Abstract
Free full text

beta-Amyloid-(1-42) is a major component of cerebrovascular amyloid deposits: implications for the pathology of Alzheimer disease.
Abstract
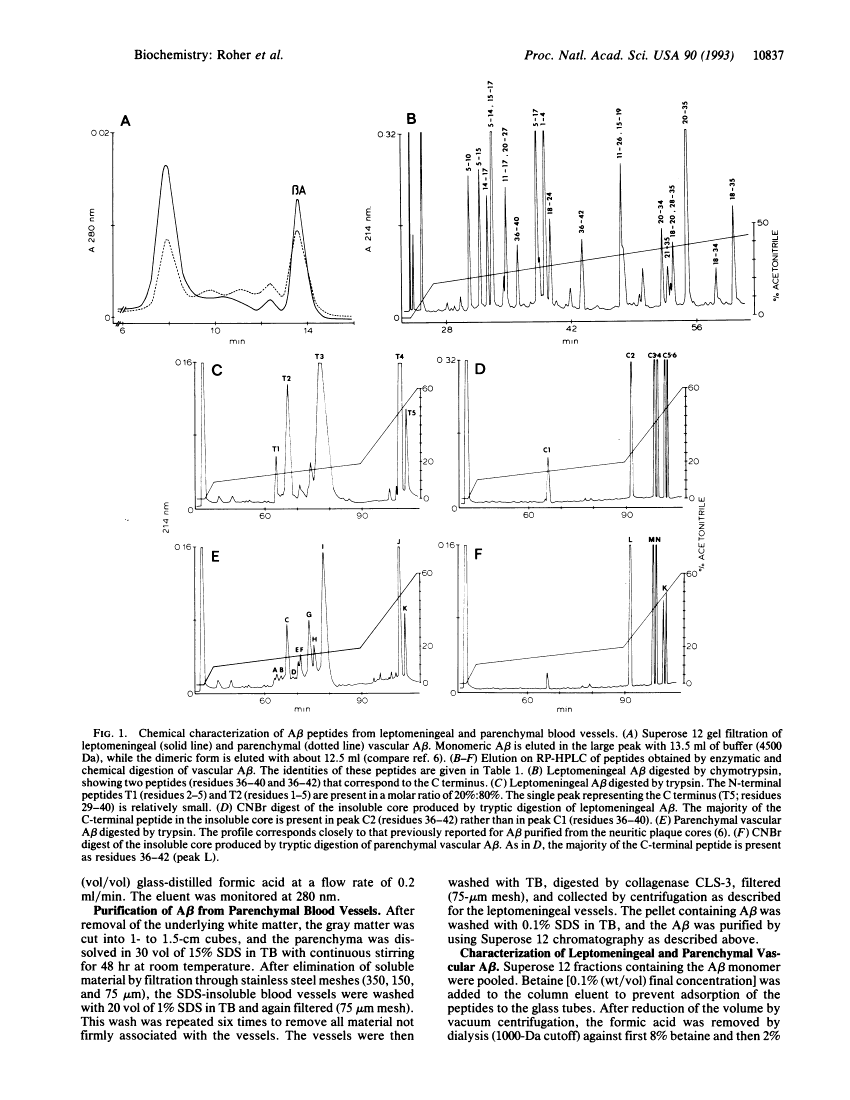
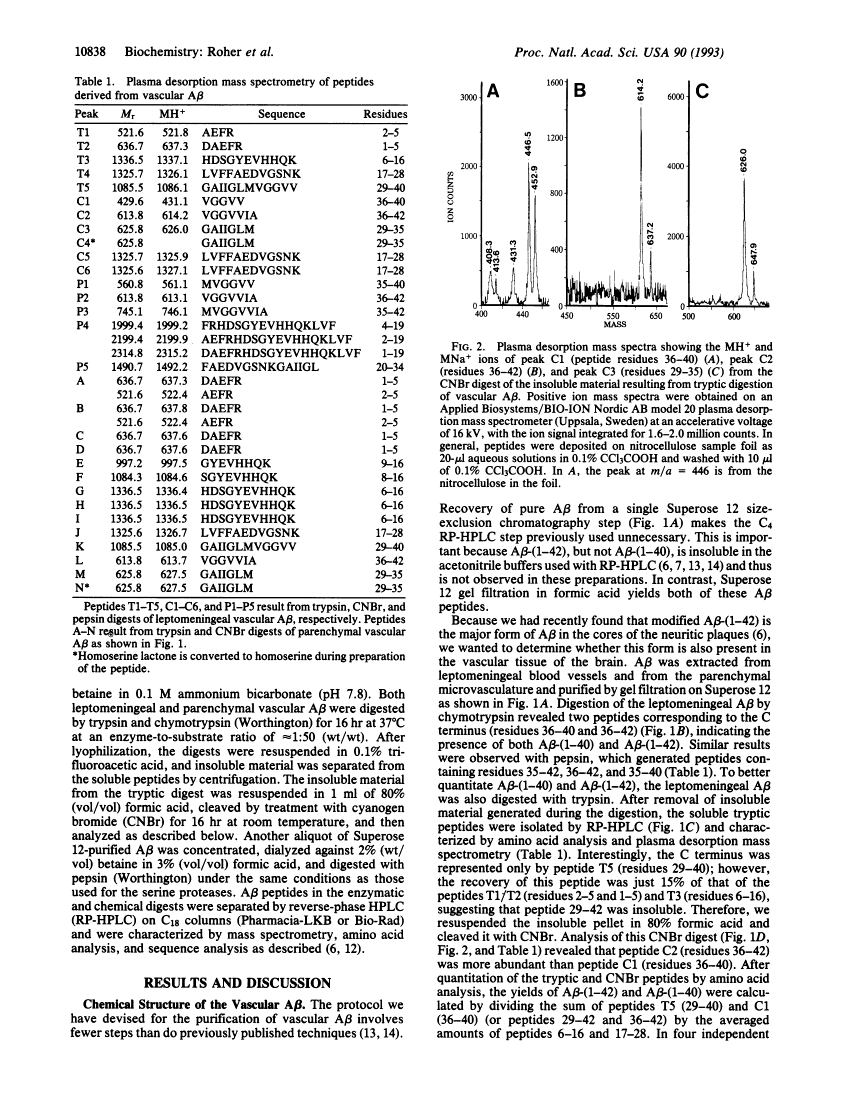
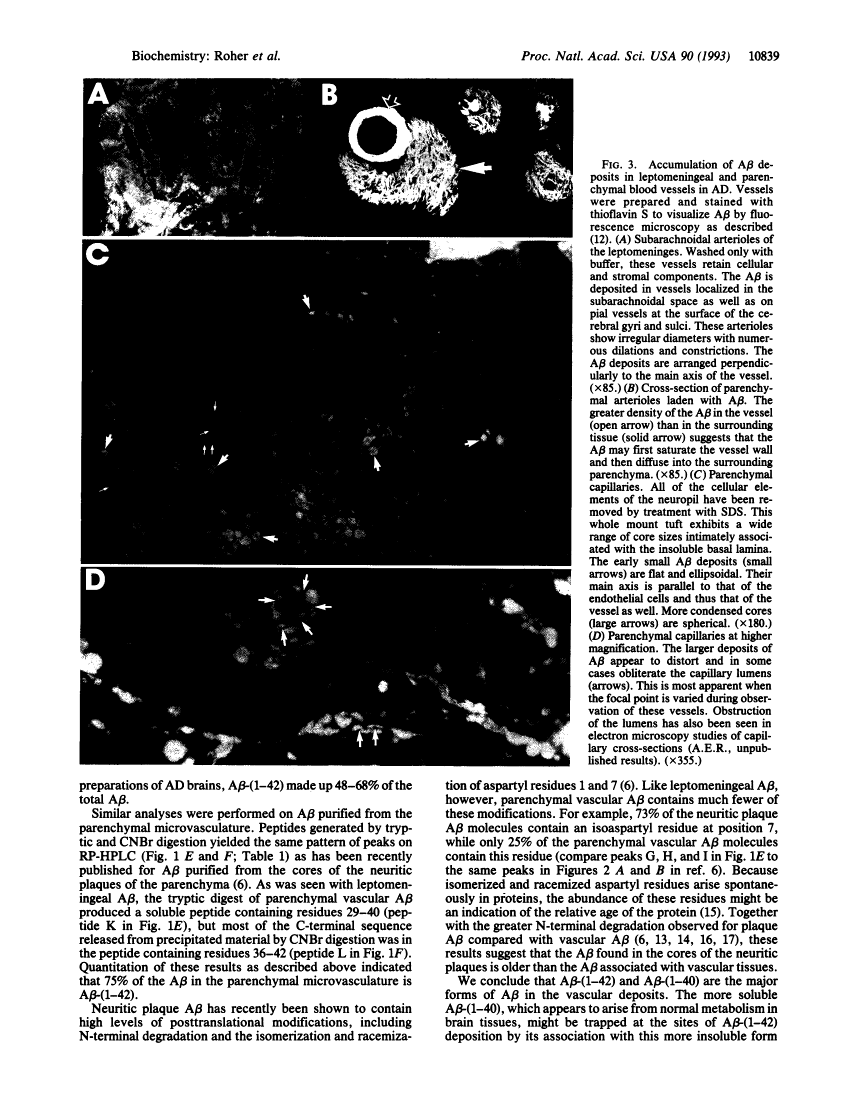
Reinvestigation of the chemical structure of beta-amyloid peptide (A beta) deposits in the vascular tissue of Alzheimer disease brains revealed that the 42-residue form A beta-(1-42), rather than the more soluble A beta-(1-40) form, is the predominant peptide. Following removal of the surrounding tissue with SDS and collagenase, A beta was solubilized in formic acid and purified by Superose 12 chromatography. Peptides generated by enzymatic and chemical digestion of the A beta were purified by HPLC and characterized by amino acid analysis, sequence analysis, and mass spectrometry. In the leptomeningeal vessels, the average ratio of A beta-(1-42)/A beta-(1-40) was 58:42, whereas in the parenchymal vessels this ratio was 75:25. Interestingly, vascular A beta contains considerably less isomerized and racemized aspartyl residues than does neuritic plaque A beta, suggesting that the vascular amyloid is "younger." The discrete nature of the bands and spherical deposits of A beta associated with arterioles and capillaries, respectively, suggests that this amyloid arises from the vascular tissue itself. Increasing A beta deposition appears to lead to the distortion and occlusion of capillaries, which may contribute significantly to the pathology of Alzheimer disease.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.4M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Selkoe DJ. The molecular pathology of Alzheimer's disease. Neuron. 1991 Apr;6(4):487–498. [Abstract] [Google Scholar]
- Joachim CL, Selkoe DJ. The seminal role of beta-amyloid in the pathogenesis of Alzheimer disease. Alzheimer Dis Assoc Disord. 1992 Spring;6(1):7–34. [Abstract] [Google Scholar]
