Abstract
Free full text

Targeting dendritic cells: a promising strategy to improve vaccine effectiveness
Abstract
Dendritic cell (DC) targeting is a novel strategy to enhance vaccination efficacy. This approach is based on the in situ delivery of antigen via antibodies that are specific for endocytic receptors expressed at the surface of DCs. Here we review the complexity of the DC subsets and the antigen presentation pathways that need to be considered in the settings of DC targeting. We also summarize current knowledge about antigen delivery to DCs via DEC-205, Clec9A and Clec12A, receptor targets that strongly enhance cellular and humoral immune responses. Finally, we discuss the intracellular trafficking criteria of the targeted receptors that may impact their effectiveness as DC targets.
Vaccination is a potent therapeutic strategy to prevent the spread of infectious disease. Current vaccines are composed of pathogen-derived antigens (purified proteins or inactivated pathogen) that give rise to antigen-specific memory B and T cells and confer long-term protection to the vaccinated individual. Most vaccines also contain an adjuvant that is critical for their effectiveness. Vaccination has considerably reduced or eliminated the spread of severe pathologies around the world. There are still many diseases, however, for which we currently cannot rely on vaccines to cure (for example, hepatitis C virus, malaria and cancer). Therefore, novel and advanced vaccination strategies are required.
Dendritic cells (DC) are major determinants of vaccination due to their role in priming T-cell immune responses against the inoculated antigen. At the periphery, DCs have the role of sentinels that capture and process antigens for presentation by major histocompatibility complex (MHC) molecules to T cells. MHC-I molecules present antigens to CD8+ T cells, whereas MHC-II molecules are specifically recognized by CD4+ T cells.1 The immune response induced by DCs depends on the environment in which the antigen is captured. In the absence of inflammatory signals, DCs are largely tolerogenic, leading to dampened T-cell responses.2 In contrast, in response to infection, inflammation or vaccine adjuvant, DCs undergo maturation that is critical to induce effective T-cell immunity. Mature DCs upregulate MHC molecules and co-stimulatory molecules and produce a variety of cytokines. Together with antigen presentation by MHC molecules, this maturation process results in the activation of naive T cells and their differentiation into effector T cells.1
The critical role of DCs in T-cell immunity and the increasing knowledge in DC biology have supported the development of DC-based vaccination strategies in recent years. A direct approach has been attempted where DCs are generated in vitro and loaded with tumor antigen before their autologous transfer into cancer patients. Aside from some minor success in clinical trials, this procedure remains laborious and onerous.3 A more simple and promising approach is based on the delivery of antigen to DC in situ.4 To this end, the desired antigen is conjugated to a monoclonal antibody that is specific for an endocytic receptor expressed by the DC. This coupling can be achieved chemically, but the reaction may impair the quality of the complexes. A better option is for genetic fusion of the antigen to the Fc portion of the antibody heavy chain. These antibodies are internalized by DCs, trafficked and degraded in the endocytic pathways, with antigenic peptides loaded onto MHC-I and/or MHC-II molecules. The benefit of using monoclonal antibodies for vaccination is that the antigen is optimally delivered to the antigen-presenting cells. Furthermore, this approach enables vaccine customization by targeting particular receptors expressed by specialized DC subsets to thereby induce the desired immune outcome. Importantly, endocytic receptors have differential potential to effectively induce T-cell immunity in the setting of DC targeting. In this review, we describe the human and mouse DC subsets and the antigen presentation pathways that interplay with DC targeting. We also focus on DEC205, Clec9A and Clec12A, DC receptors that are promising target for antibody-based vaccination. We finally examine whether specific intracellular trafficking properties of the targeted DC receptors are associated with enhanced antigen presentation outcomes.
Dendritic cell subsets
Using DC targeting for vaccination requires in the first instance an overview of the different mouse and human DC subsets and their functions in immunity. Although extensive work has been undertaken in characterizing mouse DC subsets,5 their human counterparts have remained difficult to define due to their paucity in blood and the difficulty in accessing human lymphoid organs. However, recent reports from several leading laboratories have now identified the human equivalents to mouse DCs.
Mouse and human DCs can be divided into two major subsets mostly localized in lymphoid tissues: the plasmacytoid DC (pDC) and the conventional/myeloid DC (mDC). Mouse and human pDCs have an important role during viral infection by producing large amounts of type I interferon in response to toll-like receptor (TLR)-7 and TLR-9 ligation.6 In contrast, pDC have poor antigen presentation capacity, although several studies have demonstrated that antigen targeting to endocytic receptors at the surface of pDCs induces high MHC-I and MHC-II antigen presentation.7, 8 Interestingly, mouse pDC have also been associated with tolerance.9, 10 Whether a similar tolerogenic function exists in human pDCs remains unknown.
Mouse mDCs comprise two major subsets: CD8+ DCs (and migratory CD103+ DCs) and CD8− DCs. Their human counterparts are defined as CD141+ (also named as blood DC antigen (BDCA)3+) DCs and CD1c+ (also known as BDCA1+) DCs, respectively. Common surface markers of mouse CD8+ DCs and human CD141+ DCs include the C-type lectin receptor Clec9A11, 12, 13, 14 and the chemokine (C motif) receptor 1 XCR1.15 Furthermore, both subsets share the expression of the transcription factors, interferon regulatory factor (IRF) 813 and basic leucine zipper transcription factor, ATF-like 3 (BATF3).14 Mouse CD8− DCs and human CD1c+ DCs have a highly similar transcriptional program, and both subsets express the transcription factor IRF4.16 The antigen presentation function by DCs is clearly divided between the two mouse subsets, with CD8+ DCs and CD103+ DCs having superior capacities to induce CD8+ T-cell immune responses via the presentation of extracellular antigen by MHC-I molecules (termed cross-presentation). In contrast, mouse CD8− DCs are specialized in CD4+ T-cell priming by capturing and processing extracellular antigens for MHC-II presentation.1 Such a division of antigen presentation capacity does not exist between human mDCs. Indeed, both CD141+ and CD1c+ DCs robustly cross-present antigen on MHC-I molecules.13, 14, 15, 17, 18 Furthermore, both human subsets are equally competent in MHC-II presentation.18, 19, 20
Skin contains additional DC subsets to those found in the lymphoid organs. Langerhans cells (LCs) reside both in mouse and human epidermis, and are characterized by the expression of langerin and E-cadherin.21 Mouse and human LCs are strong stimulators of CD4+ T cells.22, 23 However, whereas mouse and human LCs are strong cross-presenting cells ex vivo,24, 25 there is conflicting evidence regarding their in vivo cross-presentation capacity.26, 27 Human skin also contains CD14+ dermal DCs that closely resemble monocyte-derived macrophages.28 This subset seems to be specialized in the humoral immune response;25 however, CD14+ dermal DCs are poor stimulators of allogenic T cells and rather induce regulatory T cells.29, 30
Finally, inflammatory DCs are present in inflamed tissues and draining lymphoid organs. The mouse subset originates from monocytes and expresses macrophage-specific markers such as F4/80, CD64 and the high-affinity IgE receptor, Fc RI.31 The human counterpart, that has been identified in different inflammatory tissues has a transcriptome that closely resembles macrophages and therefore this subset is also likely derived from monocytes.32 Human monocyte-derived DCs (moDCs), that are differentiated in vitro from the culture of monocytes with granulocyte–macrophage colony-stimulating factor and interleukin (IL)-4, are also closely related to inflammatory DCs found in organs.32 Mouse inflammatory DCs activate CD4+ T cells and drive their polarization into T-helper cells (Th) 1 or Th 2.33, 34 This subset is also able to cross-present antigen.35, 36 In humans, inflammatory DCs stimulate autologous CD4+ T cells and induce IL-17 secretion, although their ability for cross-presentation remains unknown.32
