Abstract
Free full text

Mitochondria and Cancer
Abstract
Decades ago Otto Warburg observed that cancers ferment glucose in the presence of oxygen, suggesting that defects in mitochondrial respiration may be the underlying cause of cancer. We now know that the genetic events, which drive aberrant cancer cell proliferation, also alter biochemical metabolism including promoting aerobic glycolysis, but do not typically impair mitochondrial function. Mitochondria supply energy, provide building blocks for new cells, and control redox homeostasis, oncogenic signaling, innate immunity and apoptosis. Indeed, mitochondrial biogenesis and quality control are often upregulated in cancers. While some cancers have mutations in nuclear-encoded mitochondrial tricarboxylic acid (TCA) cycle enzymes that produce oncogenic metabolites, there is negative selection for pathogenic mitochondrial genome mutations. Eliminating mitochondrial DNA limits tumorigenesis and rare human tumors with mutant mitochondrial genomes are relatively benign. Thus, mitochondria play a central and multi-functional role in malignant tumor progression, and targeting mitochondria provides therapeutic opportunities.
Mitochondria are maternally inherited, cytoplasmic organelles that originated from symbiotic bacteria (Wallace, 2012). They co-evolved with their host, such that most mitochondrial proteins are nuclear encoded. Mitochondria, however, retain a small 16Kb DNA genome (mtDNA) that encodes tRNAs and rRNAs and proteins essential for respiration. Cells have hundreds of mitochondria that can be wild type or can exist of mixtures of wild type and mutant forms, a state referred to as heteroplasmy. Mitochondria are important bioenergetic and biosynthetic factories critical for normal cell function and human health. Mitochondrial diseases in humans can be devastating, affecting many tissues including the nervous system, heart, and muscle (Farrar et al., 2013). Mitochondrial diseases can be caused either by maternal inheritance of pathogenic mutant mitochondrial genomes that manifest disease upon a high degree of heteroplasmy or by nuclear inheritance of loss-of-function mutations in essential mitochondrial genes.
The observation by Otto Warburg that cancers acquire the unusual property of taking up and fermenting glucose to lactate in the presence of oxygen (aerobic glycolysis), led him to propose the mitochondrial respiration defects are the underlying basis for aerobic glycolysis and cancer (Warburg, 1956a, b). Indeed, the “Warburg effect” is the basis for tumor imaging by FDG-PET, which is in widespread clinical use (Gallamini et al., 2014). Not all tumors, however, share this property of aerobic glycolysis. It is also now apparent that mitochondrial respiration defects are not generally the cause of aerobic glycolysis, nor are they generally selected for during tumor evolution.
In most cancers, oncogenic driver mutations such as activation of K-ras, c-Myc and phosphatidylinositol-3 (PI3) kinase or loss of phosphatase and tensin homolog (Pten) and p53, not mutations that inactivate mitochondrial respiration complexes, promote glycolysis (Vander Heiden et al., 2009). Moreover, most cancers still retain mitochondrial function, including respiration. Some tumors have high levels of oxidative phosphorylation, while others that are relatively glycolytic still retain mitochondrial respiration and other functions (Zu and Guppy, 2004). When quantitated by flux analysis in cultured cells, Akt transformation does not substantially impact respiration, whereas Ras transformation reduces respiration, but nevertheless a majority ATP is still produced by oxidative phosphorylation (Fan et al., 2013; Gaglio et al., 2011; Yang et al., 2010).
Functional tests for the requirement for mitochondrial activity in cancers have revealed their importance. Inactivation of the mitochondrial transcription factor Tfam that depletes mitochondria from tumor cells impairs K-ras lung tumor growth in autochthonous models (Weinberg et al., 2010). Depleting mitochondrial DNA from tumor cells by generating ρ0 cells by poisoning mitochondrial DNA replication compromises tumorigenesis (Tan et al., 2015). Moreover, selection for restoration of the growth of mitochondrial DNA-depleted ρ0 tumors is associated with the horizontal transfer of mitochondrial genomes from host tissue and restoration of respiration. These and other findings suggest that the role of mitochondria in cancer is not as simple as Warburg envisioned. In contrast, they point to the importance of mitochondrial function to tumor growth.
Mitochondria integrate catabolism, anabolism, and signaling
Mitochondria are bioenergetic and biosynthetic organelles that take up substrates from the cytoplasm and use them to drive fatty acid oxidation (FAO), the TCA cycle, the electron transport chain (ETC) and respiration, and to synthesize amino acids, lipids, nucleotides, heme and iron sulfur clusters, as well as NADPH for their own antioxidant defense (Figure 1) (Wallace, 2012). NADH and FADH2 produced via TCA cycle turning powers the ETC, which in turn generates a proton gradient across the mitochondrial inner membrane that produces ATP through the action of the H+-ATP synthase. Dihydroorotate dehydrogenase (DHODH), which is essential for de novo pyrimidine synthesis, requires a functional respiratory chain for activity (Khutornenko et al., 2010). The reactive oxygen species (ROS) generated in mitochondria as a byproduct of the ETC can activate signal transduction pathways such as that of MAP kinase and the HIFs (in normoxia is referred to as pseudohypoxia) (Shadel and Horvath, 2015; Sullivan and Chandel, 2014). Excess ROS production can also lead to cell death. Mitochondria sequester Ca++, the selective release of which controls signaling and a broad array of cellular functions. Mitochondria act as signaling platforms for innate immunity (e.g. MAVS and by the release of mtDNA) (Weinberg et al., 2015; West et al., 2015). The BCL-2 family of proteins at the mitochondrial outer membrane control programmed cell death by apoptosis. BCL-2-realated anti-apoptotic proteins block, whereas the BAX and BAK pro-apoptotic proteins promote the release of cytochrome c from the mitochondrial inter membrane space triggers the apoptosome and caspase protease activation in the cytosol, causing cell death (Figure 1) (Czabotar et al., 2014; Moldoveanu et al., 2014). Thus, communication between mitochondria and the cell coordinates a diverse array of functions that are critical for cell metabolism, growth and survival.
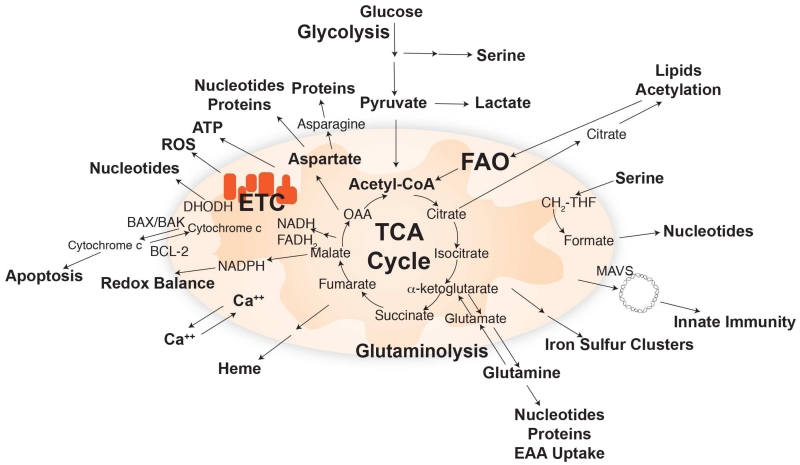
The TCA cycle uses substrates from glycolysis, fatty acid oxidation, and amino acid catabolism to generate building blocks and high-energy electrons (NADH and FADH2) to power the ETC. Major biosynthetic products produced by mitochondria and signaling pathways regulated by mitochondrial function are indicated.
In addition to being the bioenergetics power-house and signaling hub, mitochondria also function as a platform for generating biosynthesis building blocks. This requires a continuous inflow of 4 carbon (4C) units to balance the outflow of such units to amino acids and other biosynthetic products (Figure 1). This replenishment of the TCA cycle 4C units is termed anaplerosis and can occur through carboxylation of pyruvate or catabolism of glutamine and other amino acids (Comerford et al., 2014; DeBerardinis et al., 2007; Fan et al., 2009; Mashimo et al., 2014; Yuneva et al., 2007). Such provision of 4C units is in addition to the need for 2C units that are provided by acetyl-CoA, which can be formed from fatty acids, pyruvate, acetate, and many amino acids. The condensation of a 4C and 2C unit yields citrate via citrate synthase, which is solely mitochondrially localized. In addition to being an upstream precursor capable of generating all classical TCA cycle intermediates, citrate can be exported to the cytosol, where it is broken down to oxaloacetate and acetyl-CoA, which is required for lipid synthesis and protein modifications (Figure 1). In addition to 4C and 2C units, mitochondria also play a central role in the metabolism of 1C units required for purine, thymidine, and methionine synthesis. Recent literature has highlighted several advancements in the understanding of mitochondria-mediated biosynthetic pathways.
