Abstract
Free full text

One Carbon Metabolism and Epigenetics: Understanding the Specificity
Associated Data
Abstract
One carbon metabolism is a metabolic network that integrates nutrient status from the environment to yield multiple biological functions. Composed of the folate and methionine cycle, it generates S-adenosylmethionine (SAM), which is the universal methyl donor for methylation reactions, including histone and DNA methylation. Histone methylation is a crucial part of the epigenetic code and plays diverse roles in the establishment of chromatin states that mediate the regulation of gene expression. The activities of histone methyltransferases (HMTs) are dependent on intracellular levels of SAM, which fluctuate based on cellular nutrient availability, providing a link between cell metabolism and histone methylation. Here we discuss the biochemical properties of and gene regulation by histone methyltransferases and the connection to cellular metabolism. Our emphasis is on understanding the specificity of this intriguing link.
Introduction
Studies in a variety of organisms, including humans, have suggested roles for nutrient metabolism in regulating the epigenetic state in normal and disease states. Chronic diseases such as diabetes, obesity, cancer, heart disease and aging all have been linked to metabolic and epigenetic factors that play a role in pathogenesis.1–6 The maintenance of cellular homeostasis requires that alterations in nutrient availability be met by appropriate adaptations. An example is the case of cell proliferation when nutrient availability is limiting.7 These adaptations necessitate sensing mechanisms that can be modulated by nutrient availability in the environment and communicate metabolic status to affect cellular physiology.
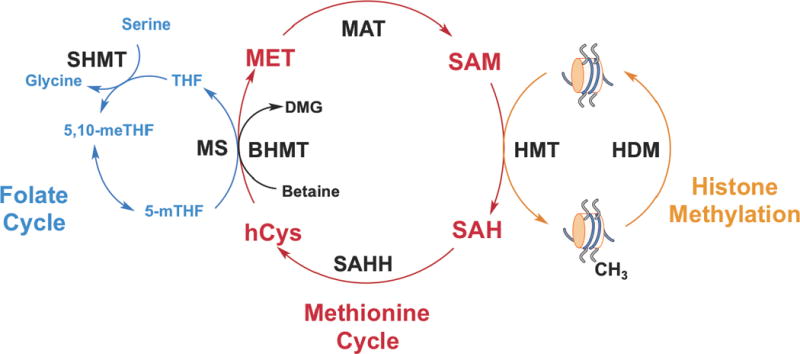
One carbon metabolism utilizes a variety of nutrients such as glucose, vitamins and amino acids to fuel a variety of metabolic pathways that utilize these one carbon units. These pathways include nucleotide metabolism, maintenance of cellular redox status, lipid biosynthesis and methylation metabolism.8 Two major components of one carbon metabolism comprise the folate and methionine cycles (Fig 1), that function to transfer single carbon units to acceptor substrates. Importantly, the methionine cycle provides a link to histone methylation through the generation of S-adenosylmethionine (SAM).
Histones can be mono-, di-, or trimethylated at lysines and arginines by histone methyltransferases (HMTs), which transfer the methyl group from SAM to the histone substrate generating SAH (Fig 1). Histone methyltransferases (HMTs) are the ‘writers’ of methylation marks on histones. Although histone methylation was discovered in 19649, bona fide HMTs weren’t described until 45 years later in 1999 and 2000 with the characterization of Co-activator Arginine Methyltransferase1 (CARM1)10 and Su-var 3–9 Homologue 1 (SUV39H1)11, respectively. Currently, there are over 30 characterized HMTs with different methylation capacities and specificities that fall into two families—lysine methyltransferases (KMTs) and protein arginine methyltransferases (PRMTs).
