Abstract
Free full text

A randomized controlled trial of personalized text message reminders to promote medication adherence among HIV-positive adolescents and young adults
Abstract
HIV-positive adolescents and young adults often experience suboptimal medication adherence, yet few interventions to improve adherence in this group have shown evidence of efficacy. We conducted a randomized trial of a two-way, personalized daily text messaging intervention to improve adherence to antiretroviral therapy (ART) among N=105 poorly adherent HIV-positive adolescents and young adults, ages 16–29. Adherence to ART was assessed via self-reported visual analogue scale (VAS; 0–100%) at 3 and 6-months for mean adherence level and proportion ≥ 90% adherent. The average effect estimate over the 6-month intervention period was significant for ≥ 90% adherence (OR=2.12, 95% CI=1.01–4.45, p<.05) and maintained at 12-months (6 months post-intervention). Satisfaction scores for the intervention were very high. These results suggest both feasibility and initial efficacy of this approach. Given study limitations, additional testing of this intervention as part of a larger clinical trial with objective and/or clinical outcome measures of adherence is warranted.
INTRODUCTION
Estimates of human immunodeficiency virus (HIV) incidence indicate that over 47,000 people in the United States (U.S.) were infected with HIV in 2013, with 37% of cases among young people aged 13–29 (1). People living with HIV, including young people, are living longer largely due to antiretroviral therapy (ART) (2). In patients with HIV, a high level of ART adherence (at least 80 percent) may be needed to suppress viral replication and avoid resistance (3–5); sub-optimal adherence can lead to poor outcomes and decreased life expectancy for HIV-related disease (6–12).
Achieving high levels of adherence is challenging because regimens may include multiple medications and side effects resulting in poor tolerability. Adolescents and young adults living with HIV, hereafter referred to as “youth” living with HIV (YLH) are a particularly vulnerable group in this regard (11, 13–19). ART adherence among YLH is well below the gold standard, as documented by evidence going back more than a decade (20). A review of 14 studies dating from the 2000s estimates a range of 30–70% adherence in the prior 30 days (21). Correlates of ART adherence among youth include key psychosocial factors, such as co-morbid mental illness, substance use and HIV-related stigma, among other factors (21). However, the most frequently cited reason for non-adherence among YLH is simply forgetting. In a recent study of YLH, (ages 12–24; n=217 perinatally-infected, n=236 behaviorally-infected), 74% reported the reason for missing doses was that they “forgot”(22). Deficits in prospective memory have also been cited as independent predictors of poor ART adherence among individuals with HIV infection even after considering established predictors such as general cognitive impairment and other co-morbidities e.g., depression (23). The first large-scale study of neurocognitive deficits in behaviorally infected adolescents and emerging adults (n=220, mean age=21, 80% male, 67% Black) found that 67% met criteria for HIV-associated neurocognitive disorders; memory deficits were among the most common (i.e., 45%–62% depending on domain). Because the study had no HIV-negative comparison group, it is not known whether these deficits may be attributable to premorbid cognitive impairments and/or socioeconomic and educational factors associated with HIV risk. These findings underscore the need for testing of memory-related adherence interventions among YLH, including novel intervention strategies such as the short messaging service (SMS) text messaging described herein.
Given the pervasiveness, low cost, and convenience of text messaging, particularly among youth, text messaging is well-suited for supporting the treatment of conditions managed over extended periods of time. Younger adults, socioeconomically disadvantaged populations, and less educated young adults have been identified as having high rates of cell phone use (24, 25). According to a Pew Research Center report on teens and technology, in 2012, 78% of adolescents had cell phones and 74% are regular users of text-messaging services (26). Several recent reviews of text messaging to promote ART adherence among adults suggest overall efficacy, but with effect size dependent on several factors (27–29). In the most recent review, effect sizes were larger for less than daily frequency of messaging, bi-directional communication, personalized message content, and messages timed with ART dosing schedule (27). Only one published study has evaluated text messaging reminders to promote medication adherence in YLH, a small single-arm proof-of-concept study, findings from which suggest potential efficacy and informed the present study (30).
Our intervention approach was informed by social cognitive theory (SCT). SCT specifies a core set of factors and mechanisms that influence health behavior with a primary emphasis on self-regulation (i.e., the accuracy and consistency of self-observation and self-monitoring) and self-reflection, including self-efficacy (31, 32). SCT holds that cognition, behavior, and environmental influences interact and are reinforcing. Self-regulatory functions, for example, are enhanced by facilitative environmental conditions (31, 32), such as reminder systems. A key developmental task for YLH is to take responsibility for management of their health care. Text messages are a supporting external influence, which is expected to enhance self-regulation, specifically the feeling of control over one’s ability to take medications as prescribed. Self-efficacy is the foundation of motivation and action (31, 32). Receipt of text messages is expected to help overcome a key barrier to adherence, forgetting to take medication, which may increase self-efficacy and thus, motivation. Among YLH, motivation and self-efficacy are strongly related to adherence (33); a relationship which is likely to be mutually reinforcing.