RI.31 The human counterpart, that has been identified in different inflammatory tissues has a transcriptome that closely resembles macrophages and therefore this subset is also likely derived from monocytes.32 Human monocyte-derived DCs (moDCs), that are differentiated in vitro from the culture of monocytes with granulocyte–macrophage colony-stimulating factor and interleukin (IL)-4, are also closely related to inflammatory DCs found in organs.32 Mouse inflammatory DCs activate CD4+ T cells and drive their polarization into T-helper cells (Th) 1 or Th 2.33, 34 This subset is also able to cross-present antigen.35, 36 In humans, inflammatory DCs stimulate autologous CD4+ T cells and induce IL-17 secretion, although their ability for cross-presentation remains unknown.32
Antigen presentation pathways
A comprehensive understanding of the antigen-processing pathways, downstream of the delivery of antigen to DC, is also critical in the design of effective DC-targeted vaccines. For MHC-II antigen presentation antigen is endocytosed and transported to late endosomes/lysosomes, where it is degraded in the acidic lumen by lysosomal proteases. Resulting peptides are loaded onto MHC-II molecules by the chaperone human leukocyte antigen DM (HLA-DM) in the endosomal compartment and MHC-II-peptide complexes are exported to the cell surface. MHC-II antigen presentation is a well-studied pathway and has been the subject of several excellent reviews, for example.37, 38, 39 In contrast, to MHC-II presentation, less is known about the trafficking of endocytosed antigen for presentation via MHC-I molecules (cross-presentation). Two major routes have been proposed referred to as the ‘cytosolic' and ‘vacuolar' pathways. The cytosolic cross-presentation pathway involves the endocytosed antigen being exported from the endosomes into the cytosol for proteasomal degradation. How antigen is transported through the endosomal membrane is not well understood. Reduction of disulfide bonds and unfolding of the endocytosed antigen is a prerequisite for antigen export to the cytosol,40 with the antigen then being rapidly refolded in the cytosol, a process that can be facilitated by heat-shock protein 90.41, 42 A role for the endoplasmic reticulum (ER)-associated degradation (ERAD) machinery has been proposed to export the endocytosed antigen through the endosomal membrane. This was based on the presence of ER-resident proteins at the surface of phagosomes, including the translocon Sec61.43 A functional study also suggested that p97, a cytosolic ATPase essential for ERAD, was also involved in protein translocation.44 After antigen breakdown by the proteasome, resulting peptides are transported through the transporter associated with antigen processing (TAP) into the ER for loading onto MHC-I molecules. There is also the possibility that peptides are reimported directly into phagosomes that contain TAP.43, 45 Trafficking of the ER-resident proteins to the endosomal compartment for cross-presentation requires Sec22b, an ER-resident soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE).46 The vacuolar cross-presentation pathway involves the antigen being processed in a proteasome and a TAP-independent mechanism. Proteolysis is achieved by lysosomal proteases and peptides are loaded onto MHC-I molecules that recycle from the cell surface.1, 47, 48
One important feature that distinguishes DCs from other antigen-presenting cells is their reduced level of antigen proteolysis in the endosomal pathway. This unique property is an important contributor to their superior ability for antigen presentation. Although antigen degradation is required for the generation of peptides for MHC molecules, complete proteolytic destruction would eliminate potential antigenic epitopes. DCs, unlike macrophages, display lower expression lysosomal proteases and a higher lysosomal pH, thereby limiting the level of antigen degradation.49, 50 The susceptibility of antigen to lysosomal proteolysis is also a critical factor that determines of MHC-I and MHC-II antigen presentation in murine DCs.50 The degree of antigen proteolysis is considered particularly important for cross-presentation. Indeed, in mice, the presence of lysosomal protease inhibitors enhances antigen cross-presentation by DCs.51, 52 Furthermore, mouse cross-presenting DCs, including CD8+ DCs, specifically express an active system to reduce antigen degradation by overexpressing NADPH oxidase (NOX)-2 at the phagosomal membrane. This NADPH oxidase produces reactive oxygen species that maintain phagosome alkalinization and promote antigen cross-presentation.53, 54 Mouse CD8+ DCs also have lower expression of lysosomal proteases compared to non-cross-presenting CD8− DCs.55 Similar to mouse DCs, human cross-presenting DCs, including CD141+ and CD1c+ DCs, also maintain an alkaline pH in their phagosomal lumen17 and this process occurs in a NOX-2 dependent manner.56
Endocytic receptors of interest for DC targeting
DEC-205
Pioneering work has demonstrated the principle of DC targeting using antigen-conjugated antibodies specific to the C-type lectin receptor DEC-205 (Figure 1). In mice, DEC-205 expression is predominant on thymic epithelial cells and DCs, including CD8+ DCs, dermal DCs and LCs.57 In humans, DEC-205 is expressed at high levels on mDCs and monocytes, at intermediate levels by B cells and at low levels on natural killer cells, T cells and pDCs.58 A major function of this endocytic receptor is suggested to involve binding dying cells for uptake and cross-presentation of debris-associated antigens by DCs.59 DEC-205 also binds to the class B CpG oligonucleotide, a synthetic TLR-9 ligand, and enables its uptake.60 Several reports have demonstrated that ex vivo targeting of mouse DCs, and more specifically CD8+ DCs, with ovalbumin (OVA)-conjugated anti-DEC-205 antibodies induces robust MHC-I cross-presentation to OVA-specific CD8+ T cells. Furthermore, these conjugates elicit high OVA presentation by MHC-II molecules, and this process is enhanced following targeting of CD8− DCs, despite their low expression of DEC205, relative to CD8+ DCs.55, 61, 62, 63 In vivo, injection of OVA-conjugated rat anti-DEC205 antibodies into mice, in the presence of an adjuvant, also induces substantial proliferation and accumulation of OVA-specific naive CD8+ and CD4+ T cells, leading to their differentiation into effector T cells.55, 61, 62, 63, 64 Interestingly, this process is concomitant with prolonged antigen presentation by MHC-I, but not by MHC-II molecules.63 Injected mice also display a strong humoral response with high levels of anti-rat and anti-OVA antibodies detected in the serum.64 Using irradiated mice reconstituted with equal ratios of DEC-205+/+ and DEC-205−/− bone marrow, we have shown that only targeted DCs, but not non-targeted DCs, are responsible for MHC-I and MHC-II presentation in response to DEC205 targeting in vivo.65 Importantly, the administration of an adjuvant, along with antigen-conjugated anti-DEC-205 antibodies, is a prerequisite to induce a robust T-cell immune response.55, 61, 62, 63, 64 In the absence of a DC maturation signal, the delivery of antigens to DEC-205 in vivo is associated with T-cell tolerance. In this case, an initial expansion of antigen-specific CD8+ and CD4+ T cells occurs, but these cells do not differentiate into effector T cells and are ultimately deleted.55, 66 Furthermore, mice that are immunized in these settings become unresponsive to rechallenge with the same unconjugated antigen.62 Interestingly, Mahnke et al.67 have reported that DEC-205 targeting under these conditions leads to the activation of CD25+ regulatory T cells, again suggesting the induction of peripheral T-cell tolerance. For B-cell-mediated immunity induced by DEC205 targeting, the requirement for an adjuvant remains unclear. Indeed, although in some studies a potent anti-rat humoral response is observed following adjuvant-free immunization with rat anti-DEC205 antibodies,68 others report very poor antibody responses in mice immunized in a similar manner.64 This may be due to the specific antibody used for targeting given that in the absence of adjuvant, significant differences in the IgG response to different anti-DEC205 antibodies are observed.68 This suggests that targeting outcomes will be impacted by inherent properties of the targeting antibodies themselves.