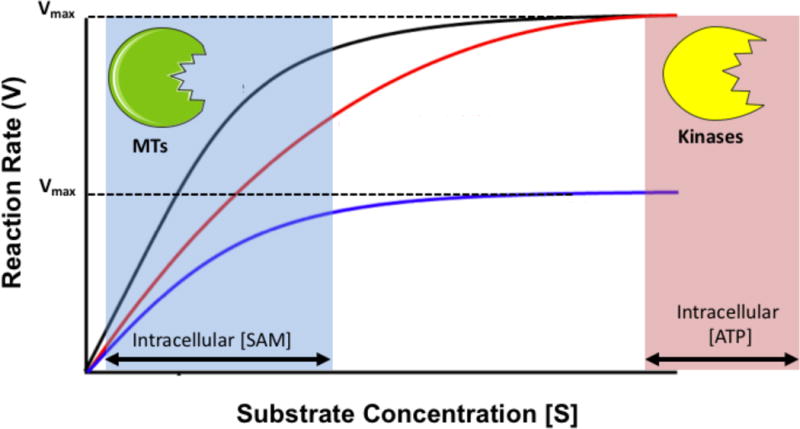
Methyltransferase activity depends on substrate concentration, as is the case with all enzymes. However in contrast to phosphorylation kinetics, where adenine triphosphate (ATP) is present in cellular concentrations far greater than the enzyme Km values (concentration of metabolite at half maximum velocity of enzyme-mediated reaction), cellular concentrations of SAM are similar to HMT Km values (Fig 2). In addition, SAH is a known inhibitor of HMTs12, suggesting the SAM/SAH ratio may also play a role in the regulation of HMT activity.13 Based solely on the biochemical characteristics of HMTs, small fluctuations in SAM concentration could significantly affect HMT activity, either increasing or decreasing histone methylation activity as has been proposed.14 This suggests a direct link from cell metabolism to histone methylation status exists which could be tested.15
Recent work has shown that altered metabolism does have an effect on histone methylation16–18, but the direct mechanistic details have yet to be fully understood. In mouse embryonic stem cells (mESC), threonine depletion contributed to decreases in the SAM/SAH ratio and histone H3 lysine 4 trimethylation (H3K4me3). However, this was observed with many other alterations such as effects on acetyl-coA metabolism indicating an indirect pathway.19, 20 Additional work on Nicotinamide N-methyltransferase (NNMT), a SAM-consuming enzyme demonstrated over expression decreased SAM and increased SAH, overall decreasing the SAM/SAH ratio and decreasing histone methylation at H3K9me2 and H3K27me3.17 Further, methionine deprivation in human stem cells decreased H3K4me3 but the observation was due in part to indirect mechanisms involving an activation of a stress response, apoptotic, and differentiation pathways.16 Despite these advances in our understanding, the precise molecular mechanisms and specific effects on gene expression have been largely uncharacterized.
In this review we will focus on methionine metabolism as an essential regulator of histone methylation and the possibility for specific gene regulation determined by the biochemical properties, specificities of HMTs and the availability of the cofactor SAM. We will concentrate on histone lysine methylation because of its importance in epigenetics and its dysregulation in disease states.
Methionine and One-Carbon Metabolism
The methionine cycle is essential for the generation of SAM through the adenylation of methionine by methionine adenosyltransferase (MAT).21–23 SAM is considered the universal methyl donor and is used by methyltransferases to methylate metabolites, RNA, DNA, and proteins, including histones. After the methyl group is transferred from SAM to an acceptor substrate, S-adenosylhomocysteine (SAH) is produced. In turn, SAH is hydrolyzed to adenine and homocysteine. In vivo, the reaction kinetics proceed forward in this direction, as long as the products are being removed through other metabolic pathways. Otherwise, the reverse reaction is more favorable increasing the pool of SAH.23 Homocysteine can enter the transulfuration pathway and condense with serine to generate cystathionine in an irreversible reaction catalyzed by cystathionine β-synthase (CBS). In addition, two enzymes can utilize homocysteine to regenerate methionine. Betaine-homocysteine methyltransferases (BHMT) transfers a methyl group from betaine, an intermediate in choline metabolism, to regenerate methionine and produce dimethylglycine. 5-methyltetrahydrofolate-homocysteine methyltransferase (MS) regenerates methionine by the transfer of the 5-methyltetrahydrofolate methyl moiety to homocysteine producing tetrahydrofolate (THF).