- Vinters HV. Cerebral amyloid angiopathy. A critical review. Stroke. 1987 Mar-Apr;18(2):311–324. [Abstract] [Google Scholar]
- Miyakawa T, Katsuragi S, Yamashita K, Ohuchi K. Morphological study of amyloid fibrils and preamyloid deposits in the brain with Alzheimer's disease. Acta Neuropathol. 1992;83(4):340–346. [Abstract] [Google Scholar]
- Yamaguchi H, Yamazaki T, Lemere CA, Frosch MP, Selkoe DJ. Beta amyloid is focally deposited within the outer basement membrane in the amyloid angiopathy of Alzheimer's disease. An immunoelectron microscopic study. Am J Pathol. 1992 Jul;141(1):249–259. [Europe PMC free article] [Abstract] [Google Scholar]
- Roher AE, Lowenson JD, Clarke S, Wolkow C, Wang R, Cotter RJ, Reardon IM, Zürcher-Neely HA, Heinrikson RL, Ball MJ, et al. Structural alterations in the peptide backbone of beta-amyloid core protein may account for its deposition and stability in Alzheimer's disease. J Biol Chem. 1993 Feb 15;268(5):3072–3083. [Abstract] [Google Scholar]
- Mori H, Takio K, Ogawara M, Selkoe DJ. Mass spectrometry of purified amyloid beta protein in Alzheimer's disease. J Biol Chem. 1992 Aug 25;267(24):17082–17086. [Abstract] [Google Scholar]
- Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schlossmacher M, Whaley J, Swindlehurst C, et al. Isolation and quantification of soluble Alzheimer's beta-peptide from biological fluids. Nature. 1992 Sep 24;359(6393):325–327. [Abstract] [Google Scholar]
- Shoji M, Golde TE, Ghiso J, Cheung TT, Estus S, Shaffer LM, Cai XD, McKay DM, Tintner R, Frangione B, et al. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1992 Oct 2;258(5079):126–129. [Abstract] [Google Scholar]
- Khachaturian ZS. Diagnosis of Alzheimer's disease. Arch Neurol. 1985 Nov;42(11):1097–1105. [Abstract] [Google Scholar]
- Mirra SS, Hart MN, Terry RD. Making the diagnosis of Alzheimer's disease. A primer for practicing pathologists. Arch Pathol Lab Med. 1993 Feb;117(2):132–144. [Abstract] [Google Scholar]