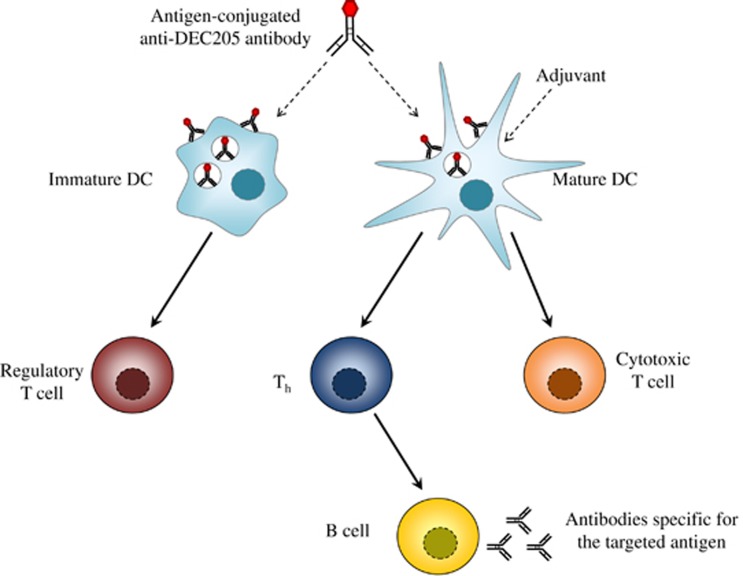
Immune responses elicited by antigen targeting to DEC-205. Adjuvant-free immunization of mice with antigen-conjugated anti-DEC-205 antibodies leads to regulatory T-cell-dependent tolerance. In contrast, co-injection of an adjuvant with anti-DEC-205 primes a robust cytotoxic T-cell immune response. It also strongly activates a CD4+ T-cell immune response leading to generation of Th cells that support the humoral response.
Most vaccination strategies are based on intradermal or intramuscular inoculation of the antigen, raising the question of the fate of the antigen-conjugated anti-DEC-205 antibodies injected via this route. A few hours after deposition under the skin, conjugates are detected at the surface of CD8+ DCs in both lymph nodes and spleen, leading to systemic antigen presentation by MHC-I and MHC-II molecules.63 In particular, the CD8+ DC subset in the skin-draining lymph nodes is targeted via the passive transport of antibodies.69 Furthermore, injected anti-DEC-205 antibodies also bind epidermal LCs and dermal DCs (encompassing langerin+ and langerin− DC). These cells migrate to the skin-draining lymph nodes, a process that is further enhanced by skin inflammation.69, 70 However, the depletion of langerin+ cells in mice does not impair the elicited CD8+ T cell immune response, raising the possibility that migrating dermal langerin− DCs and CD8+ DCs are sufficient to cross-prime CD8+ T cells in the settings of DEC-205 targeting.69
Evidence suggests that targeting DEC-205 on human DC elicits robust activation of T cells. For instance, human moDCs stained ex vivo with anti-DEC-205 antibodies conjugated to the human immunodeficiency virus (HIV) gag protein p24 are strong inducers of CD8+ T-cell proliferation and interferon-γ secretion.71 Furthermore, Birkholz et al. have genetically fused the single-chain fragment from a DEC-205 antibody to the tumor antigen melanoma-associated antigen 3. Incubation of human moDCs with this construct leads to increased secretion of IL-2 by antigen-specific CD4+ T cells compared with antigen-electroporated or peptide-pulsed moDCs.72 Others have shown that the delivery of the cancer testis antigen NY-ESO-1 to human moDCs via DEC-205 results in strong stimulation of antigen-specific CD8+ T cells compared with cells pulsed with unconjugated antigen. However, in this case no enhancement of CD4+ T-cell activation was reported in response to DEC-205 targeting.73 Importantly, few studies have used CD141+ and CD1c+ DCs, the human counterparts of mouse CD8+ and CD8− DC, to investigate antigen presentation outcomes in response to DEC-205 targeting. These two DC subsets internalize similar levels of anti-DEC-205 antibodies; however, delivering antigen to DEC-205 on CD141+ DCs, but not CD1c+ DCs, elicits higher antigen cross-presentation.19, 74 Altogether, these data demonstrate that, similar to mouse DCs, targeting antigens to DEC-205 on specific human DC subsets enhances antigen presentation outcomes.
A large body of evidence illustrates the effectiveness of DEC-205 targeting in mice to prevent the development of infectious diseases or cancer. For instance, mice that are immunized with a single dose of OVA-conjugated anti-DEC-205 antibodies, together with an adjuvant, become resistant to subsequent infection by OVA-expressing vaccinia virus, as evidenced by a reduction in virus titer and the absence of weight loss.63 Furthermore, mice that are similarly immunized and subsequently challenged with OVA-expressing tumor cells reject the tumors.63 Interestingly, this immunization strategy is also protective even if the injection is done with mice that already possess tumors nodules. Therefore, antigen-conjugated anti-DEC-205 antibodies can be used both as a prophylactic and therapeutic vaccination strategy.75 In conclusion, delivering antigen to DEC-205, along with adjuvant, is a strategy of choice to elicit strong T- and B-cell immune responses with promising applications in vaccination.