One-carbon (1C) metabolism
In activated tetrahydrofolate (THF) form, the essential vitamin folate functions to carry 1C units, which are used for methionine regeneration, and thymidine and purine synthesis. 1C metabolism plays a central role in growth and development, with folate deficiency during pregnancy resulting in neural tube defects, and anti-folates are important chemotherapy agents. The folate analog aminopterin was the first drug to produce remissions in leukemia, (Farber and Diamond, 1948) and its close relative methotrexate is still a mainstay in treatment of acute lymphocytic leukemia. The more recently discovered pemetrexed is a first-line treatment for lung cancer.
While the major pathways using 1C units are cytosolic, the enzymes generating and interconverting THF-bound 1C units exist in parallel cytosolic and mitochondrial and pathways. The primary source of 1C units is the amino acid serine, but the mitochondrial pathway can also accept a variety of other 1C donors including glycine and betaine. (Locasale, 2013). Strikingly, the mitochondrial 1C enzymes SHMT2 and MTHFD2 are among the most transcriptionally upregulated genes in cancer (Nilsson et al., 2014; Vazquez et al., 2013). SHMT2, which consumes serine to load a 1C unit on to THF, is induced in Myc-transformed cells by hypoxia (Ye et al., 2014). In human glioblastoma tumors, SHMT2 is particular strongly expressed in the ischemic cells bordering necrotic tumor regions, and, in animal models of tumor ischemia, SHMT2 expression promotes cancer cell survival (Kim et al., 2015). MTHFD2 acts immediately downstream of SHMT2, to produce formyl-THF. Mitochondrial formyl-THF can be used for at least three potentially important purposes: to facilitate initiation of mitochondrial translation by producing formyl-methionine tRNA, to generate mitochondrial NADPH through the enzyme formyl-THF dehydrogenase (ALDH1L2) and to release free formate. Recent work suggests that ALDH1L2 activity promotes mitochondrial redox homeostasis and thereby contributes to melanoma metastasis (Piskounova et al., 2015). It will be of interest to explore targeting SHMT2, MTHFD2, and ALDH1L2 for cancer therapy.
While a role for mitochondrial formate release in cancer progression is less well proven, it is likely to be of yet greater general importance. Free formate, unlike 1C-loaded THF species, can pass between the cytosol and mitochondrion (Pike et al., 2010). Thus, formate released by mitochondria can feed cytosolic 1C pools and thereby nucleotide synthesis and methylation. An important difference between the cytosolic and mitochondrial 1C pathways is cofactor usage for the dehydrogenase step: cytosolic MTHFD1 uses NADP(H) while mitochondrial MTHFD2 primarily uses NAD(H) (Shin et al., 2014). This likely results in more rapid oxidative dehydrogenase flux to formyl-THF in the mitochondrion. Accordingly, rapidly growing cells may depend on mitochondrial serine catabolism to generate formate for use in cytosolic biosynthetic pathways.
Glutamine metabolism
Glutamine is the most abundant free amino acid in human blood (400-700 μM) and the predominant TCA cycle fuel in cultured cancer cells, where the majority of intracellular glutamine is converted in mitochondria by glutaminase to glutamate (Figure 1). Glutamate can in turn be converted by glutamate dehydrogenase (GLUD) or transamination into α-ketoglutarate to enter the TCA cycle (De Vitto et al., 2015). Oxidative metabolism of α-ketoglutarate in the TCA cycle generates ATP and 4C units (e.g., oxaloacetate). Alternatively, and less commonly, α-ketoglutarate can undergo reductive carboxylation to generate isocitrate and citrate, and eventually 2C units for fat synthesis (Mullen et al., 2012). In addition to its role as a carbon source, glutamine is also a critical nitrogen source – the obligate nitrogen donor for the biosynthesis of purines, pyrimidines, NAD, asparagine, and glucosamine. Glutamine also plays important roles in transport, where glutamine concentration gradients drive the uptake of essential amino acids, and in signaling, where it activates mTOR (Nicklin et al., 2009).
Alongside altered glucose metabolism, cancer cells also increase uptake and utilization of glutamine for their anabolic needs (Wise and Thompson, 2010). The proto-oncoprotein c-Myc induces the expression of glutamine transporters SLC1A5 and SLC7A5/SLC3A2 (Nicklin et al., 2009) and glutaminase (GLS) (Gao et al., 2009). Glutamine deprivation preferentially induces apoptosis in Myc-expressing cells (Wise et al., 2008; Yuneva et al., 2007). This “glutamine addiction” is rescued by membrane-permeable variants of α-ketoglutarate, indicating that the critical need for glutamine is to support anaplerosis (Wise et al., 2008). Targeting GLS is being actively pursued for cancer therapy. Indeed, loss of one GLS allele or pharmacological GLS inhibition suppresses tumor growth in various mouse models of cancer, and this approach revealed that this may be particularly valuable for suppressing Myc-driven cancers (Shroff et al., 2015; Xiang et al., 2015).
Glutamine can be taken up from dietary sources via the small intestine. The majority of glutamine in the body, however, is synthesized by glutamine synthetase (GS), which condenses glutamate and ammonia into glutamine. Such synthesis occurs in muscle and liver as part of ammonia detoxification, and one can envision a cycle where glutamine is made in these tissues, carried by the circulation to tumors, and catabolized to release ammonia, which is carried back to muscle and liver. Some tumors, however, also upregulate GS and glutamine synthesis (He et al., 2010; van der Vos et al., 2012). In a number of breast cancer and glioblastoma cell lines, increased GS activity and de novo synthesis of glutamine is essential for cell survival, growth, and proliferation under limited supply of exogenous glutamine (Bott et al., 2015; Tardito et al., 2015). In such cases, newly synthesized glutamine mainly contributes to the biosynthesis of nucleotides and asparagine, and to the transport of essential amino acids. 13C-glucose tracing revealed that increased GS activity leads to the incorporation of glucose carbon into glutamine via α-ketoglutarate/glutamate, opposing glutamine anaplerosis (Bott et al., 2015; Tardito et al., 2015). These notions are supported by in vivo evidence that glutamine synthesis is increased in human glioblastomas (Maher et al., 2012; Marin-Valencia et al., 2012), and that glutamine oxidation is not detected in glioblastoma mouse xenograft (Mashimo et al., 2014). Moreover, while c-Myc induces GLS expression by suppressing microRNA (miR)-23a/b (Gao et al., 2009), in a number of mouse and human cell lines and cancer tissues, c-Myc induces the expression of GS via thymine DNA glycosylase (TDG)-mediated GS promoter demethylation (Bott et al., 2015). The differential expression of GS and GLS, often with inverse correlation, has been observed in Myc mouse models and in human breast cancer cell lines (Kung et al., 2011; Yuneva et al., 2012). It is likely that certain oncogenic activity such as Myc activation leads to increased demand for glutamine to support anabolic processes such as nucleotide synthesis, which can be fulfilled by increased glutamine synthesis in a cell autonomous or whole body fashion. Clarifying the circumstances in which glutamine synthesis or catabolism is essential for in vivo tumor growth will be critical to successful development of glutamine-targeted cancer therapies.
Aspartate synthesis
Both glutamine and aspartate are built from carbon skeletons that are fundamental TCA cycle intermediates, α-ketoglutarate and oxaloacetate, respectively. Both glutamine and aspartate are required not only for protein, but also for nucleotide synthesis. In terms of their roles in whole body metabolism, however, aspartate and glutamine are polar opposites. Aspartate is the least abundant of the standard 20 amino acids in the circulation, with a normal concentration in adults of less than 6 μM. Moreover, while glia actively take up aspartate via co-transport with sodium to prevent extracellular accumulation of excitatory neurotransmitter in the central nervous system, most cells have minimal ability to uptake aspartate. Thus, they are dependent on de novo aspartate synthesis, which occurs in one-step by transamination of oxaloacetate by glutamate.
Aspartate transaminase exists as both cytosolic (GOT1) and mitochondrial (GOT2) isozymes, each of which typically carries high flux due to their role in the malate-aspartate shuttle, one of the two major mechanisms for carrying high-energy electrons from the cytosol to mitochondrion. In the shuttle, aspartate is made in the mitochondria by GOT2 and converted in the cytosol into oxaloacetate by GOT1, which is then reduced by NADH to malate for import into mitochondria or for consumption by malic enzyme to make NADPH. GOT1-dependent malic enzyme-driven NADPH production is required for redox homeostasis in pancreatic cancer (Son et al., 2013).