- Roher AE, Palmer KC, Chau V, Ball MJ. Isolation and chemical characterization of Alzheimer's disease paired helical filament cytoskeletons: differentiation from amyloid plaque core protein. J Cell Biol. 1988 Dec;107(6 Pt 2):2703–2716. [Europe PMC free article] [Abstract] [Google Scholar]
- Glenner GG, Wong CW. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. [Abstract] [Google Scholar]
- Pardridge WM, Vinters HV, Yang J, Eisenberg J, Choi TB, Tourtellotte WW, Huebner V, Shively JE. Amyloid angiopathy of Alzheimer's disease: amino acid composition and partial sequence of a 4,200-dalton peptide isolated from cortical microvessels. J Neurochem. 1987 Nov;49(5):1394–1401. [Abstract] [Google Scholar]
- Stadtman ER. Covalent modification reactions are marking steps in protein turnover. Biochemistry. 1990 Jul 10;29(27):6323–6331. [Abstract] [Google Scholar]
- Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. [Europe PMC free article] [Abstract] [Google Scholar]
- Prelli F, Castaño E, Glenner GG, Frangione B. Differences between vascular and plaque core amyloid in Alzheimer's disease. J Neurochem. 1988 Aug;51(2):648–651. [Abstract] [Google Scholar]
- Barrow CJ, Yasuda A, Kenny PT, Zagorski MG. Solution conformations and aggregational properties of synthetic amyloid beta-peptides of Alzheimer's disease. Analysis of circular dichroism spectra. J Mol Biol. 1992 Jun 20;225(4):1075–1093. [Abstract] [Google Scholar]
- Knauer MF, Soreghan B, Burdick D, Kosmoski J, Glabe CG. Intracellular accumulation and resistance to degradation of the Alzheimer amyloid A4/beta protein. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7437–7441. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.90.22.10836
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc47873?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Morphological and Molecular Profiling of Amyloid-β Species in Alzheimer's Pathogenesis.
Mol Neurobiol, 24 Oct 2024
Cited by: 0 articles | PMID: 39446217