Clec9A
Clec9A (also known as DNGR-1) is a C-type lectin-like receptor. In mice, its expression is restricted to DCs with high levels measured on CD8+ DCs and lower levels on pDCs.11, 12 In humans, Clec9A is highly expressed by CD141+ DCs, the human equivalents of the CD8+ DCs, however, not by pDC.11, 12 Clec9A is a critical receptor for cross-presentation of dead cell-associated antigen by DC.76, 77 This process relies on recognition of an actin-containing cytoskeletal structure that is exposed on apoptotic and necrotic cells when the cell membrane is ruptured.77, 78 Interestingly, several studies have exploited the natural function of Clec9A in antigen presentation, using it as a target receptor for vaccine enhancement (Figure 2). In vivo injection of anti-Clec9A antibodies specifically labels CD8+ DCs and pDCs.11, 79, 80 To determine whether Clec9A was a promising receptor for DC targeting, OVA was genetically conjugated to anti-Clec9A antibodies and the resulting conjugates administrated to mice. At steady state, these conjugates induce the proliferation of OVA-specific transgenic CD8+ and CD4+ T cells, showing that antigen targeted to Clec9A is efficiently processed and presented by MHC-I and MHC-II molecules.12 Furthermore, Sancho et al.11 have shown that delivering OVA to DCs via Clec9A in vivo, together with an adjuvant, leads to a cytotoxic T lymphocyte response that actively suppresses OVA-expressing lung metastases. Similar results are observed when the vaccine is administered prior or after tumor challenge, showing that targeting antigen to Clec9A can be used as a vaccine for prophylaxis or immunotherapy.11 In another report, Joffre et al. highlight that in vivo injection of anti-Clec9A antibodies conjugated to an MHC-II-binding OVA peptide, together with an adjuvant, leads to robust CD4+ T-cell priming. In this case, the nature of the adjuvant used dictates the polarization of effector CD4+ T cells. For instance, poly I:C induces the differentiation of activated CD4+ T cells into Th1, leading to a strong humoral response. In contrast, curdlan co-injection primes a Th17 immune response.79 Adjuvant-free immunization with anti-Clec9A antibodies is reported to give rise to Foxp3+ T cells and to reduce antibody production, suggesting the induction of tolerance.79 However, in direct contrast to these data, other authors report that antigen delivery via Clec9A enhances the humoral response even in the absence of adjuvant. Mice injected with rat anti-Clec9A antibodies conjugated to OVA produce high-serum titers of anti-rat IgG and anti-OVA antibodies at steady state.12, 64 Furthermore, the use of Myd88−/− TRIF−/− mice, deficient in TLR signaling, does not impair this humoral response, ruling out possible endotoxin contamination of the anti-Clec9A antibodies acting to compensate for the absence of adjuvant.12 Park et al. have also measured the humoral response upon adjuvant-free injection of rat anti-Clec9A antibodies conjugated to hapten nitrophenol (NP). In line with the previous data, high and prolonged levels of anti-rat IgG and anti-NP antibodies are detected in the serum, encompassing all IgG isotypes.81 A strong anti-rat IgG humoral response was also induced in non-human primates immunized with rat anti-Clec9A antibodies only.82 The discrepancy between studies regarding the requirement of an adjuvant for the antibody response following Clec9A targeting may originate in the type of injected antibody. Joffre et al.79 injected a rat IgG1 anti-Clec9A antibody, whereas others have used a rat IgG2a isotype12, 64, 81, 82 that is inherently more immunogenic.82 In contrast, the adjuvant-free immunogenicity of the anti-Clec9A antibodies cannot be predicted from the targeted region of the receptor, the binding capacities to the target in vivo or the persistence of the targeting antibodies in the serum.82
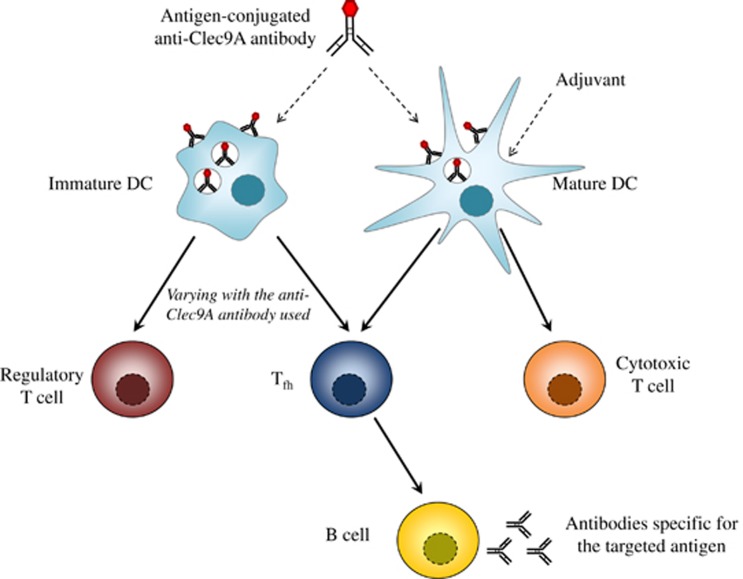
Immune responses elicited by antigen targeting to Clec9A. At steady state, some antigen-conjugated anti-Clec9A antibodies generate regulatory T cells that lead to tolerance. Alternatively, other anti-Clec9A antibodies activate a robust humoral response that involves the production of Tfh. Antigen delivery to DCs via Clec9A also elicits a strong CTL immune response that requires adjuvant administration.
The mechanism responsible for the potent humoral response induced by adjuvant-free Clec9A targeting has been deciphered. Targeting of NP to Clec9A induces transient formation of germinal centers, maturation of antibody affinity and generation of memory B cells that, altogether, characterizes a follicular response.81 In line with this data, mice immunized with OVA-conjugated anti-Clec9A antibodies in the absence of adjuvant produce high numbers of T-follicular helper cells (Tfh) that promote antibody production.64 These cells are localized in the germinal centers and express the classical Tfh markers, including chemokine (C-X-C motif) receptor 5 (CXCR5) and programmed cell death protein-1 at the cell surface, the transcription factor B-cell lymphoma 6 (Bcl6) and the cytokine IL-21.83 Furthermore, the CD4+ T cells that persist long-term after antigen targeting to Clec9A display high levels of CXCR5 and Bcl6 typical of memory Tfh. After a secondary challenge, these cells rapidly proliferate and differentiate into effector Tfh.83 In conclusion, Clec9A is a receptor that can be exploited to induce robust T- and B-cell immune responses in the settings of antibody-targeted vaccination. Importantly, the elicited humoral response does not require adjuvant administration and involves a follicular response with Tfh production.
Clec12A, like Clec9A, is also a C-type lectin-like receptor that is highly expressed by mouse CD8+ DCs and pDCs.84 Lower expression levels are also found on mouse monocytes, macrophages and B cells.84 In human, Clec12A is similarly expressed by all DC subsets and by monocytes.84 The potential of Clec12A as a target for antibody-based vaccination has been investigated. A strong humoral response is observed in mice immunized with OVA-conjugated anti-Clec12A antibodies, as evidenced by high titers of anti-rat IgG and anti-OVA antibodies.64, 84 Furthermore, targeting OVA to Clec12A induces the proliferation of OVA-specific transgenic CD8+ and CD4+ T cells; however, at a lower extent compared with antigen targeting to DEC-205 or Clec9A.64, 84 However, unlike Clec9A targeting, the B- and T-cell immune responses elicited by Clec12A targeting requires the use of an adjuvant.64, 84 Yet, even with this reagent, targeting Clec12A is inefficient at generating cytotoxic T cells.64 Overall, these results show that delivering antigen to Clec12A does not elicit robust B- and T-cell immune responses, thus limiting interest in its use in antibody-targeted vaccination.
Intracellular trafficking downstream of targeted DC receptors
Endocytic receptors expressed by DCs have differential levels of expression, internalization patterns and downstream trafficking routes, although only several of them elicit high T-cell immune responses in the setting of DC targeting. What parameters drive the ability of the receptors to exert this function? To address this question, several studies have analyzed the intracellular trafficking of DC receptors and compared it with the antigen presentation outcomes when those receptors are targeted.