We pilot tested the feasibility, acceptability and initial efficacy of a daily 2-way personalized SMS text messaging intervention on ART adherence among HIV-positive adolescents and young adults, ages 16–29, building on the findings of the small single arm study by Dowshen and colleagues (30) by extending this approach to a more rigorous randomized design, with evaluation of effects 6-months post-intervention, and exploratory assessment of potential social cognitive mediators and psychosocial moderators of the intervention effect. We hypothesized that the intervention would be feasible and acceptable, that youth randomized to the intervention would show at least 10-point improvement in adherence at 3 and 6-months post-baseline in comparison to the control (i.e., on a 0–100% self-reported visual analogue scale of medication adherence in the prior 30 days), based on empirical findings from the prior proof-of-concept study (30), and that improvement would be maintained at 9- and 12-month follow-up.
METHODS
From October 2010 to February 2014, we completed a randomized controlled trial (RCT) testing the efficacy of daily text message reminders to improve adherence among poorly adherent YLH, ages 16–29. The target sample size was determined based on estimates from the prior single arm proof-of-concept study (30). YLH were recruited at community-based health centers and other organizations using flyers and palm cards. Individuals were screened for eligibility in person or via telephone. Inclusion criteria were: 1) diagnosis with HIV (perinatally, transfusion, or behaviorally acquired); 2) on ART for ≥ 1 month with adherence problems (i.e., missed 1 dose in the past week or ≥4 doses in the last month; 3) have cell phone access; 3) report regular use of text messaging; 4) age 16–29; 5) English-speaking. YLH were excluded if they: 1) did not report regular medical follow-up, 2) reported being pregnant and on ART only due to pregnancy, and 3) unable to provide assent/consent. Participants received $40 for each visit. The protocol was approved by awardee institution’s IRB with a waiver of parental permission for participation of minors. No adverse events occurred during the study period.
Design and setting
Visits were completed at research facilities on Chicago’s north side. Participants were screened, scheduled, consented, and enrolled by the study research staff (i.e., research assistants and associates). Randomization to intervention or control conditions was generated via a computerized block random assignment (blocks of 2) at a 1:1 ratio (by L. Kuhns); allocation assignment was concealed from research staff and study participants in an opaque envelope until the end of the enrollment visit. No blinding of the study conditions was feasible and thus the study was “open label” in design. Following the initial 6-month trial period, participants were crossed over such that those randomized to the intervention arm ceased receiving the intervention and those randomized to the control condition began receiving the daily text reminders. The advantages of this additional design feature were to jointly assess sustained intervention effects in the intervention group (i.e., pre-post the intervention period), while also evaluating the intervention effect in the control group (i.e., to test replication of the direction and size of the effect), and to offer the intervention to all participants which we believed to be ethically responsive to the needs of the community.
Both study arms received HIV-related education at baseline, consisting of a 20-minute animated tutorial explaining the importance of medication adherence in HIV disease management (34); this constituted the standard-of-care adherence education in the control group. YLH randomized to the intervention completed a brief structured interview to tailor and personalize their text message reminders to their medication regimen, including the number and timing of dosages. Participants used their own cellular phones for the intervention. In the prior proof-of-concept study referenced above, this approach was well-accepted by YLH (30, 35).
Intervention
The daily text reminders were delivered by Remedy Health Media (www.remedyhealthmedia.com), which provided a user-friendly platform and interface for programing of messages. Weekly reports of sent/received and failed/invalid messages were forwarded to staff who followed up with participants (i.e., to trouble-shoot cell phone problems). Daily messages were sent to the intervention group for 6 months. The initial message was followed by a second message 15 minutes later asking whether or not participants had taken their medication. Both the initial message and follow-up messages were designed by the youth themselves and personalized to reflect content meaningful to them depending on their circumstances, i.e., by culture or other sources of identity and meaning, as well as their need for privacy and confidentiality. To protect confidentiality, staff encouraged participants to delete messages after taking medication, to use messages that would not reveal HIV status or mention medications, and provided each participant with information about phone confidentiality (e.g.. passcode protection, etc.). Message delivery was timed to coincide with individual dosing schedule. Examples of these messages designed by participants include: “Mission accomplished?”; “Will you choose life?” A set of motivational or encouraging follow-up messages were randomized depending on their affirmative or negative response (e.g., “Well done,” “You can do it!”), respectively. This 2-way personalized message system was designed as youth-friendly, including the positive feedback loop to promote self-management, extending beyond the scope of many commercial products that send 1-way “canned” reminders.
Data Collection and Measures
We collected baseline and follow-up indicators using computer-assisted administration (both interviewer and self-administration). Whenever possible, we selected measures previously tested in studies of ART adherence, particularly among youth to maximize comparability to other studies.