Review
β-Amyloids and Immune Responses Associated with Alzheimer's Disease.
Cells, 13(19):1624, 28 Sep 2024
Cited by: 0 articles | PMID: 39404388 | PMCID: PMC11475064
Review Free full text in Europe PMC
Cerebral Microbleeds Associate with Brain Endothelial Cell Activation-Dysfunction and Blood-Brain Barrier Dysfunction/Disruption with Increased Risk of Hemorrhagic and Ischemic Stroke.
Biomedicines, 12(7):1463, 01 Jul 2024
Cited by: 0 articles | PMID: 39062035 | PMCID: PMC11274519
Review Free full text in Europe PMC
Dysregulated expression of miR-140 and miR-122 compromised microglial chemotaxis and led to reduced restriction of AD pathology.
J Neuroinflammation, 21(1):167, 02 Jul 2024
Cited by: 0 articles | PMID: 38956605 | PMCID: PMC11218311
Innovative Therapeutic Strategies in Alzheimer's Disease: A Synergistic Approach to Neurodegenerative Disorders.
Pharmaceuticals (Basel), 17(6):741, 06 Jun 2024
Cited by: 0 articles | PMID: 38931409
Review
Go to all (381) article citations
Other citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Structural alterations in the peptide backbone of beta-amyloid core protein may account for its deposition and stability in Alzheimer's disease.
J Biol Chem, 268(5):3072-3083, 01 Feb 1993
Cited by: 440 articles | PMID: 8428986
Chemical characterization of A beta 17-42 peptide, a component of diffuse amyloid deposits of Alzheimer disease.
J Biol Chem, 269(15):10987-10990, 01 Apr 1994
Cited by: 127 articles | PMID: 8157623
Isolation, chemical characterization, and quantitation of A beta 3-pyroglutamyl peptide from neuritic plaques and vascular amyloid deposits.
Biochem Biophys Res Commun, 237(1):188-191, 01 Aug 1997
Cited by: 106 articles | PMID: 9266855
Relationships among the cerebral amyloid peptides and their precursors.
Ann Med, 21(2):83-87, 01 Jan 1989
Cited by: 9 articles | PMID: 2569882
Review
Funding
Funders who supported this work.
NIA NIH HHS (1)
Grant ID: P30-AG08017
NIGMS NIH HHS (1)
Grant ID: GM-26020