Intuitively, the amount of internalized antigen should correlate with antigen presentation levels, therefore highly internalized receptors should be privileged targets. However, this concept has been ruled out by several studies. For instance, Chatterjee et al. have measured the uptake of antibodies specific for DEC-205, mannose receptor and CD40 into human CD1c+ and moDCs. Anti-mannose receptor antibody was more efficiently accumulated than anti-CD40 or anti-DEC-205 antibodies, but CD40 was the best receptor to target to induce antigen cross-presentation.74 We have also analyzed the internalization parameters of different endocytic receptors expressed by mouse and human DCs, using a fluorescent DNA-based-coupled probe. By conjugating this probe to antibodies specific for receptors of interest, we have quantitatively measured the amount of antigen delivered into the intracellular compartment via the targeted molecules. Using this methodology, we observed that mature CD8+ DCs internalize a lower antigen load via DEC-205 or CD11c than via CD40. In mature CD8− DC, DEC-205, CD11c and CD40 all deliver a small amount of antigen into the endocytic pathway. However, for both mouse mDC subsets, DEC-205 was superior for MHC-I and MHC-II antigen presentation relative to the antigen load.65 Therefore, the antigen load internalized downstream of the targeted receptor does not influence the level of antigen presentation.
It has also been proposed that the speed of antigen internalization influences processing of the targeted antigen for presentation. In particular, targeting a receptor that is slowly internalized may establish an antigen depot and preserve important MHC-I epitopes from enzymatic degradation, leading to prolonged cross-presentation. This is exemplified by CD40 that is slowly internalized in human CD1c+ DCs and moDCs correlating with robust CD8+ T-cell priming following targeting of this receptor.74 However, other data do not support this notion. For instance, despite being a good target for cross-presentation, DEC-205 is rapidly lost from the cell surface of mouse mDCs7 and of human moDCs,85 suggesting an increased, rather than decreased, speed of internalization. Moreover, we have observed a rapid internalization of DEC-205, Clec9A and CD40 in mouse mDCs, whereas CD11b and CD11c are endocytosed slowly. Consistent with these data, the internalization of DEC-205 in human CD141+ and CD1c+ DCs is also fast, in contrast to CD11c that is endocytosed slowly. Yet, targeting DEC-205 over CD40 and CD11c is the more efficient route at eliciting cross-priming of CD8+ T cells.65 In conclusion, the impact of the speed of targeted receptor internalization on the antigen presentation outcome remains controversial.
The intracellular trafficking route accessed by antigen once inside the cell is a significant factor that will affect the level of antigen presentation. Therefore, it has been proposed that receptor-targeted antigen that traffics to early endosomes with reduced proteolysis is more efficiently processed for MHC-I cross-presentation. In contrast, antigen that homes into late endosomes is actively degraded by lysosomal proteases and is more likely to give rise to epitopes for MHC-II presentation. In line with this concept, internalized CD40, mannose receptor, DC-SIGN and CD11c are observed in early endosomes of human DCs and all are efficient targets to elicit antigen cross-presentation.19, 74, 86 There is evidence that in some settings internalized DEC-205 traffics to late endosomes and that antigen delivered via this route in human CD1c+ DCs and moDCs is poorly cross-presented.19, 74 This, however, contradicts the widely supported role for DEC-205 as a strong receptor to target for cross-presentation. Indeed, exceptions to the paradigm exist. For instance, antigen delivered to early endosomes via CD40 is more efficiently presented on MHC-II molecules than antigen delivered to lysosomes via DEC-205.19, 74 Furthermore, in CD141+ DCs, antigen internalized via DEC-205 also traffics to late endosomes, yet it is still efficiently cross-presented by this DC subset.19 Thus, no clear correlation has been found between the intracellular trafficking of the targeted receptor and the effectiveness of antigen presentation. In conclusion, the amount of internalized receptors, the speed of internalization and the destination of the receptor into the intracellular compartment are not reliable criteria to predict the antigen presentation outcomes in the settings of DC targeting.
Conclusion
Recent advances in the understanding of the complexity of DC subsets and how they perform antigen presentation has provided a rationale for the design of novel vaccination strategies based on antigen-conjugated antibodies. DEC-205 is a very promising target given the robust T-cell and B-cell immune responses elicited in mice upon antigen delivery to this receptor, together with adjuvant. Targeting antigen to human DCs via DEC-205 ex vivo also efficiently primes T cells. Translating these findings into clinical applications is underway with several anti-DEC-205 antibodies being currently tested as prophylactic or therapeutic vaccines (https://clinicaltrials.gov). Clec9A is another target of interest, in particular due to its ability to induce a strong antibody response. This humoral response involves the formation of germinal centers and the generation of Tfh cells. Interestingly, some of the anti-Clec9A antibodies used are active without the need of an adjuvant. This feature is likely related to the immunogenicity of the antibody backbone itself. This property is of interest for future translation into human trials in order to prevent possible side effects associated with adjuvant use.
Understanding the rules that dictate DC targeting effectiveness is critical to the design of antibody-based vaccinations. However, currently no correlations can be drawn between antigen presentation outcomes and the intracellular trafficking of antigen delivered to DCs via specific receptors, including antigen load, speed of receptor internalization or the destination of internalized receptor in the endocytic pathway. Attention should be paid to the targeted DC subset in order to elicit the desired antigen presentation outcome. This is particularly true for the cross-priming of CD8+ T cells by delivering antigen to DC with higher cross-presentation capacities. Other factors may have a role in the effectiveness of DC targeting, such as the inflammatory environment or the type of antibody used. Therefore, more work is required to decipher the molecular and cellular processes that underpin effective DC targeting. This knowledge will likely help to identify novel successful targets for antibody-based vaccination.
References
- Segura E, Villadangos JA. Antigen presentation by dendritic cells in vivo. Curr Opin Immunol 2009; 21: 105–110. [Abstract] [Google Scholar]
- Probst HC, Muth S, Schild H. Regulation of the tolerogenic function of steady-state DCs. Eur J Immunol 2014; 44: 927–933. [Abstract] [Google Scholar]