Recent work reveals that another essential role of GOT1 in cell proliferation is to support aspartate synthesis in conditions where the ETC is impaired. CRISPR-based screening identified GOT1 as required for cell growth in the presence of pharmacological ETC inhibition (Birsoy et al., 2015). The requirement for GOT1 could be circumvented by providing sufficient exogenous aspartate, or by providing an electron acceptor to restore the NAD+/NADH ratio. Classically, growth of ETC-deficient cells is supported by pyruvate, which serves both as the substrate for pyruvate carboxylase to make oxaloacetate and as an electron acceptor. Ketobutyrate, which only fulfills the electron acceptor role, can also support aspartate synthesis and cell growth (Sullivan et al., 2015). Thus, through the accumulation of mitochondrial NADH, ETC deficiency depletes oxaloacetate and impairs mitochondrial aspartate synthesis, rendering cells dependent on GOT1 or exogenous aspartate or electron acceptors.
The mitochondrial genome in cancer
Acquired nuclear somatic and germ line mutations in about one hundred genes are known to provide a selective advantage in cancer (Stratton et al., 2009). Increases in the mutation frequency due to DNA repair defects, replication errors, carcinogen exposure or aging promote cancer by increasing the occurrence of mutations in these genes. These cancer driver mutations and their functional consequence to cancer growth are often well understood and are the subject of targeted anti-cancer drug development. In contrast, the status and role of mutations in the mitochondrial genome in cancer has been less clear. Studies with small sample sizes using the low sensitivity of past sequencing technologies to assess heteroplasmic genomes have rendered association of somatically acquired mitochondrial genome mutations with cancer unclear. Moreover, the now commonplace advanced nuclear sequencing studies usually ignore the mitochondrial genome.
Two recent large-scale efforts to examine the mutational landscape of the mitochondrial genome in over 2000 human cancers across more than 30 tumor types compared with normal tissue from the same patient by massively parallel DNA sequencing has revealed important insights (Ju et al., 2014; Stewart et al., 2015). These studies identified many somatic substitutions with strong replicative strand bias, with C to T and A to G transversions on the mitochondrial heavy strand indicative of mitochondrial polymerase G errors as the major cause of mutations. This is surprising, given the close association of mitochondrial ROS production with mutations, which is apparently dwarfed by the frequency of polymerase errors that occur during the normal process of genome replication. More importantly, missense mutations are selectively neutral and drift toward homoplasmy, whereas there is negative selection for deleterious, pathogenic mutations that remain heteroplamic (Ju et al., 2014; Stewart et al., 2015). These findings clearly indicate that there is generally selective pressure in human cancers for retention of mitochondrial genome function.
Mitochondrial quality control in cancer
For cells to function properly, there needs to be communication back and forth between mitochondria and the nucleus, referred to as antegrade and retrograde signaling (Chandel, 2015). Nuclear genes control mitochondrial biogenesis to synthesize more mitochondria and meet increased metabolic demand. Opposing biogenesis is another layer of nuclear control by the autophagy and mitophagy genes. The lack of accumulation of pathogenic mitochondrial genome mutations in human cancers suggests engagement of active mechanisms for mitochondrial quality control that prevent the accumulation of defective mitochondria. Mitophagy is a specialized from of autophagy that selectively degrades and eliminates superfluous or damaged mitochondria (Randow and Youle, 2014). Biogenesis and mitophagy work in concert to regulate mitochondrial mass, function and quality. In turn, defective mitochondria provide the “eat me” signal directly to the mitophagy machinery by membrane depolarization and a cascade of phosphorylation and ubiquitation of mitochondrial proteins, while reduced cellular energy output (as occurs if the total number of mitochondria is insufficient) triggers mitochondrial biogenesis via the general energy sensor AMP kinase and by other mechanisms.
Depolarization of the outer mitochondrial membrane triggers the activation of the kinase PINK1, which phosphorylates ubiquitin causing the recruitment of the E3 ligase PARKIN to the mitochondrial outer membrane (Lazarou et al., 2015). PARKIN further ubiquitinylates mitochondrial proteins, which recruit the autophagy machinery to capture, engulf and degrade these specific mitochondria in lysosomes. Several other PARKIN-independent mitophagy receptor proteins include BNIP3 (Daido et al., 2004), NIX (Schweers et al., 2007), FUNDC1 (Liu et al., 2012), and BCL2L13 (Murakawa et al., 2015). Although it is reasonable to speculate a role of mitophagy in cancer, direct evidence that mitophagy is involved in tumorigenesis is lacking. The role of mitochondria quality control in cancer is mostly implicated by information obtained using genetic models with manipulation of bona fide autophagy genes.
Ras-driven cancers upregulate basal autophagy by activating the MiTF/TFE family of basic helix–loop–helix leucine zipper transcription factors that promote autophagy and lysosome biogenesis (Guo et al., 2011; Perera et al., 2015; Yang et al., 2011). Defects in autophagy produced by knocking out essential autophagy genes in autochthonous mouse models for cancer causes the accumulation of defective mitochondria and other autophagy substrates, and impairs mitochondrial respiration, cell growth, and survival while increasing cell death and senescence (Guo et al., 2013a; Guo and White, 2013; Guo et al., 2013b; Huo et al., 2013; Rao et al., 2014; Rosenfeldt et al., 2013; Strohecker et al., 2013; Xie et al., 2015; Yang et al., 2014). Systemic, acute autophagy ablation in mice with established Ras-driven lung cancer produces substantial tumor regression prior to significant damage to most normal tissues, suggesting that some tumors are particularly autophagy-dependent (Karsli-Uzunbas et al., 2014). Importantly, in contrast to the carcinomas generated with autophagy intact, autophagy deficient tumors resemble benign oncocytomas, which are tumors characterized by the accumulation of defective mitochondria (Guo et al., 2013a; Karsli-Uzunbas et al., 2014; Strohecker et al., 2013). Thus autophagy is required for tumors to progress from benign to malignant growth. This is also consistent with the requirement for autophagy in the malignant progression of Ras-driven benign pancreatic PANIN lesions (Rosenfeldt et al., 2013; Yang et al., 2014). Thus autophagy is induced by and required for overcoming a barrier to malignant growth in a variety of cancer types.
As a major role of autophagy is mitochondrial quality control and possibly also providing substrates for mitochondrial metabolism, autophagy may promote tumorigenesis and malignancy by these mechanisms. Direct demonstration of this is lacking, as is the identity of specific substrates provided by autophagy-mediated recycling and the metabolic pathways they support is not yet known. Autophagy deficiency in tumor cells causes glutamine dependency suggesting that glutamine may be one autophagy-supplied substrate (Guo et al., 2013a; Strohecker et al., 2013). It is also not known if autophagy eliminates mitochondria with pathogenic mitochondrial genome mutations in tumor cells to preserve the functioning pool of mitochondria or whether the defects in respiration observed without autophagy are the result of protein damage or substrate limitation. In neurons, however, mitophagy is important for the removal of damaged mitochondrial proteins in the setting of mitochondrial genome damage (Pickrell et al., 2015).
Oncocytomas accumulate defective mitochondria
Oncocytomas are rare, benign tumors of most epithelia characterized by the vast accumulation of defective mitochondria due to pathogenic mtDNA mutations (Gasparre et al., 2011). In mouse models, loss of autophagy results in oncocytoma tumors: genetic deletion of an essential autophagy gene causes Ras- and Braf-driven lung carcinomas to become benign oncocytomas (Guo et al., 2013a; Karsli-Uzunbas et al., 2014; Strohecker et al., 2013). Oncocytomas provide an example of tumors with defective mitochondrial respiration caused directly by pathogenic mitochondrial genome mutations. The existence of oncocytomas raises many questions. Why do oncocytomas accumulate defective mitochondria? Why are oncocytomas benign? Is there a role for defective mitochondria in either initiating tumorigenesis or limiting progression to benign disease?
A recent comprehensive analysis of the mutational landscape of human renal oncocytomas revealed that there are two main subtypes (Joshi et al., 2015). Type 1 is characterized by CCND1 rearrangements whereas Type 2 is aneuploid with loss of chromosome 1, and X or Y and/or 14 and 21, and displays male gender bias. There are few and no recurrent somatic mutations in the nuclear genome in either subtype. Type 2 oncocytoma may be a precursor of the more aggressive eosinophilic chromophobe renal cell carcinoma (ChRCC) based on similar transcriptomes and copy number variations (Joshi et al., 2015). What both renal oncocytoma subtypes share in common is recurrent pathogenic mutations in the mitochondrial genome, which may lead to ROS production that could contribute oncogenic signaling or perhaps to accumulation of oncometabolites.