Demographic Variables
Participants were asked their date-of-birth, birth sex, gender identity, race/ethnicity, education level, history of childhood government assistance (i.e., AFDC/TANF), and mode of infection.
Primary and Secondary Outcome Measures: Medication Adherence and Viral Load
Because of the pilot nature of the study, self-reported adherence was used as the primary outcome, measured using a visual analogue scale (VAS) to rate adherence for each medication taken in the last 30 days on a scale of 0–100%. Among YLH, self-reported measures of adherence have been found to correlate moderately and significantly with viral load (20), including the VAS (i.e., r=−0.5) (18, 36); in our sample the correlation was significant, but relatively low at Spearman-rho=−0.24 (p=.02). Average adherence was computed to include all ART medications. A second dichotomous variable indicating ≥90% adherence was computed to provide a “gold standard” adherence measure for each participant. Viral load was a secondary outcome abstracted from medical records, measured in RNA copies per milliliter, with ≤ 75 copies per mL defined as viral suppression.
Medication-taking Self-efficacy, Outcome Expectancy and Motivation
We measured social cognitive constructs theorized to reinforce the effect of the text messaging intervention on adherence (i.e., as mediators), including adherence self-efficacy, treatment outcome expectancy, and medication motivation. Adherence self-efficacy, and outcome expectancies were measured using the HIV Medication Taking Self-efficacy Scale (37), which assess confidence to take medications and the expectation that taking medication will result in improved health. We measured perceived control of medication adherence and medication motivation using the Self-Regulation of Medication Adherence Battery (SRMAAB) which measures medication motivation, and perceived control of medication adherence (38).
Substance Abuse, Depressive Symptoms and HIV Stigma
We measured HIV-related stigma, depressive symptoms and substance use as moderators of intervention effects. Stigma was operationalized with a 10-item scale created and tested for YLH (39). The stigma scale was analyzed as both a continuous and binary variable dichotomized about the median (median=3.4). We measured depressive symptoms with the Brief Symptom Inventory (BSI), a multi-item scale of mental health problems experienced in the last 7 days (40). A standard cutoff score of >63 on the depression subscale was used to indicate high depressive symptomology. Problem alcohol and drug use was assessed with the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) (41). The ASSIST covers 10 substances (in the previous 3 months) and assesses frequency of use and associated problems for each. For moderation analyses, dummy variables were created for each substance corresponding to a rating of moderate to high-risk (vs. none/low).
Intervention Acceptability and Satisfaction
Participants were asked the frequency of receipt of text messages, the degree to which they found the messages intrusive/bothersome, and whether the messages met their privacy expectations. We used an adapted version of the 8-item Client Satisfaction Questionnaire (42) to measure satisfaction, (e.g., “How would you rate the quality of the text messaging intervention?”; “Did the text messaging intervention meet your expectations?”).
Data Analysis
Intervention effects were evaluated using a modified intention-to-treat (ITT) approach. ITT modifications included withdrawing participants found to be ineligible based on evidence post-randomization (n=4 withdrawn). Baseline characteristics of intervention and control group participants were compared to assess randomization using Chi-square tests for categorical variables and Wilcoxon rank-sum tests for continuous variables. To assess intervention effects, we compared the intervention and control groups at 3 and 6-months post-baseline using generalized linear models (GLM) with link functions, applying the generalized estimating equation (GEE) extension of GLM to account for within-subject correlation of repeated measures. We fit models with an indicator