The amount of intracellular SAM depends largely on the availability of methionine, an essential amino acid sourced from the diet. The concentration of methionine circulating throughout the body in the serum is around 30μM in adults but may vary widely and change with disease.24, 25 It was recently found that methionine was observed to be the most variable serum amino acid with dietary factors being a major source of the variation.26 SAM concentration, on the other hand, is very low in the serum (<0.5μM)27 and is contained mostly in intracellular pools ranging from below 10μM up to 90μM under normal conditions and is dependent on tissue type.28
Histone Methyltransferases and their Biochemical Properties
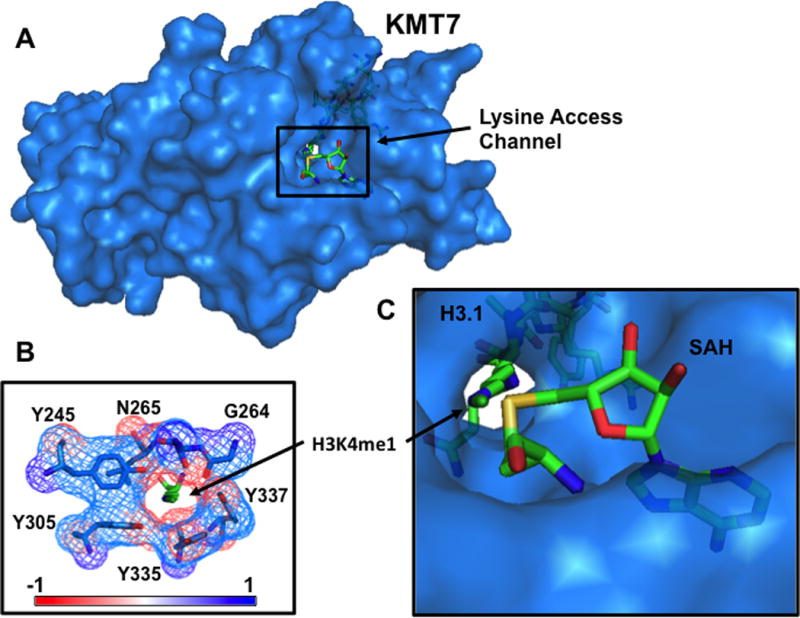
Histones can be methylated at basic residues, most notably, lysine and arginine. In addition, lysine can be mono-, di-, or trimethylated and arginine can be mono-methylated, or symmetrically or asymmetrically dimethylated. All characterized lysine-specific methyltransferases contain a SET (Su(var), Enhancer of Zeste and Trithorax) domain except Disruptor of Telomeric Silencing-like 1 (DOT1L), which methylates the globular domain of H3 at Lysine 79 (H3K79) putatively only when the nucleosome is intact29. The SET domain spans 130 amino acids forming a tunnel that connects the cofactor binding site to the substrate binding site on the opposite side. This lysine access channel determines the number of methyl groups that can be transferred during a reaction for a given HMT and depends on the position of the methylated lysine (Fig 3).30, 31 Before transfer, the ε-amino group on the lysine substrate is deprotonated and points towards the SAM methyl group at ~180º suggesting an SN2 reaction mechanism of transfer. No matter the type of HMT, they utilize the universal methyl donor, S-adenosylmethionine (SAM) to methylate histones releasing S-adenosylhomocysteine (SAH) in the process allowing for regulation by cell metabolism.
The regulation of HMT activity would depend on the processing of methionine to form SAM, SAM availability to HMTs, and the concentration of HMTs in the cell. Many HMTs have been carefully studied and characterized (Summarized in Table 1). Interestingly, the Km, SAM for the methylation reactions performed by HMTs falls within the range of observed intracellular SAM concentrations. Notably, there is also range of kinetic parameters across the family of HMTs suggesting that a change in metabolic flux would affect only select HMT activity.