Primary oncocytoma cells are defective for respiration and ROS production, are highly glycolytic, and have a transcriptional signature of p53 and AMP kinase activation, suggesting that loss of respiration may activate a metabolic checkpoint that limits tumor growth to benign disease (Joshi et al., 2015). Moreover, oncocytomas have disruption of the Golgi and vesicle trafficking, particularly loss of processing and activation of major lysosomal cathepsin proteases, accompanied by accumulation of autophagy substrates. Therefore, chronic energy crisis in oncocytomas caused by pathogenic mitochondrial mutations and defects in oxidative phosphorylation, may block trafficking and cause profound cellular dysfunction, limiting these tumors to benign disease (Joshi et al., 2015). Oncocytomas could also have deficits in the synthesis of other important building blocks such as amino acids and they may be expected to require reductive carboxylation of α-ketoglutarate to generate citrate. These hypotheses will be interesting to test in the future.
One long-standing idea is that pathogenic mitochondrial mutations cause oncocytomas, since some tumors are near homoplastic for a mutant mitochondrial genome (Gasparre et al., 2011), a finding that has held up in more recent genomic analyses (Joshi et al., 2015; Lang et al., 2015). Although they share the property of pathogenic mitochondrial mutations, both oncocytoma subtypes also have nuclear genome mutations, specifically CCND1 rearrangements and whole chromosome copy number losses, which can explain why they are tumors. Why they accumulate mtDNA mutations is less clear. One possibility is that pathogenic mitochondrial mutations are selected for because they produce ROS that activates HIFs and perhaps other oncogenic signaling pathways. The mutational signature in the mitochondrial genome in oncocytomas reflects ROS damage in addition to the expected signature of polymerase errors, but as respiration is defective, there is no ROS production or HIF transcriptional signature in tumors (Joshi et al., 2015). Thus, evoking this mechanism would require a temporal sequence of partial mitochondrial dysfunction, ROS production and transient activation of oncogenic signaling that initiates tumor growth followed later by complete loss of respiration and benign disease.
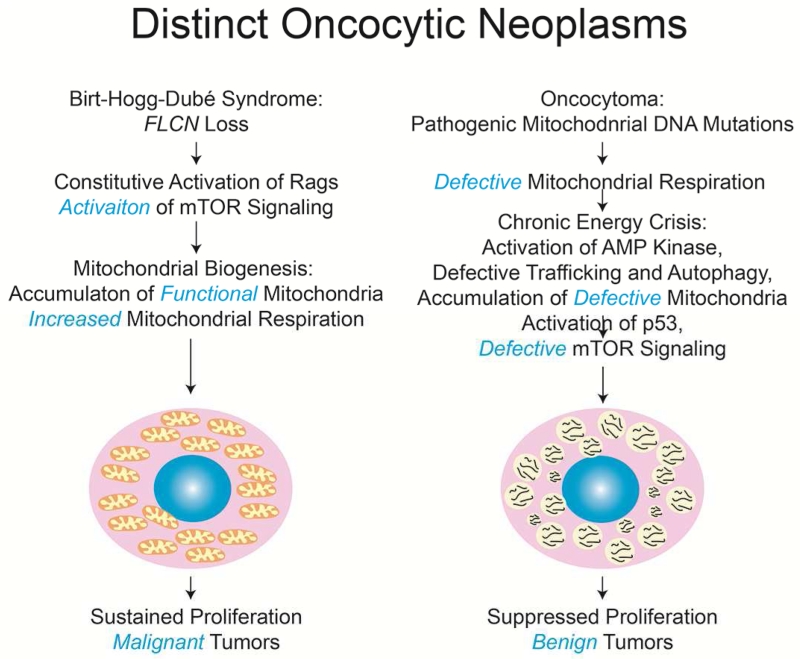
Different subtypes of mitochondria-rich tumors
Striking numerical increases in mitochondria is a characteristic of distinct tumor types with opposing clinical outcomes. Oncocytomas accumulate vast overabundance of mutant mitochondria, and as a result, are well known for their hallmark expansive, eosinophilic cytoplasm. This “oncocytic feature” associated with mitochondrial accumulation in tumor cells is also a characteristic of other tumors, although the significance is unknown.
Oncocytic tumor types include those arising in individuals with Birt-Hogg-Dube (BHD) syndrome that develop spontaneous renal and other tumors (Hasumi et al., 2012; Linehan et al., 2013). Most cases of BHD are caused by mutations in the FLCN tumor suppressor gene that encodes Foliculin, a GTPase activating protein (GAP) for the Rag GTPases (Tsun et al., 2013). Rags promote mTOR activation when amino acids are present, and this activity of the Rags is normally suppressed by FLCN. Loss of FLCN thereby promotes mTOR activation and signaling of cell growth that is a common oncogenic mechanism. Although FLCN may have other functions, its loss removes a repressive signal on mTOR in the absence of amino acids to produce constitutive signaling of cell growth. In sharp contrast to oncocytomas, the vast majority of which are benign, tumors resulting from FLCN loss progress to malignancy (Linehan et al., 2013). So why are tumors that accumulate mitochondria both benign and malignant?
Malignant tumors accumulate respiration functional mitochondria
In contrast to benign oncocytomas, oncocytic renal BHD tumors resulting from FLCN loss accumulate morphological normal, respiration-proficient mitochondria (Lang et al., 2015). Cells from these FLCN-deficient tumors have higher rates of respiration than normal cells, likely explained by the increased number of normal mitochondria (Hasumi et al., 2012). Thus there are two classes of mitochondria-rich oncocytic tumors, those that have mitochondrial defects that are benign, and those where mitochondrial function remains intact that are malignant (Figure 2). This fits with the concept of a tumor-promoting role for mitochondria. The reason why FLCN-deficient tumors accumulate mitochondria is not known, but mTOR promotes mitochondrial biogenesis and inhibits mitochondrial elimination by autophagy. The transcriptional signature for mitochondrial biogenesis is present in FLCN-deficient tumors and it would be interesting to explore the functional role of this and also the potential role of repressed mitophagy.
Mutations in TCA cycle enzymes promote cancer
Mutations in the nuclear encoded TCA cycle enzymes isocitrate dehydrogenase (IDH) 2, succinate dehydrogenase (SDH)× (SDHA-D and SDHAF2), and fumarate hydratase (FH) are found in human cancers (Gaude and Frezza, 2014). IDH2 mutations, like those in the closely related cytosolic protein IDH1, are genetically dominant, with active site mutations in a single allele producing a neomorphic activity where α-ketoglutarate is reduced to the oncometabolite 2-hydroxyglutarate (2-HG) (Dang et al., 2009; Losman and Kaelin, 2013). There are many α-ketoglutarate-dependent enzymes, particularly dioxygenases, and 2-HG is a competitive inhibitor of these enzymes, particularly, hypoxia-inducible factor (HIF) prolyl hydroxylases (PHDs), JmjC domain- containing histone demethylases (part of the JMJD family) and the ten-eleven translocation (TET) family of 5methyl cytosine (5mC) DNA hydroxylases. Oncometabolite-driven changes in the epigenome suppress differentiation and promote proliferation (Dang et al., 2009; Lu and Thompson, 2012; Parker and Metallo, 2015). 2-HG has also been found to inhibit the PHDs that degrade the HIFs and may thereby have a role in cancer. The exact dioxygenase and cancer pathway affected by 2-HG may depend on the cancer type. Nonetheless this provides a clear example of how a mutation in a mitochondrial TCA cycle enzyme can contribute to cancer through production of an oncometabolite.
Loss of function mutations in FH and SDH may act similarly by producing the accumulation of succinate, which also antagonizes the function of these α-ketoglutarate-dependent dioxygenases. Loss of FH also causes the accumulation of fumarate and the inactivation of proteins through aberrant succinylation of proteins such as the KEAP1 tumor suppressor that results in activation of the oncoprotein NRF2 (Adam et al., 2011; Adam et al., 2014). Fumarate also causes succinylation of glutathione, elevated ROS production and NRF2 activation, which can contribute to oncogenesis (Sullivan et al., 2013). Although these findings revealed new cancer-causing paradigms with significant translational implications, they also raise the question of how cancer cells adapt to these significant alterations in an important mitochondrial metabolic activity.