for intervention group, time, and a group-by-time interaction assessing whether the intervention and control groups differed over time, with the primary hypothesis assessed by significance of the group by time interaction. In the absence of significant interaction, models were refitted without the interaction to assess group differences across the follow-up period, controlling for the baseline value of the outcome. We compared adherence rates in each arm at 9 and 12 months using measurement at the 6-month time point as the baseline to assess: (1) intervention effects in the control group after crossing over to receive the intervention and (2) durability of intervention effects in the original intervention arm. Mediational analysis were conducted to test the statistical significance of the indirect effects of social cognitive factors on adherence using methods outlined by MacKinnon and colleagues (43, 44) that have been adapted for binary outcomes (45) and longitudinal designs (46). Potential effect modifiers of the association between intervention group assignment and adherence and changes in adherence over time within the intervention group were assessed by testing the statistical significance of interactions of the effect modifier with intervention group and/or with time. Analyses were conducted in SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
Sample Characteristics
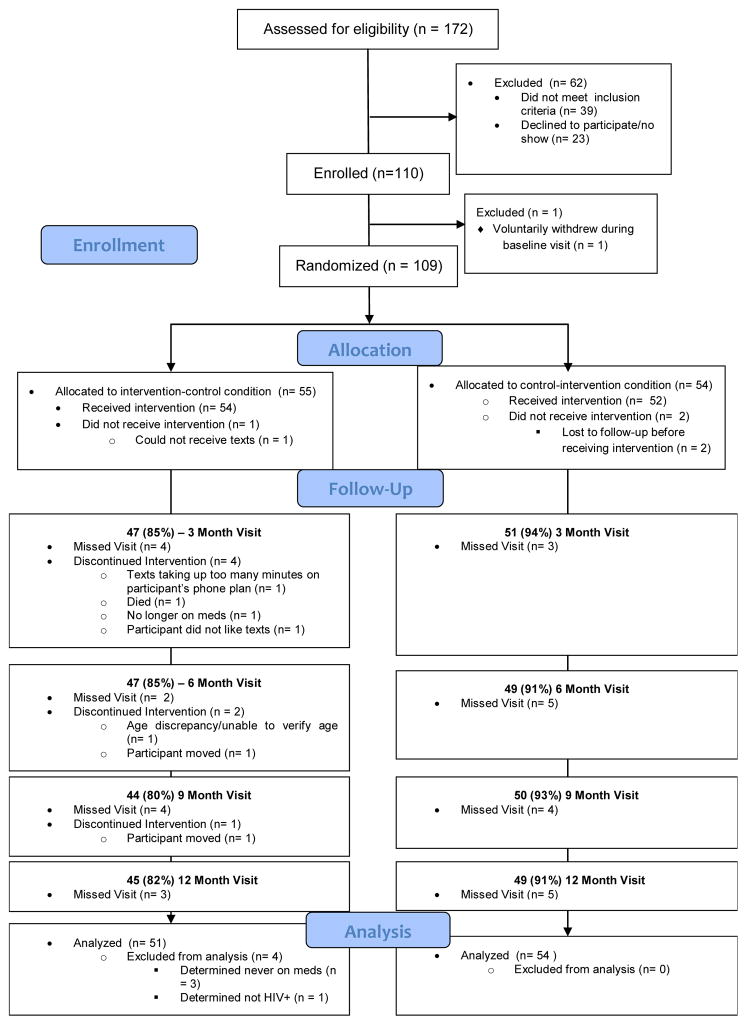
172 individuals were assessed for eligibility of which 133 (77%) were eligible. Twenty-three eligible participants (17%) were not enrolled due to non-response to attempts to schedule/re-schedule an enrollment visit. Thus 109 participants were enrolled and randomized (one eligible participant signed consent, but withdrew voluntarily prior to randomization; n=55 intervention, n=54 control); four randomized participants were found to be ineligible post-randomization (n=4 intervention) and were withdrawn from analysis, thus N=105 were included in subsequent analyses (Figure 1, CONSORT diagram). Participants had a baseline average ART adherence of 67%; only 26% were at or above 90% (Table I). Average age was 24 years. A majority of participants were male (80%), Black (74%), and behaviorally-infected (82%). More than two-thirds of the sample reported moderate to high risk of substance abuse/dependence largely driven by marijuana use (61% of those at moderate/high risk); 41% of the sample had high levels of depressive symptoms. In comparing the intervention and control groups at baseline, only the number of medications prescribed was significantly different between the two groups (NB: baseline number of medications was controlled in GEE analyses). There were no statistically significant differences in the intervention and control groups for demographic characteristics, medication adherence, viral load or mode of infection and no significant differences in rates of 3- or 6-month follow-up, which was 90% and 88% in the sample overall at 3- and 6-month respectively.
Table I
Baseline Comparison of Intervention and Control Groups
| Totala, N=105 n (%) | Intervention, N=51 n (%) | Control, N=54 n (%) | p- valueb | |
|---|---|---|---|---|
| VAS, continuous | 0.343 | |||
 M (SD) M (SD) | 66.6 (25.0) | 68.3 (24.9) | 65.0 (25.2) | |
 Median (Range) Median (Range) | 70 (50–90) | 80 (0–95) | 70 (0–100) | |
| Adherence | 0.692 | |||
 ≥90% ≥90% | 27 (25.7) | 14 (27.5) | 13 (24.1) | |
 <90% <90% | 78 (74.3) | 37 (72.6) | 41 (75.9) | |
| Age | 0.916 | |||
 M (SD) M (SD) | 24.1 (2.9) | 24.1 (3.2) | 24.1 (2.7) | |
 Median (Range) Median (Range) | 23 (18–29) | 23 (18–29) | 23 (18–29) | |
| Log VL | 0.480 | |||
 M (SD) M (SD) | 2.47 (1.41) | 2.48 (1.56) | 2.46 (1.25) | |
 Median (Range) Median (Range) | 1.68 (1.30–5.98) | 1.68 (1.30–5.98) | 1.61 (1.30–5.97) | |
| VL ≤75 copies/ml | 0.272 | |||
 Yes Yes | 61 (65.6) | 34 (70.8) | 27 (60.0) | |
 No No | 32 (34.4) | 14 (29.2) | 18 (40.0) | |
| Sex at birth | 0.608 | |||
 Male Male | 86 (81.9) | 41 (80.4) | 45 (83.3) | |
 Female Female | 18 (17.1) | 10 (19.6) | 8 (14.8) | |
 Intersex Intersex | 1 (1.0) | 0 (0.0) | 1 (1.9) | |
| Gender identity | 0.703 | |||
