effects of CR (11), we found that frailty scores did not significantly change with CR in male and female DBA/2J mice. Earlier studies did find there was an effect of 40% CR in DBA2/J mice on longevity (29), and on delaying age-related pathologies (30). The lack of change in FI scores for calorie restricted DBA/2J mice in this study would suggest that CR may not delay age-related decline, or increase the healthspan of this strain of mice.
The difference in frailty scores between the short-lived DBA/2J strain and the C57BL/6J strain was not as substantial as expected. This cohort of mice were younger (19 months) than the RSV-fed mice (24 months) so perhaps the effects of interventions on the FI may be more clearly seen with increasing age. We observed less variability in frailty scores at 24 months of age in our study (coefficient of variation for male AL-fed C57BL/6J was 0.16 at 24 months and 0.30 at 19 months), which is consistent with previous findings in mice (8) and humans (31). On the other hand, it may indicate that although the DBA/2J strain are up to 6 months short-lived than the C57BL/6J strain (12), perhaps they have a comparatively longer healthspan, as indicated by moderate frailty scores.
It is also possible that the FI scale may need to be tailored to include strain-specific ageing deficits for use in different mouse strains. The high prevalence of factors like hearing loss in the 19-month old DBA/2J mice both in this study and in much younger mice (2–3 weeks) in other studies (32) suggest that this deficit saturates too early to be useful as a deficit in assessment of a FI in this strain. Early saturation has previously been identified as a reason an item should be excluded as a deficit in clinical frailty studies (33).
There were some interesting changes in the specific items of the FI across the strain and diet groups. CR brought the body weight and temperature scores of both DBA/2J and C57BL/6J mice back to the “normal” 12-week old mouse range, compared to the AL-fed groups. This is consistent with previous studies in which AL feeding caused young and middle-aged mice of both strains to gain weight, and this effect was stopped by CR (34). Temperature is also known to increase with feeding and decrease with CR (35) and decreased core temperature is also associated with increased longevity (36).
One of the secondary aims of this study was to assess the applicability of the Whitehead and colleagues (8) FI to a different C57BL/6J mouse cohort. We successfully used the FI to test mice from a different facility and cohort to the original article (7), with dietary and pharmaceutical interventions. The FI was convenient to use, with easily available equipment and fast assessment of each mouse using the Mouse Frailty Assessment Form (© Susan Howlett, 2013). It allows assessment of the effect of interventions in mice of the same chronological age on biological age.
The correlation between the functional outcomes of latency to fall for rotarod, wire hang and cage top and the FI was investigated for a subset of cohort 1 C57BL/6J and DBA/2J mice (Figure 2). There were no significant associations between any of the measured functional outcomes and FI. This is consistent with human data, as clinically the patients identified as frail with the FI, are not always identified as frail with the phenotype approach that includes measures of functional performance, and vice versa (37,38). Furthermore, as this study was completed as part of a larger study, the functional outcome testing was done at 16 months, while the frailty assessment was done at 19 months. The smaller sample size and the difference in time for assessment may explain the lack of correlation, and it would be interesting to explore this further, with both functional assessment and FI assessment completed at the same time.
The two raters had excellent inter-rater reliability as assessed with the intraclass correlation coefficient. The percentage correlation for the specific items of the FI was calculated to determine if there were certain items on which raters were more likely to disagree (Table 2). The items of the scale which had the worst agreement across raters were coat condition, body condition score, mouse grimace scale, vision loss, and forelimb grip strength. These items all seem to have an element of subjectivity, where the rater is required to judge the reaction of the mouse compared to “normal” rather than objective criteria such as “more than 25% hair loss” for example. The majority of these items were also identified by Feridooni and colleagues (9) as those with the most discrepancies between raters, and they found that after a discussion period between raters about discrepancies in scoring, inter-rater correlation increased. The use of a training and/or discussion period, or a more detailed scoring manual may have further improved the inter rater correlation in this study. There is currently much research into the search for frailty biomarkers clinically (39), which could provide additional objective items for the FI, but would add complexity and cost to the assessment.