Targeting of mitochondrial metabolism for cancer therapy
Understanding of the role of mitochondria in cancer is revealing novel approaches to targeted therapy. Targeting 1C and glutamine metabolism and aspartate synthesis is being explored, as discussed above. The complex I inhibitor, metformin, a biguanide drug commonly used in the treatment of diabetes, has anticancer activity in diabetics, and it is of great interest to determine if this is more widely applicable (Pollak, 2013). One of the most important questions regarding metformin is whether its anticancer activity arises from reduced systemic glucose and insulin levels, or alternatively through targeting of the tumor ETC. Recently, it is been shown that expression of a metformin-resistant variant of Complex I within a Ras-driven colon cancer xenograft, rendered the tumor insensitive to metformin (Wheaton et al., 2014). Thus, at least within this model system, metformin impairs tumor growth by blocking tumor-intrinsic metabolism. While this could involve redox changes within the tumor, such as elevated NADH and reduced mitochondrial ROS production, the simplest explanation involves a dependence of the tumor on oxidative phosphorylation to make ATP.
Tumors cells with sensitivity to low glucose and reduced oxidative phosphorylation are sensitized to biguanides that inhibit complex I (Birsoy et al., 2014). Certain cancer stem cells, such as Ras-driven pancreatic cancer stem cells, appear to be particularly reliant on oxidative phosphorylation (Lonardo et al., 2013). Such cells are enriched after genetic extinction of Ras signaling, raising the possibility of synergy between oncogene-targeting agents and metformin (Viale et al., 2014).
Promoting mitochondrial ROS production to induce cancer cell death may enhance the activity of chemotherapy (Yun et al., 2015). Inhibiting autophagy and mitophagy can interfere with mitochondrial metabolism by blocking mitochondrial quality control and/or substrate supply (White, 2015; White et al., 2015). This is currently being explored in clinical trials by interfering with lysosome function (White et al., 2015). Targeting the MiTF/TFE family of transcription factors and master regulators of autophagy and lysosomal biogenesis activated in many cancers (Ferguson, 2015) may provide another option and may identify tumors potentially sensitive to autophagy inhibition.
Tumors where TCA cycle enzymes are mutated has also generated novel approaches. Tumors with mutations in TCA cycle enzymes require reductive carboxylation to generate the necessary TCA cycle intermediates, thus their metabolism is distinct from most normal tissues identifying a metabolic vulnerability (Cardaci et al., 2015; Mullen et al., 2012). The most promising is targeting the neomorphic activity of mutant IDH (Rohle et al., 2013; Wang et al., 2013). FH mutations cause a dependency on heme oxygenase (HO) to run a partial TCA cycle and NADH production important for viability (Frezza et al., 2011). Similarly, pyruvate carboxylase (PC) enables aspartate synthesis in SDH-deficient tumor cells creating a metabolic vulnerability (Cardaci et al., 2015). More generally, tumors with defective mitochondrial function, including benign oncocytomas and SDH and FH-deficient malignant tumors, require up-regulated glycolysis to meet their energy demands. Accordingly, such tumors may be particularly sensitive to inhibition of glucose uptake or glycolysis. Such inhibition may be close to what Warburg envisioned 80 years ago. Yet greater opportunities, however, lie in the bulk of tumors that do not conform to Warburg’s initial hypothesis of mitochondrial dysfunction, in which case is the secret may be to target effectively the mitochondria themselves.
Acknowledgements
E. White is supported by the NIH under award numbers R01CA163591, R01CA130893, R01CA188096, and R01CA193970, in addition to P30CA72720 to the Rutgers Cancer Institute of New Jersey. W.-X. Zong is supported by the NIH under award numbers R01CA129536 and R01GM97355. J.D. Rabinowitz is supported by R01CA163591 and SU2C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Potential Conflicts of Interest: EW is on the SAB of Forma Therapeutics. JDR is a co-founder and on the SAB of Raze Therapeutics and on the SAB of Kadmon Pharmaceuticals.
Author Contributions
All authors contributed to conceiving, writing and editing this review.
References
- Adam J, Hatipoglu E, O’Flaherty L, Ternette N, Sahgal N, Lockstone H, Baban D, Nye E, Stamp GW, Wolhuter K, et al. Renal cyst formation in Fh1-deficient mice is independent of the Hif/Phd pathway: roles for fumarate in KEAP1 succination and Nrf2 signaling. Cancer cell. 2011;20:524–537. [Europe PMC free article] [Abstract] [Google Scholar]
- Adam J, Yang M, Soga T, Pollard PJ. Rare insights into cancer biology. Oncogene. 2014;33:2547–2556. [Abstract] [Google Scholar]
- Birsoy K, Possemato R, Lorbeer FK, Bayraktar EC, Thiru P, Yucel B, Wang T, Chen WW, Clish CB, Sabatini DM. Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. Nature. 2014;508:108–112. [Europe PMC free article] [Abstract] [Google Scholar]
- Birsoy K, Wang T, Chen WW, Freinkman E, Abu-Remaileh M, Sabatini DM. An Essential Role of the Mitochondrial Electron Transport Chain in Cell Proliferation Is to Enable Aspartate Synthesis. Cell. 2015;162:540–551. [Europe PMC free article] [Abstract] [Google Scholar]
- Bott AJ, Peng IC, Fan Y, Faubert B, Zhao L, Li J, Neidler S, Sun Y, Jaber N, Krokowski D, et al. Oncogenic Myc Induces Expression of Glutamine Synthetase through Promoter Demethylation. Cell metabolism. 2015;22:1068–1077. [Europe PMC free article] [Abstract] [Google Scholar]
- Cardaci S, Zheng L, MacKay G, van den Broek NJ, MacKenzie ED, Nixon C, Stevenson D, Tumanov S, Bulusu V, Kamphorst JJ, et al. Pyruvate carboxylation enables growth of SDH-deficient cells by supporting aspartate biosynthesis. Nature cell biology. 2015;17:1317–1326. [Europe PMC free article] [Abstract] [Google Scholar]
- Chandel NS. Evolution of Mitochondria as Signaling Organelles. Cell metabolism. 2015;22:204–206. [Abstract] [Google Scholar]
- Comerford SA, Huang Z, Du X, Wang Y, Cai L, Witkiewicz AK, Walters H, Tantawy MN, Fu A, Manning HC, et al. Acetate dependence of tumors. Cell. 2014;159:1591–1602. [Europe PMC free article] [Abstract] [Google Scholar]
- Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. [Abstract] [Google Scholar]
- Daido S, Kanzawa T, Yamamoto A, Takeuchi H, Kondo Y, Kondo S. Pivotal role of the cell death factor BNIP3 in ceramide-induced autophagic cell death in malignant glioma cells. Cancer Res. 2004;64:4286–4293. [Abstract] [Google Scholar]
- Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. [Europe PMC free article] [Abstract] [Google Scholar]
- De Vitto H, Perez-Valencia J, Radosevich JA. Glutamine at focus: versatile roles in cancer. Tumour Biol. 2015 [Abstract] [Google Scholar]
- DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19345–19350. [Europe PMC free article] [Abstract] [Google Scholar]
- Fan J, Kamphorst JJ, Mathew R, Chung MK, White E, Shlomi T, Rabinowitz JD. Glutamine-driven oxidative phosphorylation is a major ATP source in transformed mammalian cells in both normoxia and hypoxia. Mol Syst Biol. 2013;9:712. [Europe PMC free article] [Abstract] [Google Scholar]
- Fan TW, Lane AN, Higashi RM, Farag MA, Gao H, Bousamra M, Miller DM. Altered regulation of metabolic pathways in human lung cancer discerned by (13)C stable isotope-resolved metabolomics (SIRM) Mol Cancer. 2009;8:41. [Europe PMC free article] [Abstract] [Google Scholar]