 Male Male | 84 (80.0) | 40 (78.4) | 44 (81.5) | |
 Female Female | 18 (17.1) | 10 (19.6) | 8 (14.8) | |
 Transgender M to F Transgender M to F | 3 (2.9) | 1 (2.0) | 2 (3.7) | |
| Race/Ethnicity | 0.855 | |||
 White White | 5 (4.8) | 3 (5.9) | 2 (3.7) | |
 Black Black | 78 (74.3) | 39 (76.5) | 39 (72.2) | |
 Hispanic Hispanic | 8 (7.6) | 3 (5.9) | 5 (9.3) | |
 Other Other | 14 (13.3) | 6 (11.8) | 8 (14.8) | |
| Education | 0.329 | |||
 < HS < HS | 14 (13.3) | 9 (17.7) | 5 (9.3) | |
 HS or GED HS or GED | 25 (23.8) | 11 (21.6) | 14 (25.9) | |
 Some college Some college | 48 (45.7) | 20 (39.2) | 28 (51.9) | |
 Associates degree/Trade school Associates degree/Trade school | 8 (7.6) | 6 (11.8) | 2 (3.7) | |
 Bachelor’s degree or higher Bachelor’s degree or higher | 10 (9.5) | 5 (9.8) | 5 (9.3) | |
| Ever received AFDC/TANF | 0.976 | |||
 Yes Yes | 72 (71.3) | 35 (71.4) | 37 (71.2) | |
 No No | 29 (28.7) | 14 (28.6) | 15 (28.9) | |
| Mode of Infection | 0.700 | |||
 Behavioral Behavioral | 86 (81.9) | 40 (78.4) | 46 (85.2) | |
 Perinatal Perinatal | 10 (9.5) | 6 (11.8) | 4 (7.4) | |
 Other Other | 9 (8.6) | 5 (9.8) | 4 (7.4) | |
| Completed 3 month follow-up | 0.090 | |||
 Yes Yes | 94 (89.5) | 43 (84.3) | 51 (94.4) | |
 No No | 11 (10.5) | 8 (15.7) | 3 (5.6) | |
| Completed 6 month follow-up | 0.318 | |||
 Yes Yes | 92 (87.6) | 43 (84.3) | 49 (90.7) | |
 No No | 13 (12.4) | 8 (15.7) | 5 (9.3) | |
| Marijuana use (ASSIST) | 0.398 | |||
 Moderate-High risk Moderate-High risk | 51 (60.7) | 23 (56.1) | 28 (65.1) | |
 Low risk Low risk | 33 (39.3) | 18 (43.9) | 15 (34.9) | |
| Depression | ||||
 BSI Depression Score, M (SD) BSI Depression Score, M (SD) | 59.4 (11.2) | 59.7 (12.3) | 59.2 (10.2) | 0.773 |
 BSI >63, n (%) BSI >63, n (%) | 43 (41.0) | 22 (43.1) | 21 (38.9) | 0.658 |
 BSI ≤63, n (%) BSI ≤63, n (%) | 62 (59.0) | 29 (56.9) | 33 (61.1) | |
| Medications | ||||
 Number of ARVs, M (SD) Number of ARVs, M (SD) | 2.1 (1.0) | 2.5 (1.0) | 1.8 (1.0) | 0.002 |
 ≥ 2 meds vs. 1, n (%) ≥ 2 meds vs. 1, n (%) | 64 (61.1) | 39 (76.5) | 25 (46.3) | 0.002 |
 Total drug duration in months, M (SD) Total drug duration in months, M (SD) | 23.7 (42.7) | 21.3 (27.7) | 25.9 (53.3) | 0.496 |
Abbreviations: VAS Visual Analogue Scale; VL viral load; M mean; SD standard deviation; ASSIST Alcohol, Smoking, Substance Involvement Screening Test, BSI Brief Symptom Inventory, AFDC/TANF AIDS for Families with Dependent Children/Temporary Assistance for Needy Families
Intervention Dosage and Satisfaction
Over the 6-month intervention period, 9,586 reminder messages were sent with 8,512 successfully received (89%). All participants who successfully received messages responded to the reminder message at least once, with a 58% average response rate (i.e., the total number of “yes” and “no” responses divided by the total number of messages sent). Two participants changed the content of their reminder message and 17 participants (33%) changed their mobile number at least once during the intervention period. Participants rated the intervention highly in terms of accessibility and satisfaction. Of intervention participants who completed the 6-month follow-up visit (N = 43), 100.0% reported they would recommend the text messaging intervention for a friend in need of similar help, 81% reported wanting to continue getting the text messages after conclusion of the study, and 95% were satisfied with the intervention overall. This level of satisfaction was also reflected in open-text comments collected immediate post-intervention. In response to the question, “What could we have done differently in this research study to improve your experience?” the overwhelming majority of responses recommended no changes in statements such as, “I find the txt helpful and it’s worked perfect for me, thx;” “nothing, you’ve done well;” “everything OK;” “great study.” The few suggestions for changes included, for example, a preference by one participant for one message daily (i.e., just the reminder message), one respondent indicated a preference for texts every other day, and another respondent recommended a 3rd message, i.e., initial reminder plus two follow-up messages.