Table 1
Kinetic properties of histone methyltransferases
| Histone | HMT | Modification | Km(SAM) [μM] | References |
|---|---|---|---|---|
| H3K4 | KMT2A (MLL1) | me1, me2, me3 | 10.4 ± 3.1 | Ref. 67 |
| KMT2B (MLL2) | me1, me2, me3 | 4.5 ± 0.82 | Ref. 68 | |
| KMT2C (MLL3) | me1, me2, me3 | 0.85 ± 0.19 | Ref. 68 | |
| KMT7 (SET7/9) |     me3 me3 | 6.0 ± 1.4 | Ref. 26 | |
| H3K9 | KMT1A (SUV39H1) | me1, me2 | 12.3 ± 0.6 | Ref. 69 |
| KMT1B (SUV39H2) | me1, me2 | 0.74 ± 0.23 | Ref. 68 | |
| KMT1C (EHMT2) |   me2, me3 me2, me3 | 1.8 ± 0.2 | Ref. 70 | |
| H3K27 | EZH1 | me1, me2, me3 | 1.24 ± 0.15 | Ref. 68 |
| KMT6 (EZH2) | me1, me2, me3 | 1.64 ± 0.26 | Ref. 68 |
Histone Methylation Readers, Chromatin Organization, and Gene Expression
Lysine methylation does not significantly alter the local chemical environment and leads to modest if not insignificant changes in DNA and gene accessibility, in contrast to acetylation and phosphorylation. Therefore, “readers” of histone methylation are important since they are able to bind specific histone methylation marks to propagate their effects to gene expression.32 Several conserved histone methyl binding motifs are categorized broadly into two groups, those containing Chromodomains and those that are part of the Royal superfamily.33,32 These domains are present in “reader” proteins that bind histone methylation sites, like CHD1 (chromodomain helices DNA-bnding protein 1)34, HP1 (heterochromatin protein 1)35, PC (polycomb protein)36, and p53BP1 (p53-binding protein 1)37 which allow HMTs to regulate a range of cellular processes including, transcription, RNA processing, and DNA methylation, all of which affect gene expression by chromatin remodeling and transcriptional machinery recruitment.
With the advances in sequencing and chromatin immunoprecipitation (ChIP), we now have a vast repository of data showing the genomic locations of associated proteins including modified histones.38–41 H3K4me3 has been shown to correlate well with active gene transcription and is concentrated around transcriptional start sites.42, 43 ChIP-seq has confirmed earlier studies and consistently identifies H3K4me3 at promoters as a predictive mark of actively transcribed genes.40 Recent analyses have also identified the shape of the peak around the transcriptional start site to be a major feature of genes involved in key biological processes such as aging and tumor suppression.44, 45 H3K4me3 is recognized by TAF3 (TATA Box Binding Protein (TBP) Associated Factor, 140kD) a subunit of TFIID (Transcription Factor II D) suggesting a broad role in RNAP II dependent gene transcription.46 In addition, H3K4me1 has been implicated in marking enhancer regions and depletion at enhancers abolishes long range effects on gene regulation.47
H3K9 methylation, participates in both constitutive and facultative heterochromatin formation and maintenance.48–51 HP1 is recruited to sites of H3K9me3 by interactions with the trimethyl moiety on H3K9 and SUV39H1.52 HP1 then forms a multimeric complex as SUV39H1 methylates more H3K9 recruiting more HP1 in a positive feedback loop that continues to maintain these heterochromatin regions.53
In addition, H3K27me is correlated with gene inactivation and silencing.41 In contrast to other histone methylation events, only two enzymes (EZH1 and EZH2) methylate H3K27 and both are associated with the Polycomb Repressive Complexes (PRCs).54 The PRC is responsible for transcriptional repression by ubiquitination of Histone H2A Lysine 119 (H2A119ub1)55, recruitment of DNA methyltransferases (DNMTs)56 and chromatin compaction.57 Thus with each of these types of regulation by different histone methylation events, metabolism offers several selective modes of interaction to mediate histone methylation and downstream consequences. For instance, a change in SAM levels could trigger a cascade that would propagate through the activity of the HMTs to the recruitment of readers to reach the end point of differential gene expression.