- Farber S, Diamond LK. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid. The New England journal of medicine. 1948;238:787–793. [Abstract] [Google Scholar]
- Farrar GJ, Chadderton N, Kenna PF, Millington-Ward S. Mitochondrial disorders: aetiologies, models systems, and candidate therapies. Trends in genetics: TIG. 2013;29:488–497. [Abstract] [Google Scholar]
- Ferguson SM. Beyond indigestion: emerging roles for lysosome-based signaling in human disease. Current opinion in cell biology. 2015;35:59–68. [Europe PMC free article] [Abstract] [Google Scholar]
- Frezza C, Zheng L, Folger O, Rajagopalan KN, MacKenzie ED, Jerby L, Micaroni M, Chaneton B, Adam J, Hedley A, et al. Haem oxygenase is synthetically lethal with the tumour suppressor fumarate hydratase. Nature. 2011;477:225–228. [Abstract] [Google Scholar]
- Gaglio D, Metallo CM, Gameiro PA, Hiller K, Danna LS, Balestrieri C, Alberghina L, Stephanopoulos G, Chiaradonna F. Oncogenic K-Ras decouples glucose and glutamine metabolism to support cancer cell growth. Mol Syst Biol. 2011;7:523. [Europe PMC free article] [Abstract] [Google Scholar]
- Gallamini A, Zwarthoed C, Borra A. Positron Emission Tomography (PET) in Oncology. Cancers (Basel) 2014;6:1821–1889. [Europe PMC free article] [Abstract] [Google Scholar]
- Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT, et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–765. [Europe PMC free article] [Abstract] [Google Scholar]
- Gasparre G, Romeo G, Rugolo M, Porcelli AM. Learning from oncocytic tumors: Why choose inefficient mitochondria? Biochimica et biophysica acta. 2011;1807:633–642. [Abstract] [Google Scholar]
- Gaude E, Frezza C. Defects in mitochondrial metabolism and cancer. Cancer & metabolism. 2014;2:10. [Europe PMC free article] [Abstract] [Google Scholar]
- Guo JY, Chen HY, Mathew R, Fan J, Strohecker AM, Karsli-Uzunbas G, Kamphorst JJ, Chen G, Lemons JM, Karantza V, et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes & development. 2011;25:460–470. [Europe PMC free article] [Abstract] [Google Scholar]
- Guo JY, Karsli-Uzunbas G, Mathew R, Aisner SC, Kamphorst JJ, Strohecker AM, Chen G, Price S, Lu W, Teng X, et al. Autophagy suppresses progression of K-ras-induced lung tumors to oncocytomas and maintains lipid homeostasis. Genes & development. 2013a;27:1447–1461. [Europe PMC free article] [Abstract] [Google Scholar]
- Guo JY, White E. Autophagy is required for mitochondrial function, lipid metabolism, growth, and fate of KRAS(G12D)-driven lung tumors. Autophagy. 2013;9:1636–1638. [Europe PMC free article] [Abstract] [Google Scholar]
- Guo JY, Xia B, White E. Autophagy-mediated tumor promotion. Cell. 2013b;155:1216–1219. [Europe PMC free article] [Abstract] [Google Scholar]
- Hasumi H, Baba M, Hasumi Y, Huang Y, Oh H, Hughes RM, Klein ME, Takikita S, Nagashima K, Schmidt LS, et al. Regulation of mitochondrial oxidative metabolism by tumor suppressor FLCN. J Natl Cancer Inst. 2012;104:1750–1764. [Europe PMC free article] [Abstract] [Google Scholar]
- He Y, Hakvoort TB, Kohler SE, Vermeulen JL, de Waart DR, de Theije C, ten Have GA, van Eijk HM, Kunne C, Labruyere WT, et al. Glutamine synthetase in muscle is required for glutamine production during fasting and extrahepatic ammonia detoxification. The Journal of biological chemistry. 2010;285:9516–9524. [Europe PMC free article] [Abstract] [Google Scholar]
- Huo Y, Cai H, Teplova I, Bowman-Colin C, Chen G, Price S, Barnard N, Ganesan S, Karantza V, White E, et al. Autophagy opposes p53-mediated tumor barrier to facilitate tumorigenesis in a model of PALB2-associated hereditary breast cancer. Cancer discovery. 2013;3:894–907. [Europe PMC free article] [Abstract] [Google Scholar]
- Joshi S, Tolkunov D, Aviv H, Hakimi AA, Yao M, Hsieh JJ, Ganesan S, Chan CS, White E. The Genomic Landscape of Renal Oncocytoma Identifies a Metabolic Barrier to Tumorigenesis. Cell Rep. 2015;13:1895–1908. [Europe PMC free article] [Abstract] [Google Scholar]
- Ju YS, Alexandrov LB, Gerstung M, Martincorena I, Nik-Zainal S, Ramakrishna M, Davies HR, Papaemmanuil E, Gundem G, Shlien A, et al. Origins and functional consequences of somatic mitochondrial DNA mutations in human cancer. eLife. 2014;3 [Europe PMC free article] [Abstract] [Google Scholar]
- Karsli-Uzunbas G, Guo JY, Price S, Teng X, Laddha SV, Khor S, Kalaany NY, Jacks T, Chan CS, Rabinowitz JD, et al. Autophagy is required for glucose homeostasis and lung tumor maintenance. Cancer discovery. 2014;4:914–927. [Europe PMC free article] [Abstract] [Google Scholar]
- Khutornenko AA, Roudko VV, Chernyak BV, Vartapetian AB, Chumakov PM, Evstafieva AG. Pyrimidine biosynthesis links mitochondrial respiration to the p53 pathway. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12828–12833. [Europe PMC free article] [Abstract] [Google Scholar]
- Kim D, Fiske BP, Birsoy K, Freinkman E, Kami K, Possemato RL, Chudnovsky Y, Pacold ME, Chen WW, Cantor JR, et al. SHMT2 drives glioma cell survival in ischaemia but imposes a dependence on glycine clearance. Nature. 2015;520:363–367. [Europe PMC free article] [Abstract] [Google Scholar]
- Kung HN, Marks JR, Chi JT. Glutamine synthetase is a genetic determinant of cell type-specific glutamine independence in breast epithelia. PLoS Genet. 2011;7:e1002229. [Europe PMC free article] [Abstract] [Google Scholar]
- Lang M, Vocke CD, Merino MJ, Schmidt LS, Linehan WM. Mitochondrial DNA mutations distinguish bilateral multifocal renal oncocytomas from familial Birt-Hogg-Dube tumors. Mod Pathol. 2015;28:1458–1469. [Europe PMC free article] [Abstract] [Google Scholar]
- Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, Sideris DP, Fogel AI, Youle RJ. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 2015;524:309–314. [Europe PMC free article] [Abstract] [Google Scholar]
- Linehan WM, Srinivasan R, Garcia JA. Non-clear cell renal cancer: disease-based management and opportunities for targeted therapeutic approaches. Semin Oncol. 2013;40:511–520. [Europe PMC free article] [Abstract] [Google Scholar]
- Liu L, Feng D, Chen G, Chen M, Zheng Q, Song P, Ma Q, Zhu C, Wang R, Qi W, et al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nature cell biology. 2012;14:177–185. [Abstract] [Google Scholar]
- Locasale JW. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nature reviews. Cancer. 2013;13:572–583. [Europe PMC free article] [Abstract] [Google Scholar]
- Lonardo E, Cioffi M, Sancho P, Sanchez-Ripoll Y, Trabulo SM, Dorado J, Balic A, Hidalgo M, Heeschen C. Metformin targets the metabolic achilles heel of human pancreatic cancer stem cells. PloS one. 2013;8:e76518. [Europe PMC free article] [Abstract] [Google Scholar]
- Losman JA, Kaelin WG., Jr. What a difference a hydroxyl makes: mutant IDH, (R)-2-hydroxyglutarate, and cancer. Genes & development. 2013;27:836–852. [Europe PMC free article] [Abstract] [Google Scholar]
- Lu C, Thompson CB. Metabolic regulation of epigenetics. Cell metabolism. 2012;16:9–17. [Europe PMC free article] [Abstract] [Google Scholar]
- Maher EA, Marin-Valencia I, Bachoo RM, Mashimo T, Raisanen J, Hatanpaa KJ, Jindal A, Jeffrey FM, Choi C, Madden C, et al. Metabolism of [U-13 C]glucose in human brain tumors in vivo. NMR Biomed. 2012;25:1234–1244. [Europe PMC free article] [Abstract] [Google Scholar]
- Marin-Valencia I, Yang C, Mashimo T, Cho S, Baek H, Yang XL, Rajagopalan KN, Maddie M, Vemireddy V, Zhao Z, et al. Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell metabolism. 2012;15:827–837. [Europe PMC free article] [Abstract] [Google Scholar]
- Mashimo T, Pichumani K, Vemireddy V, Hatanpaa KJ, Singh DK, Sirasanagandla S, Nannepaga S, Piccirillo SG, Kovacs Z, Foong C, et al. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell. 2014;159:1603–1614. [Europe PMC free article] [Abstract] [Google Scholar]