Intervention Efficacy
From baseline to 3-month follow-up, the difference in mean adherence between the intervention and control group was 7 percentage points (95% CI: 0.91–13.9) and the odds ratio for ≥ 90% adherence was 2.57 (95% CI: 1.01–6.54), indicating a significant difference between the two study arms (Table II). This effect attenuated thereafter and was not significantly different at 6-months. Over the 6-month intervention period, the average effect for mean adherence level was not significant, however, the average effect for the proportion ≥90% adherence was significant with an odds ratio of 2.12 (95% CI: 1.01–4.45). The percent of the intervention group with ≥90% adherence increased from 28% at baseline to 64% at 3-months and attenuated slightly to 61% at 6-month follow-up; comparable rates in the control group were 24% at baseline and 43% and 51% at 3- and 6-month follow-up respectively. There was no significant difference in either log viral load or viral suppression between the two arms at either 3 or 6-month follow-up, although information on viral load was unavailable for over half the sample at 6 months (due to resource limitations of the funding mechanism). We found no evidence of mediation by social cognitive factors or moderation by HIV stigma, depressive symptoms or substance use during the intervention period.
Table II
Effects of the Intervention on Primary and Secondary Outcomes at 3 and 6 months
| Intervention, N=51 | Control, N=54 | Effect Estimates | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted No. (%)or Mean (SD) | Unadjusted No. (%)or Mean (SD) | 3-month vs. Baseline | 6 month vs. Baseline | Average effect, baseline to 6 mos | |||||
| Baseline | 3 month | 6 month | Baseline | 3 month | 6 month | OR or Mean Diffa (95% CI) | OR or Mean Diffa (95% CI) | OR or Mean Diffb (95% CI) | |
| n | 51 | 44 | 43 | 54 | 51 | 49 | |||
| VAS, M (SD) | 68.3 (24.9) | 85.7 (11.9) | 79.8 (21.2) | 65.0 (25.2) | 76.7 (21.4) | 80.8 (16.2) | 7.38 (0.91–13.9)* | −0.89 (−8.57–6.79) | 3.54 (−2.03–9.11) |
| VAS ≥90, n (%) | 14 (27.5) | 28 (63.6) | 26 (60.5) | 13 (24.1) | 22 (43.1) | 25 (51.0) | 2.57 (1.01–6.54)* | 1.68 (0.69–4.09) | 2.12 (1.01–4.45)* |
| n | 48 | 27 | 20 | 45 | 33 | 23 | |||
| Log viral load, M (SD) | 2.5 (1.6) | 2.0 (1.2) | 2.2 (1.4) | 2.5 (1.3) | 2.0 (0.9) | 2.2 (1.0) | 0.01 (−0.47–0.50) | −0.06 (−0.87–0.75) | 0.04 (−0.39–0.47) |
| Undetectable Viral load, n (%) | 34 (70.8) | 20 (74.1) | 16 (80.0) | 27 (60.0) | 24 (72.7) | 13 (56.5) | 0.70 (0.14–3.45) | 2.42 (0.51–11.4) | 0.77 (0.24–2.49) |
Durability of Intervention Effects
To assess durability of effects in the intervention arm and evidence of effect in the control group (pre-post), study conditions crossed over at the 6-month follow-up visit. There was no significant difference in either mean adherence or 90% adherence in the intervention group (i.e., the group initially randomized to intervention) at 9- and 12-month follow-up compared to the 6-month time point. The proportion with 90% adherence in the intervention group was 58%, and 61% at 9-, and 12-month follow-up respectively (p=0.6 for post-intervention vs. intervention period; data not shown), indicating durability of the intervention effect. Furthermore, there was an observed improvement in adherence in the initial control group following the crossover, with the proportion with 90% adherence increasing from 51% at 6 months (“baseline”) to 52% at 9 months and 65% at 12 months (p=0.07 for post vs. pre-intervention). We assessed the adherence trajectory (baseline to 12 months) in the intervention group by levels of HIV stigma, depressive symptoms and substance abuse (Table III). No differences in adherence were found according to levels of HIV stigma, however, differential trajectories were observed for participants reporting both high levels of depressive symptoms and moderate-to-high levels of marijuana use in comparison to participants reporting neither (NB: alcohol and other substances were also explored with no evidence of moderation). Because of the potential for compound effects of depressive symptoms and marijuana abuse, we combined these categories for further analysis. Despite small cell sizes, we found participants in the intervention condition who reported both high levels of depressive symptoms and marijuana use (notably almost 32% of participants in the intervention arm) had significantly lower adherence over the observation period in comparison to those with neither condition (exposure by linear trend p=.005).