Histone Methyltransferases and Their Target Genes
Genetic studies in Drosophila identified two groups of genes that suppress and enhance the position-effect variegation phenotype, referred to as the trithorax (trx) and Polycomb (Pc) genes, respectively.58, 59. Many of these genes encode proteins, which contain a SET domain. SET1, the first identified HMT with a SET domain was characterized in yeast and further studies identified the Complex of Proteins Associated with SET1 (COMPASS).60, 61 In humans there are two homologous complexes that associate with SET1a and SET1b in addition to four COMPASS-like complexes that associate with MLL1-4.62
SET1 is recruited to actively transcribed genes by an interaction at the C-terminal Domain (CTD) of RNAPII during elongation and is responsible for broader H3K4me3 marking across the gene.63, 64 In humans, SET1a and SET1b localize to different euchromatic structures within the nucleus suggesting distinct roles in regulating gene expression.65 RNAi-mediated knockdown of Set1a decreased promoter H3K4me2 accompanied by decreased expression of oncogenes, Myc and BRCA1.66
The MLL family of histone methyltransferases was identified in humans because of their significant role in Leukemias.67 MLL-1 is present at active promoters with 90% overlap with RNAPII suggesting a broad role in regulating active transcription.68 In addition, ChIP-chip experiments suggest a specific role in regulating miRNAs and the late HoxA cluster (HoxA7, HoxA9, HoxA10, HoxA11, and HoxA13)68. In contrast, MLL-2 and MLL-3 do not alter bulk H3K4me3 after more than 80% knockdown.69 Instead, MLL-2 seems to functions at developmentally regulated genes with characteristic bivalent promoters marked by H3K27me3 and H3K4me3 including all four Hox gene clusters.69 ChIP-seq analysis of MLL4 demonstrated a unique subset of enhancers that are dynamically regulated by MLL4 mono- and dimethylation at different points during differentiation.70 MLL4 co-localizes with transcription factors (PPARγ and C/EBPs) that determine cell lineage during adipogenesis. In addition, deletion of MLL4 decreases H3K4me1, H3K4me2, RNAPII and Mediator occupancy at active enhancers.
H3K9 methyltransferases belong to the KMT1 family with the exception of PRDM2 (KMT8). Supressor of Variegation 3–9 Homolog 1 (SUV39H1) and 2 (SUV39H2) have redundant function and methylate pericentric regions to maintain chromatin organization.51 On the other hand, H3K9 Euchromatic Histone-Lysine N-Methyltransferase 2 (EHMT2/KMT1C) ChIP-seq data shows enrichment at developmental genes and co-localization with PRC2 at a subset of genes suggesting cross talk between H3K9me and H3K27me. Interestingly, co-localization of EHMT2 and PRC2 subunits are enriched at genes important in neuronal development.71 Lastly, H3K27 methylation occurs via EZH1 or EZH2. In mouse hair follicle, single mutants EZH1−/− or EZH2−/− showed no phenotypic difference, suggesting functional redundancy as concluded by the authors. However a group of genes associated with cell cycle progression, cell death and regulation of biological/cellular processes had increased expression and decreased H3K27me2/3 after knockdown of EZH2 by siRNA.72
The activity of each of these enzymes is affected differently by the availability of methionine and status of one carbon metabolism. In each of the genetic studies, it is tempting to speculate that the observed phenotypes that demonstrate the requirements of each HMT would depend on the nutritional environment and metabolic status of the experimental background. For example, in conditions of SAM levels, one might observe different dependencies of different MLL enzymes with differential requirements on the maintenance of histone methylation. Furthermore, the target list of genes mediated by these enzymes may be grossly different in conditions of different one carbon metabolism since it is likely that the marks surrounding a given gene would not be altered in an identical manner to what would be observed in a neighboring gene. Comparative ChIP-seq analysis is likely to reveal new principles into how this specificity would be maintained.