- Moldoveanu T, Follis AV, Kriwacki RW, Green DR. Many players in BCL-2 family affairs. Trends in biochemical sciences. 2014;39:101–111. [Europe PMC free article] [Abstract] [Google Scholar]
- Mullen AR, Wheaton WW, Jin ES, Chen PH, Sullivan LB, Cheng T, Yang Y, Linehan WM, Chandel NS, DeBerardinis RJ. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2012;481:385–388. [Europe PMC free article] [Abstract] [Google Scholar]
- Murakawa T, Yamaguchi O, Hashimoto A, Hikoso S, Takeda T, Oka T, Yasui H, Ueda H, Akazawa Y, Nakayama H, et al. Bcl-2-like protein 13 is a mammalian Atg32 homologue that mediates mitophagy and mitochondrial fragmentation. Nature communications. 2015;6:7527. [Europe PMC free article] [Abstract] [Google Scholar]
- Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. [Europe PMC free article] [Abstract] [Google Scholar]
- Nilsson R, Jain M, Madhusudhan N, Sheppard NG, Strittmatter L, Kampf C, Huang J, Asplund A, Mootha VK. Metabolic enzyme expression highlights a key role for MTHFD2 and the mitochondrial folate pathway in cancer. Nature communications. 2014;5:3128. [Europe PMC free article] [Abstract] [Google Scholar]
- Parker SJ, Metallo CM. Metabolic consequences of oncogenic IDH mutations. Pharmacol Ther. 2015;152:54–62. [Europe PMC free article] [Abstract] [Google Scholar]
- Perera RM, Stoykova S, Nicolay BN, Ross KN, Fitamant J, Boukhali M, Lengrand J, Deshpande V, Selig MK, Ferrone CR, et al. Transcriptional control of autophagy-lysosome function drives pancreatic cancer metabolism. Nature. 2015 [Europe PMC free article] [Abstract] [Google Scholar]
- Pickrell AM, Huang CH, Kennedy SR, Ordureau A, Sideris DP, Hoekstra JG, Harper JW, Youle RJ. Endogenous Parkin Preserves Dopaminergic Substantia Nigral Neurons following Mitochondrial DNA Mutagenic Stress. Neuron. 2015;87:371–381. [Europe PMC free article] [Abstract] [Google Scholar]
- Pike ST, Rajendra R, Artzt K, Appling DR. Mitochondrial C1-tetrahydrofolate synthase (MTHFD1L) supports the flow of mitochondrial one-carbon units into the methyl cycle in embryos. The Journal of biological chemistry. 2010;285:4612–4620. [Europe PMC free article] [Abstract] [Google Scholar]
- Piskounova E, Agathocleous M, Murphy MM, Hu Z, Huddlestun SE, Zhao Z, Leitch AM, Johnson TM, DeBerardinis RJ, Morrison SJ. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 2015;527:186–191. [Europe PMC free article] [Abstract] [Google Scholar]
- Pollak M. Potential applications for biguanides in oncology. The Journal of clinical investigation. 2013;123:3693–3700. [Europe PMC free article] [Abstract] [Google Scholar]
- Randow F, Youle RJ. Self and nonself: how autophagy targets mitochondria and bacteria. Cell Host Microbe. 2014;15:403–411. [Europe PMC free article] [Abstract] [Google Scholar]
- Rao S, Tortola L, Perlot T, Wirnsberger G, Novatchkova M, Nitsch R, Sykacek P, Frank L, Schramek D, Komnenovic V, et al. A dual role for autophagy in a murine model of lung cancer. Nature communications. 2014;5:3056. [Abstract] [Google Scholar]
- Rohle D, Popovici-Muller J, Palaskas N, Turcan S, Grommes C, Campos C, Tsoi J, Clark O, Oldrini B, Komisopoulou E, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science. 2013;340:626–630. [Europe PMC free article] [Abstract] [Google Scholar]
- Rosenfeldt MT, O’Prey J, Morton JP, Nixon C, MacKay G, Mrowinska A, Au A, Rai TS, Zheng L, Ridgway R, et al. p53 status determines the role of autophagy in pancreatic tumour development. Nature. 2013;504:296–300. [Abstract] [Google Scholar]
- Schweers RL, Zhang J, Randall MS, Loyd MR, Li W, Dorsey FC, Kundu M, Opferman JT, Cleveland JL, Miller JL, et al. NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19500–19505. [Europe PMC free article] [Abstract] [Google Scholar]
- Shadel GS, Horvath TL. Mitochondrial ROS Signaling in Organismal Homeostasis. Cell. 2015;163:560–569. [Europe PMC free article] [Abstract] [Google Scholar]
- Shin M, Bryant JD, Momb J, Appling DR. Mitochondrial MTHFD2L is a dual redox cofactor-specific methylenetetrahydrofolate dehydrogenase/methenyltetrahydrofolate cyclohydrolase expressed in both adult and embryonic tissues. The Journal of biological chemistry. 2014;289:15507–15517. [Europe PMC free article] [Abstract] [Google Scholar]
- Shroff EH, Eberlin LS, Dang VM, Gouw AM, Gabay M, Adam SJ, Bellovin DI, Tran PT, Philbrick WM, Garcia-Ocana A, et al. MYC oncogene overexpression drives renal cell carcinoma in a mouse model through glutamine metabolism. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:6539–6544. [Europe PMC free article] [Abstract] [Google Scholar]
- Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, Perera RM, Ferrone CR, Mullarky E, Shyh-Chang N, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496:101–105. [Europe PMC free article] [Abstract] [Google Scholar]
- Stewart JB, Alaei-Mahabadi B, Sabarinathan R, Samuelsson T, Gorodkin J, Gustafsson CM, Larsson E. Simultaneous DNA and RNA Mapping of Somatic Mitochondrial Mutations across Diverse Human Cancers. PLoS Genet. 2015;11:e1005333. [Europe PMC free article] [Abstract] [Google Scholar]
- Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719–724. [Europe PMC free article] [Abstract] [Google Scholar]
- Strohecker AM, Guo JY, Karsli-Uzunbas G, Price SM, Chen GJ, Mathew R, McMahon M, White E. Autophagy sustains mitochondrial glutamine metabolism and growth of BrafV600E-driven lung tumors. Cancer discovery. 2013;3:1272–1285. [Europe PMC free article] [Abstract] [Google Scholar]
- Sullivan LB, Chandel NS. Mitochondrial reactive oxygen species and cancer. Cancer & metabolism. 2014;2:17. [Europe PMC free article] [Abstract] [Google Scholar]
- Sullivan LB, Gui DY, Hosios AM, Bush LN, Freinkman E, Vander Heiden MG. Supporting Aspartate Biosynthesis Is an Essential Function of Respiration in Proliferating Cells. Cell. 2015;162:552–563. [Europe PMC free article] [Abstract] [Google Scholar]
- Sullivan LB, Martinez-Garcia E, Nguyen H, Mullen AR, Dufour E, Sudarshan S, Licht JD, Deberardinis RJ, Chandel NS. The proto-oncometabolite fumarate binds glutathione to amplify ROS-dependent signaling. Molecular cell. 2013;51:236–248. [Europe PMC free article] [Abstract] [Google Scholar]
- Tan AS, Baty JW, Dong LF, Bezawork-Geleta A, Endaya B, Goodwin J, Bajzikova M, Kovarova J, Peterka M, Yan B, et al. Mitochondrial genome acquisition restores respiratory function and tumorigenic potential of cancer cells without mitochondrial DNA. Cell metabolism. 2015;21:81–94. [Abstract] [Google Scholar]
- Tardito S, Oudin A, Ahmed SU, Fack F, Keunen O, Zheng L, Miletic H, Sakariassen PO, Weinstock A, Wagner A, et al. Glutamine synthetase activity fuels nucleotide biosynthesis and supports growth of glutamine-restricted glioblastoma. Nature cell biology. 2015;17:1556–1568. [Europe PMC free article] [Abstract] [Google Scholar]
- Tsun ZY, Bar-Peled L, Chantranupong L, Zoncu R, Wang T, Kim C, Spooner E, Sabatini DM. The folliculin tumor suppressor is a GAP for the RagC/D GTPases that signal amino acid levels to mTORC1. Molecular cell. 2013;52:495–505. [Europe PMC free article] [Abstract] [Google Scholar]
- van der Vos KE, Eliasson P, Proikas-Cezanne T, Vervoort SJ, van Boxtel R, Putker M, van Zutphen IJ, Mauthe M, Zellmer S, Pals C, et al. Modulation of glutamine metabolism by the PI(3)K-PKB-FOXO network regulates autophagy. Nature cell biology. 2012;14:829–837. [Abstract] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. [Europe PMC free article] [Abstract] [Google Scholar]