- Tacken PJ, de Vries IJM, Torensma R, Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol 2007; 7: 790–802. [Abstract] [Google Scholar]
- Caminschi I, Shortman K. Boosting antibody responses by targeting antigens to dendritic cells. Trends Immunol 2012; 33: 71–77. [Abstract] [Google Scholar]
- Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol 2007; 7: 19–30. [Abstract] [Google Scholar]
- Swiecki M, Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol 2015; 15: 471–485. [Europe PMC free article] [Abstract] [Google Scholar]
- Moffat JM, Segura E, Khoury G, Caminschi I, Cameron PU, Lewin SR et al. Targeting antigen to bone marrow stromal cell-2 expressed by conventional and plasmacytoid dendritic cells elicits efficient antigen presentation. Eur J Immunol 2013; 43: 595–605. [Abstract] [Google Scholar]
- Tel J, Benitez-Ribas D, Hoosemans S, Cambi A, Adema GJ, Figdor CG et al. DEC-205 mediates antigen uptake and presentation by both resting and activated human plasmacytoid dendritic cells. Eur J Immunol 2011; 41: 1014–1023. [Abstract] [Google Scholar]
- Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C et al. Plasmacytoid dendritic cells mediate oral tolerance. Immunity 2008; 29: 464–475. [Europe PMC free article] [Abstract] [Google Scholar]
- de Heer HJ, Hammad H, Soullié T, Hijdra D, Vos N, Willart MAM et al. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med 2004; 200: 89–98. [Europe PMC free article] [Abstract] [Google Scholar]
- Sancho D, Mourão-Sá D, Joffre OP, Schulz O, Rogers NC, Pennington DJ et al. Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J Clin Invest 2008; 118: 2098–2110. [Abstract] [Google Scholar]
- Caminschi I, Proietto AI, Ahmet F, Kitsoulis S, Shin Teh J, Lo JCY et al. The dendritic cell subtype-restricted C-type lectin Clec9A is a target for vaccine enhancement. Blood 2008; 112: 3264–3273. [Europe PMC free article] [Abstract] [Google Scholar]
- Poulin LF, Salio M, Griessinger E, Anjos-Afonso F, Craciun L, Chen J-L et al. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8alpha+ dendritic cells. J Exp Med 2010; 207: 1261–1271. [Europe PMC free article] [Abstract] [Google Scholar]
- Poulin LF, Reyal Y, Uronen-Hansson H, Schraml BU, Sancho D, Murphy KM et al. DNGR-1 is a specific and universal marker of mouse and human Batf3-dependent dendritic cells in lymphoid and nonlymphoid tissues. Blood 2012; 119: 6052–6062. [Abstract] [Google Scholar]
- Bachem A, Güttler S, Hartung E, Ebstein F, Schaefer M, Tannert A et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med 2010; 207: 1273–1281. [Europe PMC free article] [Abstract] [Google Scholar]
- Schlitzer A, McGovern N, Teo P, Zelante T, Atarashi K, Low D et al. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity 2013; 38: 970–983. [Europe PMC free article] [Abstract] [Google Scholar]
- Segura E, Durand M, Amigorena S. Similar antigen cross-presentation capacity and phagocytic functions in all freshly isolated human lymphoid organ-resident dendritic cells. J Exp Med 2013; 210: 1035–1047. [Europe PMC free article] [Abstract] [Google Scholar]
- Segura E, Valladeau-Guilemond J, Donnadieu M-H, Sastre-Garau X, Soumelis V, Amigorena S. Characterization of resident and migratory dendritic cells in human lymph nodes. J Exp Med 2012; 209: 653–660. [Europe PMC free article] [Abstract] [Google Scholar]
- Cohn L, Chatterjee B, Esselborn F, Smed-Sörensen A, Nakamura N, Chalouni C et al. Antigen delivery to early endosomes eliminates the superiority of human blood BDCA3+ dendritic cells at cross presentation. J Exp Med 2013; 210: 1049–1063. [Europe PMC free article] [Abstract] [Google Scholar]
- Yu CI, Becker C, Metang P, Marches F, Wang Y, Toshiyuki H et al. Human CD141+ dendritic cells induce CD4+ T cells to produce type 2 cytokines. J Immunol 2014; 193: 4335–4343. [Europe PMC free article] [Abstract] [Google Scholar]
- Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol 2008; 8: 935–947. [Abstract] [Google Scholar]
- Igyártó BZ, Haley K, Ortner D, Bobr A, Gerami-Nejad M, Edelson BT et al. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity 2011; 35: 260–272. [Europe PMC free article] [Abstract] [Google Scholar]
- van der Vlist M, de Witte L, de Vries RD, Litjens M, de Jong MAWP, Fluitsma D et al. Human Langerhans cells capture measles virus through Langerin and present viral antigens to CD4+ T cells but are incapable of cross-presentation. Eur J Immunol 2011; 41: 2619–2631. [Abstract] [Google Scholar]
- Stoitzner P, Tripp CH, Eberhart A, Price KM, Jung JY, Bursch L et al. Langerhans cells cross-present antigen derived from skin. Proc Natl Acad Sci USA 2006; 103: 7783–7788. [Europe PMC free article] [Abstract] [Google Scholar]
- Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity 2008; 29: 497–510. [Europe PMC free article] [Abstract] [Google Scholar]
- Holcmann M, Stoitzner P, Drobits B, Luehrs P, Stingl G, Romani N et al. Skin Inflammation Is Not Sufficient to Break Tolerance Induced against a Novel Antigen. J Immunol 2009; 183: 1133–1143. [Abstract] [Google Scholar]
- Bursch LS, Rich BE, Hogquist KA. Langerhans cells are not required for the CD8 T cell response to epidermal self-antigens. J Immunol 2009; 182: 4657–4664. [Europe PMC free article] [Abstract] [Google Scholar]
- McGovern N, Schlitzer A, Gunawan M, Jardine L, Shin A, Poyner E et al. Human dermal CD14+ cells are a transient population of monocyte-derived macrophages. Immunity 2014; 41: 465–477. [Europe PMC free article] [Abstract] [Google Scholar]
- Banchereau J, Thompson-Snipes L, Zurawski S, Blanck J-P, Cao Y, Clayton S et al. The differential production of cytokines by human Langerhans cells and dermal CD14(+) DCs controls CTL priming. Blood 2012; 119: 5742–5749. [Europe PMC free article] [Abstract] [Google Scholar]
- Morelli AE, Rubin JP, Erdos G, Tkacheva OA, Mathers AR, Zahorchak AF et al. CD4+ T cell responses elicited by different subsets of human skin migratory dendritic cells. J Immunol 2005; 175: 7905–7915. [Abstract] [Google Scholar]
- Segura E, Amigorena S. Inflammatory dendritic cells in mice and humans. Trends Immunol 2013; 34: 440–445. [Abstract] [Google Scholar]
- Segura E, Touzot M, Bohineust A, Cappuccio A, Chiocchia G, Hosmalin A et al. Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity 2013; 38: 336–348. [Abstract] [Google Scholar]
- Nakano H, Lin KL, Yanagita M, Charbonneau C, Cook DN, Kakiuchi T et al. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat Immunol 2009; 10: 394–402. [Europe PMC free article] [Abstract] [Google Scholar]
- Kool M, Soullié T, van Nimwegen M, Willart MAM, Muskens F, Jung S et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med 2008; 205: 869–882. [Europe PMC free article] [Abstract] [Google Scholar]
- Ballesteros-Tato A, León B, Lund FE, Randall TD. Temporal changes in dendritic cell subsets, cross-priming and costimulation via CD70 control CD8(+) T cell responses to influenza. Nat Immunol 2010; 11: 216–224. [Europe PMC free article] [Abstract] [Google Scholar]