Table III
Mean VAS in the Intervention Arm by Time and Co-Morbid Condition(s)*
| Intervention Arm | Exposure x linear time | ||||||
|---|---|---|---|---|---|---|---|
| n (%) at baseline | Baseline | 3 Month | 6 Month | 9 Month | 12 Month | interaction p-value | |
| Marijuana/Depression | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | ||
| Both marijuana, depression | 13 (31.7) | 73.5 (18.9) | 85.0 (10.0) | 71.7 (26.6) | 59.3 (38.6) | 69.1 (25.5) | 0.005 |
| Marijuana only | 5 (12.2) | 67.0 (28.6) | 88.3 (7.1) | 84.4 (20.7) | 75.2 (27.8) | 76.7 (26.9) | 0.178 |
| Depression only | 10 (24.4) | 44.0 (28.8) | 72.5 (23.6) | 77.5 (18.9) | 57.5 (35.9) | 72.5 (28.7) | 0.9 |
| Neither | 13 (31.7) | 74.4 (24.0) | 89.5 (8.3) | 86.7 (8.7) | 96.1 (4.9) | 96.3 (5.2) | Ref Global test of interaction p-value=0.024 |
DISCUSSION
To our knowledge this pilot study is the first RCT in the published literature demonstrating the initial efficacy of a daily, tailored and personalized 2-way SMS text messaging reminder system to improve HIV medication adherence among YLH in the U.S. Our sample’s mean age was 24 and was otherwise largely reflective of the epidemic among YLH in the U.S with the majoriy being male, Black, behaviorally-infected and of low socioeconomic status. Additionally, levels of co-morbid substance use and mental health problems were high among the sample. With regard to initial efficacy, we found that participants in the intervention arm were more than 2.6 times as likely as those in the control to report ≥90% adherence at 3-months. This is a relatively large effect size when compared to other behavioral-interventions targeting HIV medication adherence among adults (47) and youth (21, 48) in the published literature. Beyond 3 months there was a general plateau of intervention effects and some attenuation of the effects out to 6 and 12 months; however overall 61% of the intervention group was ≥90% adherent at 6-months versus 51% in the control group and this level of adhence was maintained at 12-months in the intervention group (6 months post-intervention). This suggests both durability of effects and the potential for additional intervention at 3 months to optimize or further bolster current intervention effects.
With regard to mediation and moderation of the intervention effect, we did not find evidence of mediation by social cognitive factors or moderation by psychosocial factors during the intervention period. However, the adherence trajectory in the intervention arm was modified by a combination of marijuana use and depressive symptoms over the 12-month study period. The moderation of the adherence trajectory among participants who reported both moderate/high marijuana use and depressive symptoms is a potentially important finding. These conditions are all-too common among YLH and therefore this finding warrants further inquiry as part of a larger RCT with a more diverse sample.
These findings further demonstrate the critical need for low-intensity interventions that are responsive to the social realites of YLH and scalable in clinical or community-based settings. Participants used their own cell phones and while there were interruptions in cell phone service (33% of intervention participants reported at least one change in their cell phone number), 89% of messages were successfully sent as intended. Satisfaction with the intervention was also high, with 95% reporting that they were satisfied with the intervention overall.
Limitations
The reliance on a self-report measure of adherence and the lack of complete data for a biological outcome are limitations that should be considered for interpretation of findings in this pilot study. While the correlation between self-reported adherence (via the VAS) and viral load in our sample was relatively low, self-reported measures continue to be very important to the assesment of adherence for several reasons. First, the use of viral load in the assessment of adherence among adolescents and young adults may be less helpful than it once was given the widespread use of ART early in the disease course (e.g. early treatment in the treatment as prevention model of care). In our sample, 66% of participants were virally supressed at baseline (see Table I). However, early adherence patterns are important predictors of viralogic outcomes over time in YLH (7), thus establishing good adherence early on in the disease process is of critical importance, prior to the emergence of viral resistance.
An additional limitation in this study was collection of data from a convenience sample of adolescents and young adults living in one geographic area, thus findings may not generalize to all urban YLH. As well, 17% of volunteers who screened eligible did not enroll due to non-response; these youth may be the more likely to be non-adherent than those who enrolled. Thus despite the low baseline adherence rate (67%), our study may not have included youth most likely to need medication adherence intervention. Finally, the null finding regarding both mediation and moderation during the intervention period may have been due to lack of power as the sample size was quite small to detect these effects. Furthermore, the theorized mediation effect is one of secondary reinforcement rather than a primary mechanism of action, which may further limit power to detect an effect, i.e., the intervention reminder triggers adherence behavior which is theorized to increase self-efficacy, outcome expectancy, and motivation to support durability of the intervention effect.