- Vazquez A, Tedeschi PM, Bertino JR. Overexpression of the mitochondrial folate and glycine-serine pathway: a new determinant of methotrexate selectivity in tumors. Cancer Res. 2013;73:478–482. [Europe PMC free article] [Abstract] [Google Scholar]
- Viale A, Pettazzoni P, Lyssiotis CA, Ying H, Sanchez N, Marchesini M, Carugo A, Green T, Seth S, Giuliani V, et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature. 2014;514:628–632. [Europe PMC free article] [Abstract] [Google Scholar]
- Wallace DC. Mitochondria and cancer. Nature reviews. Cancer. 2012;12:685–698. [Europe PMC free article] [Abstract] [Google Scholar]
- Wang F, Travins J, DeLaBarre B, Penard-Lacronique V, Schalm S, Hansen E, Straley K, Kernytsky A, Liu W, Gliser C, et al. Targeted inhibition of mutant IDH2 in leukemia cells induces cellular differentiation. Science. 2013;340:622–626. [Abstract] [Google Scholar]
- Warburg O. On respiratory impairment in cancer cells. Science. 1956a;124:269–270. [Abstract] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956b;123:309–314. [Abstract] [Google Scholar]
- Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, Kalyanaraman B, Mutlu GM, Budinger GR, Chandel NS. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:8788–8793. [Europe PMC free article] [Abstract] [Google Scholar]
- Weinberg SE, Sena LA, Chandel NS. Mitochondria in the regulation of innate and adaptive immunity. Immunity. 2015;42:406–417. [Europe PMC free article] [Abstract] [Google Scholar]
- West AP, Khoury-Hanold W, Staron M, Tal MC, Pineda CM, Lang SM, Bestwick M, Duguay BA, Raimundo N, MacDuff DA, et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature. 2015;520:553–557. [Europe PMC free article] [Abstract] [Google Scholar]
- Wheaton WW, Weinberg SE, Hamanaka RB, Soberanes S, Sullivan LB, Anso E, Glasauer A, Dufour E, Mutlu GM, Budigner GS, et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. eLife. 2014;3:e02242. [Europe PMC free article] [Abstract] [Google Scholar]
- White E. The role for autophagy in cancer. The Journal of clinical investigation. 2015;125:42–46. [Europe PMC free article] [Abstract] [Google Scholar]
- White E, Mehnert JM, Chan CS. Autophagy, Metabolism, and Cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2015;21:5037–5046. [Europe PMC free article] [Abstract] [Google Scholar]
- Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18782–18787. [Europe PMC free article] [Abstract] [Google Scholar]
- Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trends in biochemical sciences. 2010;35:427–433. [Europe PMC free article] [Abstract] [Google Scholar]
- Xiang Y, Stine ZE, Xia J, Lu Y, O’Connor RS, Altman BJ, Hsieh AL, Gouw AM, Thomas AG, Gao P, et al. Targeted inhibition of tumor-specific glutaminase diminishes cell-autonomous tumorigenesis. The Journal of clinical investigation. 2015;125:2293–2306. [Europe PMC free article] [Abstract] [Google Scholar]
- Xie X, Koh JY, Price S, White E, Mehnert JM. Atg7 overcomes senescence and promotes growth of BRAFV600E-driven melanoma. Cancer discovery. 2015 [Europe PMC free article] [Abstract] [Google Scholar]
- Yang A, Rajeshkumar NV, Wang X, Yabuuchi S, Alexander BM, Chu GC, Von Hoff DD, Maitra A, Kimmelman AC. Autophagy Is Critical for Pancreatic Tumor Growth and Progression in Tumors with p53 Alterations. Cancer discovery. 2014 [Europe PMC free article] [Abstract] [Google Scholar]
- Yang D, Wang MT, Tang Y, Chen Y, Jiang H, Jones TT, Rao K, Brewer GJ, Singh KK, Nie D. Impairment of mitochondrial respiration in mouse fibroblasts by oncogenic H-RAS(Q61L) Cancer Biol Ther. 2010;9:122–133. [Europe PMC free article] [Abstract] [Google Scholar]
- Yang S, Wang X, Contino G, Liesa M, Sahin E, Ying H, Bause A, Li Y, Stommel JM, Dell’antonio G, et al. Pancreatic cancers require autophagy for tumor growth. Genes & development. 2011;25:717–729. [Europe PMC free article] [Abstract] [Google Scholar]
- Ye J, Fan J, Venneti S, Wan YW, Pawel BR, Zhang J, Finley LW, Lu C, Lindsten T, Cross JR, et al. Serine catabolism regulates mitochondrial redox control during hypoxia. Cancer discovery. 2014;4:1406–1417. [Europe PMC free article] [Abstract] [Google Scholar]
- Yun J, Mullarky E, Lu C, Bosch KN, Kavalier A, Rivera K, Roper J, Chio II, Giannopoulou EG, Rago C, et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science. 2015;350:1391–1396. [Europe PMC free article] [Abstract] [Google Scholar]
- Yuneva M, Zamboni N, Oefner P, Sachidanandam R, Lazebnik Y. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol. 2007;178:93–105. [Europe PMC free article] [Abstract] [Google Scholar]
- Yuneva MO, Fan TW, Allen TD, Higashi RM, Ferraris DV, Tsukamoto T, Mates JM, Alonso FJ, Wang C, Seo Y, et al. The metabolic profile of tumors depends on both the responsible genetic lesion and tissue type. Cell metabolism. 2012;15:157–170. [Europe PMC free article] [Abstract] [Google Scholar]
- Zu XL, Guppy M. Cancer metabolism: facts, fantasy, and fiction. Biochemical and biophysical research communications. 2004;313:459–465. [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.molcel.2016.02.011
Read article for free, from open access legal sources, via Unpaywall:
http://www.cell.com/article/S1097276516000952/pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.molcel.2016.02.011
Article citations
Non-cell autonomous regulation of cell-cell signaling and differentiation by mitochondrial ROS.
J Cell Biol, 223(12):e202401084, 13 Nov 2024
Cited by: 0 articles | PMID: 39535785 | PMCID: PMC11561560
LncRNA mediated metabolic reprogramming: the chief culprits of solid tumor malignant progression: an update review.
Nutr Metab (Lond), 21(1):89, 08 Nov 2024
Cited by: 0 articles | PMID: 39516895 | PMCID: PMC11549785
Review Free full text in Europe PMC
Insights into Metabolic Reprogramming in Tumor Evolution and Therapy.
Cancers (Basel), 16(20):3513, 17 Oct 2024
Cited by: 0 articles | PMID: 39456607 | PMCID: PMC11506062
Review Free full text in Europe PMC
Integrative multi-omics analysis unveils the connection between transcriptomic characteristics associated with mitochondria and the tumor immune microenvironment in lower-grade gliomas.
Sci Rep, 14(1):23675, 10 Oct 2024
Cited by: 0 articles | PMID: 39390013 | PMCID: PMC11467307
A novel role for WZ3146 in the inhibition of cell proliferation via ERK and AKT pathway in the rare EGFR G719X mutant cells.
Sci Rep, 14(1):22895, 02 Oct 2024
Cited by: 0 articles | PMID: 39358400 | PMCID: PMC11447065
Go to all (571) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Decoding Warburg's hypothesis: tumor-related mutations in the mitochondrial respiratory chain.
Oncotarget, 6(39):41582-41599, 01 Dec 2015
Cited by: 26 articles | PMID: 26462158 | PMCID: PMC4747175
Review Free full text in Europe PMC
Mitochondria and cancer chemoresistance.
Biochim Biophys Acta Bioenerg, 1858(8):686-699, 01 Feb 2017
Cited by: 167 articles | PMID: 28161329
Review
Mitochondria: The metabolic switch of cellular oncogenic transformation.
Biochim Biophys Acta Rev Cancer, 1876(1):188534, 29 Mar 2021
Cited by: 26 articles | PMID: 33794332
Review
The Warburg effect and its cancer therapeutic implications.
J Bioenerg Biomembr, 39(3):267-274, 01 Jun 2007
Cited by: 176 articles | PMID: 17551814
Review
Funding
Funders who supported this work.
NCI NIH HHS (12)
Grant ID: P30 CA072720
Grant ID: P30CA72720
Grant ID: R01CA188096
Grant ID: R01 CA163591
Grant ID: R01 CA188096
Grant ID: R01CA129536
Grant ID: R01 CA129536
Grant ID: R01 CA130893
Grant ID: R01 CA193970
Grant ID: R01CA130893
Grant ID: R01CA163591
Grant ID: R01CA193970
NIGMS NIH HHS (2)
Grant ID: R01 GM097355
Grant ID: R01GM97355