- Segura E, Albiston AL, Wicks IP, Chai SY, Villadangos JA. Different cross-presentation pathways in steady-state and inflammatory dendritic cells. Proc Natl Acad Sci USA 2009; 106: 20377–20381. [Europe PMC free article] [Abstract] [Google Scholar]
- Roche PA, Furuta K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat Rev Immunol 2015; 15: 203–216. [Abstract] [Google Scholar]
- Watts C. Capture and processing of exogenous antigens for presentation on MHC molecules. Annu Rev Immunol 1997; 15: 821–850. [Abstract] [Google Scholar]
- Villadangos JA, Schnorrer P, Wilson NS. Control of MHC class II antigen presentation in dendritic cells: a balance between creative and destructive forces. Immunol Rev 2005; 207: 191–205. [Abstract] [Google Scholar]
- Singh R, Cresswell P. Defective Cross-Presentation of Viral Antigens in GILT-Free Mice. Science 2010; 328: 1394–1398. [Europe PMC free article] [Abstract] [Google Scholar]
- Giodini A, Cresswell P. Hsp90-mediated cytosolic refolding of exogenous proteins internalized by dendritic cells. EMBO J 2008; 27: 201–211. [Abstract] [Google Scholar]
- Imai T, Kato Y, Kajiwara C, Mizukami S, Ishige I, Ichiyanagi T et al. Heat shock protein 90 (HSP90) contributes to cytosolic translocation of extracellular antigen for cross-presentation by dendritic cells. Proc Natl Acad Sci USA 2011; 108: 16363–16368. [Europe PMC free article] [Abstract] [Google Scholar]
- Houde M, Bertholet S, Gagnon E, Brunet S, Goyette G, Laplante A et al. Phagosomes are competent organelles for antigen cross-presentation. Nature 2003; 425: 402–406. [Abstract] [Google Scholar]
- Ackerman AL, Giodini A, Cresswell P. A role for the endoplasmic reticulum protein retrotranslocation machinery during crosspresentation by dendritic cells. Immunity 2006; 25: 607–617. [Abstract] [Google Scholar]
- Guermonprez P, Saveanu L, Kleijmeer M, Davoust J, van Endert P, Amigorena S. ER–phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature 2003; 425: 397–402. [Abstract] [Google Scholar]
- Cebrian I, Visentin G, Blanchard N, Jouve M, Bobard A, Moita C et al. Sec22b Regulates Phagosomal Maturation and Antigen Crosspresentation by Dendritic Cells. Cell 2011; 147: 1355–1368. [Abstract] [Google Scholar]
- Neefjes J, Jongsma MLM, Paul P, Bakke O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat Rev Immunol 2011; 11: 823–836. [Abstract] [Google Scholar]
- Blum JS, Wearsch PA, Cresswell P. Pathways of antigen processing. Annu Rev Immunol 2013; 31: 443–473. [Europe PMC free article] [Abstract] [Google Scholar]
- Trombetta ES, Ebersold M, Garrett W, Pypaert M, Mellman I. Activation of lysosomal function during dendritic cell maturation. Science 2003; 299: 1400–1403. [Abstract] [Google Scholar]
- Delamarre L, Couture R, Mellman I, Trombetta ES. Enhancing immunogenicity by limiting susceptibility to lysosomal proteolysis. J Exp Med 2006; 203: 2049–2055. [Europe PMC free article] [Abstract] [Google Scholar]
- Accapezzato D, Visco V, Francavilla V, Molette C, Donato T, Paroli M et al. Chloroquine enhances human CD8+ T cell responses against soluble antigens in vivo. J Exp Med 2005; 202: 817–828. [Europe PMC free article] [Abstract] [Google Scholar]
- Belizaire R, Unanue ER. Targeting proteins to distinct subcellular compartments reveals unique requirements for MHC class I and II presentation. Proc Natl Acad Sci USA 2009; 106: 17463–17468. [Abstract] [Google Scholar]
- Savina A, Jancic C, Hugues S, Guermonprez P, Vargas P, Moura IC et al. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell 2006; 126: 205–218. [Abstract] [Google Scholar]
- Savina A, Peres A, Cebrian I, Carmo N, Moita C, Hacohen N et al. The small GTPase Rac2 controls phagosomal alkalinization and antigen crosspresentation selectively in CD8(+) dendritic cells. Immunity 2009; 30: 544–555. [Abstract] [Google Scholar]
- Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S et al. Differential antigen processing by dendritic cell subsets in vivo. Science 2007; 315: 107–111. [Abstract] [Google Scholar]
- Mantegazza AR, Savina A, Vermeulen M, Pérez L, Geffner J, Hermine O et al. NADPH oxidase controls phagosomal pH and antigen cross-presentation in human dendritic cells. Blood 2008; 112: 4712–4722. [Europe PMC free article] [Abstract] [Google Scholar]
- Inaba K, Swiggard WJ, Inaba M, Meltzer J, Mirza A, Sasagawa T et al. Tissue distribution of the DEC-205 protein that is detected by the monoclonal antibody NLDC-145. I. Expression on dendritic cells and other subsets of mouse leukocytes. Cell Immunol 1995; 163: 148–156. [Abstract] [Google Scholar]
- Kato M, McDonald KJ, Khan S, Ross IL, Vuckovic S, Chen K et al. Expression of human DEC-205 (CD205) multilectin receptor on leukocytes. Int Immunol 2006; 18: 857–869. [Abstract] [Google Scholar]
- Shrimpton RE, Butler M, Morel A-S, Eren E, Hue SS, Ritter MA. CD205 (DEC-205): a recognition receptor for apoptotic and necrotic self. Mol Immunol 2009; 46: 1229–1239. [Europe PMC free article] [Abstract] [Google Scholar]
- Lahoud MH, Ahmet F, Zhang J-G, Meuter S, Policheni AN, Kitsoulis S et al. DEC-205 is a cell surface receptor for CpG oligonucleotides. Proc Natl Acad Sci USA 2012; 109: 16270–16275. [Europe PMC free article] [Abstract] [Google Scholar]
- Castro FVV, Tutt AL, White AL, Teeling JL, James S, French RR et al. CD11c provides an effective immunotarget for the generation of both CD4 and CD8 T cell responses. Eur J Immunol 2008; 38: 2263–2273. [Abstract] [Google Scholar]
- Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med 2002; 196: 1627–1638. [Europe PMC free article] [Abstract] [Google Scholar]
- Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S-I, Soares H et al. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med 2004; 199: 815–824. [Europe PMC free article] [Abstract] [Google Scholar]
- Lahoud MH, Ahmet F, Kitsoulis S, Wan SS, Vremec D, Lee C-N et al. Targeting antigen to mouse dendritic cells via Clec9A induces potent CD4 T cell responses biased toward a follicular helper phenotype. J Immunol 2011; 187: 842–850. [Abstract] [Google Scholar]
- Reuter A, Panozza SE, Macri C, Dumont C, Li J, Liu H et al. Criteria for dendritic cell receptor selection for efficient antibody-targeted vaccination. J Immunol 2015; 194: 2696–2705. [Abstract] [Google Scholar]
- Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med 2001; 194: 769–779. [Europe PMC free article] [Abstract] [Google Scholar]
- Mahnke K, Qian Y, Knop J, Enk AH. Induction of CD4+/CD25+ regulatory T cells by targeting of antigens to immature dendritic cells. Blood 2003; 101: 4862–4869. [Abstract] [Google Scholar]
- Pugholm LH, Petersen LR, Søndergaard EKL, Varming K, Agger R. Enhanced Humoral Responses Induced by Targeting of Antigen to Murine Dendritic Cells. Scand J Immunol 2015; 82: 515–522. [Abstract] [Google Scholar]