Conclusions
While some HMTs appear to exhibit regulation of broad chromatin regions (ex. MLL1), most have demonstrated a preference for a subset of genes that are specifically regulated. Since the activity of each HMT is also governed by substrate availability (SAM or histone), gene specific regulation is likely altered under different metabolic conditions. For example, if the SAM concentration is suppressed due to insufficient amounts of methionine and choline in the diet, this change in the intracellular concentration of SAM would in turn decrease the activity of a subset of methyltransferases based on SAM Km values and other relevant kinetic parameters. In this regard, KMT2A (MLL1), with a Km, SAM of 12.3μM, should be more affected by decreases in SAM than EZH1, with a Km,SAM of 1.24μM. This could have specific consequences on gene expression. Since MLL1 is a general H3K4me3 methyltransferase, one might hypothesize an overall decrease in H3K4me3 deposition across the genome specifically at promoter regions while H3K27me3 is largely unaffected. The loss of H3K4me3 at promoters has been linked to decreases in Transcription Factor II D (TFIID) binding, an important player in Pol II recruitment, through its TAF3 subunit, ultimately leading to decreased gene expression.73 Although there are many factors to consider in this regulation, mathematical modeling allows one to parse many simultaneous interactions and would be a useful tool to determine the contribution of each HMT to the regulation of global and specific gene expression. It will be important to determine how methionine metabolism is altered in different physiological states, (dieting, cancer, etc.), and the consequences on histone methylation and gene expression to determine the specificity of this sensing mechanism. Future work providing deeper mechanistic connections between metabolism and epigenetics will provide insight into the link between metabolic status, histone methylation and the effect on gene expression—whether transient or permanent—providing a molecular basis for how environmental factors, such as diet, can influence gene expression via cell metabolism.
Literature Cited
Full text links
Read article at publisher's site: https://doi.org/10.1111/nyas.12956
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4801744?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1111/nyas.12956
Article citations
Vitamin B6 Pathway Maintains Glioblastoma Cell Survival in 3D Spheroid Cultures.
Int J Mol Sci, 25(19):10428, 27 Sep 2024
Cited by: 0 articles | PMID: 39408757 | PMCID: PMC11476381
Association between dietary choline intake and asthma and pulmonary inflammation and lung function: NHANES analysis 2009-2018.
J Health Popul Nutr, 43(1):143, 09 Sep 2024
Cited by: 0 articles | PMID: 39252146 | PMCID: PMC11386084
Maternal Malnutrition and Elevated Disease Risk in Offspring.
Nutrients, 16(16):2614, 08 Aug 2024
Cited by: 1 article | PMID: 39203750 | PMCID: PMC11357549
Review Free full text in Europe PMC
Subcellular one carbon metabolism in cancer, aging and epigenetics.
Front Epigenet Epigenom, 2:1451971, 31 Jul 2024
Cited by: 1 article | PMID: 39239102 | PMCID: PMC11375787
Behind the Genetics: The Role of Epigenetics in Infertility-Related Testicular Dysfunction.
Life (Basel), 14(7):803, 26 Jun 2024
Cited by: 0 articles | PMID: 39063558 | PMCID: PMC11277947
Review Free full text in Europe PMC
Go to all (186) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Histone Methylation Dynamics and Gene Regulation Occur through the Sensing of One-Carbon Metabolism.
Cell Metab, 22(5):861-873, 24 Sep 2015
Cited by: 333 articles | PMID: 26411344 | PMCID: PMC4635069
Intrinsic catalytic properties of histone H3 lysine-9 methyltransferases preserve monomethylation levels under low S-adenosylmethionine.
J Biol Chem, 299(7):104938, 17 Jun 2023
Cited by: 2 articles | PMID: 37331600 | PMCID: PMC10404681
Methyl-Metabolite Depletion Elicits Adaptive Responses to Support Heterochromatin Stability and Epigenetic Persistence.
Mol Cell, 78(2):210-223.e8, 23 Mar 2020
Cited by: 41 articles | PMID: 32208170 | PMCID: PMC7191556
Methyl Donors, Epigenetic Alterations, and Brain Health: Understanding the Connection.
Int J Mol Sci, 24(3):2346, 25 Jan 2023
Cited by: 12 articles | PMID: 36768667 | PMCID: PMC9917111
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NCI NIH HHS (5)
Grant ID: R01 CA193256
Grant ID: R21 CA201963
Grant ID: R00CA168997
Grant ID: R01CA193256
Grant ID: R00 CA168997
NIGMS NIH HHS (2)
Grant ID: T32 GM007273
Grant ID: T32GM007273