Strengths of this study include the rigorous and blinded data collection, and quality of the animal cohorts. Some of the limitations of this study include the lack of temperature scores for one mouse cohort, and the time and sample size differences between the functional outcome scoring and the FI scoring. It would also be interesting, in future studies, to assess the FI score on the same mice over time, or pre- and post-interventions, and to assess the association of frailty prospectively with other markers of healthspan and lifespan.
In summary, this study was the first to investigate the effect of mouse strain and dietary and pharmaceutical interventions on this novel clinical FI in mice. RSV treatment and CR reduced FI in ageing male C57BL/6J mice. This study also used the mouse clinical FI in a different mouse cohort, tested the index in a different mouse strain, and investigated the inter-rater reliability across two raters. The clinical mouse FI provides an invaluable tool for studies in ageing mice to assess the impact of interventions on frailty or the impact of frailty on response to interventions.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
A.E.K is supported by a National Health and Medical Research Council (NHMRC) biomedical postgraduate scholarship. S.E.H. is supported by a grant from the Canadian Institutes of Health Research (MOP 126018). R.d.C is supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health. This work was also supported by Geoff and Elaine Penney Ageing Research Unit.
Acknowledgments
We would like to thank Jillian Patterson for her assistance with the statistical analysis.
References
Articles from The Journals of Gerontology Series A: Biological Sciences and Medical Sciences are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/gerona/glu315
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/biomedgerontology/article-pdf/71/3/333/16744903/glu315.pdf
Citations & impact
Impact metrics
Article citations
Late-life dietary folate restriction reduces biosynthesis without compromising healthspan in mice.
Life Sci Alliance, 7(10):e202402868, 23 Jul 2024
Cited by: 0 articles | PMID: 39043420 | PMCID: PMC11266815
Quantification of healthspan in aging mice: introducing FAMY and GRAIL.
Geroscience, 46(5):4203-4215, 17 May 2024
Cited by: 1 article | PMID: 38755467 | PMCID: PMC11336093
The intersection of frailty and metabolism.
Cell Metab, 36(5):893-911, 12 Apr 2024
Cited by: 3 articles | PMID: 38614092
Review
Metabolic dysfunction and the development of physical frailty: an aging war of attrition.
Geroscience, 46(4):3711-3721, 24 Feb 2024
Cited by: 3 articles | PMID: 38400874 | PMCID: PMC11226579
Review Free full text in Europe PMC
Gut microbiota influence frailty syndrome in older adults: mechanisms and therapeutic strategies.
Biogerontology, 25(1):107-129, 27 Dec 2023
Cited by: 2 articles | PMID: 38150088
Review
Go to all (79) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Identification of tissue-specific transcriptional markers of caloric restriction in the mouse and their use to evaluate caloric restriction mimetics.
Aging Cell, 16(4):750-760, 26 May 2017
Cited by: 24 articles | PMID: 28556428 | PMCID: PMC5506434
Reliability of a Frailty Index Based on the Clinical Assessment of Health Deficits in Male C57BL/6J Mice.
J Gerontol A Biol Sci Med Sci, 70(6):686-693, 09 Sep 2014
Cited by: 45 articles | PMID: 25205762 | PMCID: PMC4425849
A clinical frailty index in aging mice: comparisons with frailty index data in humans.
J Gerontol A Biol Sci Med Sci, 69(6):621-632, 19 Sep 2013
Cited by: 235 articles | PMID: 24051346 | PMCID: PMC4022099
Frailty index as a biomarker of lifespan and healthspan: Focus on pharmacological interventions.
Mech Ageing Dev, 180:42-48, 26 Mar 2019
Cited by: 35 articles | PMID: 30926563 | PMCID: PMC7307802
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Canadian Institutes of Health Research (1)
Grant ID: MOP 126018
 1
,
2
1
,
2