- Flacher V, Tripp CH, Haid B, Kissenpfennig A, Malissen B, Stoitzner P et al. Skin langerin+ dendritic cells transport intradermally injected anti-DEC-205 antibodies but are not essential for subsequent cytotoxic CD8+ T cell responses. J Immunol 2012; 188: 2146–2155. [Europe PMC free article] [Abstract] [Google Scholar]
- Flacher V, Tripp CH, Stoitzner P, Haid B, Ebner S, Del Frari B et al. Epidermal Langerhans cells rapidly capture and present antigens from C-type lectin-targeting antibodies deposited in the dermis. J Invest Dermatol 2010; 130: 755–762. [Europe PMC free article] [Abstract] [Google Scholar]
- Bozzacco L, Trumpfheller C, Siegal FP, Mehandru S, Markowitz M, Carrington M et al. DEC-205 receptor on dendritic cells mediates presentation of HIV gag protein to CD8+ T cells in a spectrum of human MHC I haplotypes. Proc Natl Acad Sci USA 2007; 104: 1289–1294. [Europe PMC free article] [Abstract] [Google Scholar]
- Birkholz K, Schwenkert M, Kellner C, Gross S, Fey G, Schuler-Thurner B et al. Targeting of DEC-205 on human dendritic cells results in efficient MHC class II-restricted antigen presentation. Blood 2010; 116: 2277–2285. [Abstract] [Google Scholar]
- Tsuji T, Matsuzaki J, Kelly MP, Ramakrishna V, Vitale L, He L-Z et al. Antibody-targeted NY-ESO-1 to mannose receptor or DEC-205 in vitro elicits dual human CD8+ and CD4+ T cell responses with broad antigen specificity. J Immunol 2011; 186: 1218–1227. [Abstract] [Google Scholar]
- Chatterjee B, Smed-Sörensen A, Cohn L, Chalouni C, Vandlen R, Lee B-C et al. Internalization and endosomal degradation of receptor-bound antigens regulate the efficiency of cross presentation by human dendritic cells. Blood 2012; 120: 2011–2020. [Abstract] [Google Scholar]
- Mahnke K, Qian Y, Fondel S, Brueck J, Becker C, Enk AH. Targeting of antigens to activated dendritic cells in vivo cures metastatic melanoma in mice. Cancer Res 2005; 65: 7007–7012. [Abstract] [Google Scholar]
- Sancho D, Joffre OP, Keller AM, Rogers NC, Martínez D, Hernanz-Falcón P et al. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature 2009; 458: 899–903. [Europe PMC free article] [Abstract] [Google Scholar]
- Zhang J-G, Czabotar PE, Policheni AN, Caminschi I, Wan SS, Kitsoulis S et al. The dendritic cell receptor Clec9A binds damaged cells via exposed actin filaments. Immunity 2012; 36: 646–657. [Abstract] [Google Scholar]
- Ahrens S, Zelenay S, Sancho D, Hanč P, Kjær S, Feest C et al. F-actin is an evolutionarily conserved damage-associated molecular pattern recognized by DNGR-1, a receptor for dead cells. Immunity 2012; 36: 635–645. [Abstract] [Google Scholar]
- Joffre OP, Sancho D, Zelenay S, Keller AM, Reis e Sousa C. Efficient and versatile manipulation of the peripheral CD4+ T-cell compartment by antigen targeting to DNGR-1/CLEC9A. Eur J Immunol 2010; 40: 1255–1265. [Europe PMC free article] [Abstract] [Google Scholar]
- Idoyaga J, Lubkin A, Fiorese C, Lahoud MH, Caminschi I, Huang Y et al. Comparable T helper 1 (Th1) and CD8 T-cell immunity by targeting HIV gag p24 to CD8 dendritic cells within antibodies to Langerin, DEC205, and Clec9A. Proc Natl Acad Sci USA 2011; 108: 2384–2389. [Europe PMC free article] [Abstract] [Google Scholar]
- Park H-Y, Light A, Lahoud MH, Caminschi I, Tarlinton DM, Shortman K. Evolution of B cell responses to Clec9A-targeted antigen. J Immunol 2013; 191: 4919–4925. [Abstract] [Google Scholar]
- Li J, Ahmet F, Sullivan LC, Brooks AG, Kent SJ, De Rose R et al. Antibodies targeting Clec9A promote strong humoral immunity without adjuvant in mice and non-human primates. Eur J Immunol 2015; 45: 854–864. [Abstract] [Google Scholar]
- Kato Y, Zaid A, Davey GM, Mueller SN, Nutt SL, Zotos D et al. Targeting Antigen to Clec9A Primes Follicular Th Cell Memory Responses Capable of Robust Recall. J Immunol 2015; 195: 1006–1014. [Abstract] [Google Scholar]
- Lahoud MH, Proietto AI, Ahmet F, Kitsoulis S, Eidsmo L, Wu L et al. The C-type lectin Clec12A present on mouse and human dendritic cells can serve as a target for antigen delivery and enhancement of antibody responses. J Immunol 2009; 182: 7587–7594. [Abstract] [Google Scholar]
- Butler M, Morel A-S, Jordan WJ, Eren E, Hue S, Shrimpton RE et al. Altered expression and endocytic function of CD205 in human dendritic cells, and detection of a CD205-DCL-1 fusion protein upon dendritic cell maturation. Immunology 2007; 120: 362–371. [Abstract] [Google Scholar]
- Tacken PJ, Ginter W, Berod L, Cruz LJ, Joosten B, Sparwasser T et al. Targeting DC-SIGN via its neck region leads to prolonged antigen residence in early endosomes, delayed lysosomal degradation, and cross-presentation. Blood 2011; 118: 4111–4119. [Abstract] [Google Scholar]
Articles from Clinical & Translational Immunology are provided here courtesy of Australasian Society of Immunology
Full text links
Read article at publisher's site: https://doi.org/10.1038/cti.2016.6
Read article for free, from open access legal sources, via Unpaywall:
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1038/cti.2016.6
Citations & impact
Impact metrics
Article citations
Recent Review on Biological Barriers and Host-Material Interfaces in Precision Drug Delivery: Advancement in Biomaterial Engineering for Better Treatment Therapies.
Pharmaceutics, 16(8):1076, 16 Aug 2024
Cited by: 0 articles | PMID: 39204421 | PMCID: PMC11360117
Review Free full text in Europe PMC
Crosstalking with Dendritic Cells: A Path to Engineer Advanced T Cell Immunotherapy.
Front Syst Biol, 4:1372995, 29 Apr 2024
Cited by: 0 articles | PMID: 38911455 | PMCID: PMC11192543
FcRn regulates antigen presentation in dendritic cells downstream of DEC205-targeted vaccines.
NPJ Vaccines, 9(1):76, 09 Apr 2024
Cited by: 1 article | PMID: 38594284 | PMCID: PMC11003989
Single-shot dendritic cell targeting SARS-CoV-2 vaccine candidate induces broad, durable and protective systemic and mucosal immunity in mice.
Mol Ther, 32(7):2299-2315, 06 May 2024
Cited by: 1 article | PMID: 38715364
Immunoprotection of FliBc chimeric fiber2 fusion proteins targeting dendritic cells against Fowl adenovirus serotype 4 infection.
Poult Sci, 103(4):103474, 08 Feb 2024
Cited by: 1 article | PMID: 38387285 | PMCID: PMC10899072
Go to all (94) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Antibody-mediated targeting of antigen to C-type lectin-like receptors Clec9A and Clec12A elicits different vaccination outcomes.
Mol Immunol, 81:143-150, 12 Dec 2016
Cited by: 10 articles | PMID: 27978488
Direct Delivery of Antigens to Dendritic Cells via Antibodies Specific for Endocytic Receptors as a Promising Strategy for Future Therapies.
Vaccines (Basel), 4(2):E8, 28 Mar 2016
Cited by: 43 articles | PMID: 27043640 | PMCID: PMC4931625
Review Free full text in Europe PMC
CLEC12A-Mediated Antigen Uptake and Cross-Presentation by Human Dendritic Cell Subsets Efficiently Boost Tumor-Reactive T Cell Responses.
J Immunol, 197(7):2715-2725, 26 Aug 2016
Cited by: 29 articles | PMID: 27566820
Enhanced effects of DNA vaccine against botulinum neurotoxin serotype A by targeting antigen to dendritic cells.
Immunol Lett, 190:118-124, 09 Aug 2017
Cited by: 6 articles | PMID: 28802641