Conclusion
Limitations not withstanding, this approach holds promise as a low-intensity intervention with potential for positive impact on medication adherence among YLH. This intervention has considerable public health implications as a practical and scalable tool that can be implemented in primary care clinics across the U.S and may help youth living with HIV lead healthier lives. However, additional inquiry and research with a larger, more geographically diverse sample and objective measures of adherence is warranted.
Acknowledgments
We thank the participants of the “TXTXT” study for their time and effort. Special thanks to Camdin Gray and Jennifer Leininger for their contributions to sample recruitment and retention. Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number R34DA031053. Additional support was received from the Stanley Manne Children’s Research Institute at Ann & Robert H. Lurie Children’s Hospital of Chicago (Lurie Children’s). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Approval to conduct this study was received from the Institutional Review Board (IRB) at Lurie Children’s under protocol #2011-14398.
Footnotes
Clinical trials registration: This trial has been registered at www.clinicaltrials.gov (identifier NCT01354210)
No financial disclosures were reported by the authors of this paper.
Drs. Garofalo and Kuhns jointly developed the study rationale, aims, and design. Dr. Garofalo supervised the implementation of the study and drafted and revised the final manuscript. Dr. Kuhns drafted the methods section, assisted with interpretation of findings, and critically reviewed the manuscript. Dr. Hotton conducted all data analyses, created all tables, drafted the statistical methods and results sections, and critically reviewed the manuscript.
Ms. Johnson and Ms. Muldoon oversaw data entry and data quality and critically reviewed the manuscript; additionally Ms. Muldoon drafted figure 1. Mr. Rice assisted with the background literature review and critically reviewed the final manuscript. All authors approved the final manuscript as submitted.
Conflict of Interest/Financial Disclosure: No financial disclosures or conflicts of interest were reported by the authors of this paper.
References
Full text links
Read article at publisher's site: https://doi.org/10.1007/s10461-015-1192-x
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4788595?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1007/s10461-015-1192-x
Article citations
User Engagement With mHealth Interventions to Promote Treatment Adherence and Self-Management in People With Chronic Health Conditions: Systematic Review.
J Med Internet Res, 26:e50508, 24 Sep 2024
Cited by: 0 articles | PMID: 39316431 | PMCID: PMC11462107
Review Free full text in Europe PMC
A Web-Based Antiretroviral Therapy Adherence Intervention (Thrive With Me) in a Community-Recruited Sample of Sexual Minority Men Living With HIV: Results of a Randomized Controlled Study.
J Med Internet Res, 26:e53819, 30 Sep 2024
Cited by: 0 articles | PMID: 39348677 | PMCID: PMC11474139
Acceptability of an automated directly observed therapy (DOT) application for PrEP adherence support among young men who have sex with men: a qualitative exploration.
AIDS Care, 36(11):1704-1718, 02 Sep 2024
Cited by: 0 articles | PMID: 39222964
The impact of using reinforcement learning to personalize communication on medication adherence: findings from the REINFORCE trial.
NPJ Digit Med, 7(1):39, 19 Feb 2024
Cited by: 0 articles | PMID: 38374424 | PMCID: PMC10876539
Adjunctive interventions: change methods directed at recipients that support uptake and use of health innovations.
Implement Sci, 19(1):10, 08 Feb 2024
Cited by: 6 articles | PMID: 38331832 | PMCID: PMC10854146
Go to all (92) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT01354210
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Feasibility of interactive text message response (ITR) as a novel, real-time measure of adherence to antiretroviral therapy for HIV+ youth.
AIDS Behav, 17(6):2237-2243, 01 Jul 2013
Cited by: 45 articles | PMID: 23546844
Positive STEPS - a randomized controlled efficacy trial of an adaptive intervention for strengthening adherence to antiretroviral HIV treatment among youth: study protocol.
BMC Public Health, 18(1):867, 13 Jul 2018
Cited by: 15 articles | PMID: 30001703 | PMCID: PMC6043988
Positive Strategies to Enhance Problem-Solving Skills (STEPS): A Pilot Randomized, Controlled Trial of a Multicomponent, Technology-Enhanced, Customizable Antiretroviral Adherence Intervention for HIV-Infected Adolescents and Young Adults.
AIDS Patient Care STDS, 33(1):21-24, 01 Jan 2019
Cited by: 13 articles | PMID: 30601059 | PMCID: PMC6338456
Mobile Telephone Text Messaging for Medication Adherence in Chronic Disease: A Meta-analysis.
JAMA Intern Med, 176(3):340-349, 01 Mar 2016
Cited by: 363 articles | PMID: 26831740
Review
Funding
Funders who supported this work.
NIDA NIH HHS (1)
Grant ID: R34 DA031053
National Institute on Drug Abuse (1)
Grant ID: R34DA031053