Abstract
Free full text

The MERTK/FLT3 inhibitor MRX-2843 overcomes resistance-conferring FLT3 mutations in acute myeloid leukemia
Abstract
FMS-like tyrosine kinase 3–targeted (FLT3-targeted) therapies have shown initial promise for the treatment of acute myeloid leukemia (AML) expressing FLT3-activating mutations; however, resistance emerges rapidly. Furthermore, limited options exist for the treatment of FLT3-independent AML, demonstrating the need for novel therapies that reduce toxicity and improve survival. MERTK receptor tyrosine kinase is overexpressed in 80% to 90% of AMLs and contributes to leukemogenesis. Here, we describe MRX-2843, a type 1 small-molecule tyrosine kinase inhibitor that abrogates activation of both MERTK and FLT3 and their downstream effectors. MRX-2843 treatment induces apoptosis and inhibits colony formation in AML cell lines and primary patient samples expressing MERTK and/or FLT3-ITD, with a wide therapeutic window compared with that of normal human cord blood cells. In murine orthotopic xenograft models, once-daily oral therapy prolonged survival 2- to 3-fold over that of vehicle-treated controls. Additionally, MRX-2843 retained activity against quizartinib-resistant FLT3-ITD–mutant proteins with clinically relevant alterations at the D835 or F691 loci and prolonged survival in xenograft models of quizartinib-resistant AML. Together, these observations validate MRX-2843 as a translational agent and support its clinical development for the treatment of AML.
Introduction
Acute myeloid leukemia (AML) continues to have a poor prognosis, despite therapeutic advancements over the past several decades, as survival rates are only 60% to 70% in pediatric patients and less than 50% in adult patients (1, 2). Older patients have progressively worse outcomes that are at least partly due to an inability to tolerate aggressive cytotoxic chemotherapy (3). In pediatric patients, these therapies can lead to devastating long-term side effects, including growth hormone deficiency, neurocognitive abnormalities, and infertility (4). These observations demonstrate the need for novel, better-tolerated therapies in AML patients and have led to the development of a number of targeted agents over the past decade. For instance, numerous small-molecule inhibitors targeting the often-mutated FMS-like tyrosine kinase 3 (FLT3) are currently in clinical trials.
Internal tandem duplication (ITD) mutations that confer constitutive kinase activation in FLT3 (FLT3-ITD) are observed in 20% to 30% of adults and 10% to 15% of children with AML and are associated with poor prognosis (5, 6). Although many patients have a good initial response to FLT3 inhibition, sustained responses have been less successful. In a phase II study of quizartinib monotherapy in patients with relapsed or refractory FLT3-ITD AML, a 44% composite complete remission was achieved; however, development of resistance was rapid, and the median duration of response was only approximately 11 weeks (7). Subsequent studies utilizing saturation mutagenesis in cell culture assays identified 3 residues in the FLT3 kinase domain that conferred resistance to quizartinib — 2 within the kinase activation loop (D835 and Y842) and 1 at a gatekeeper position (F691). Mutations at 2 of these loci — D835 and F691 — were subsequently identified in patients who relapsed while on quizartinib monotherapy, confirming the clinical relevance of these loci (8). In addition, the acquisition of mutations at D835 has also been reported after treatment with sorafenib, a first-generation FLT3 inhibitor (9). Although tyrosine kinase inhibitors (TKIs) that retain activity against either the D835Y activation loop mutation (crenolanib) or the F691L gatekeeper mutation (ponatinib) have been developed, inhibitors that retain equal activity against both mutations have not been reported (10, 11). While these previous studies have validated FLT3 inhibition as a therapeutic strategy for the treatment of patients with AML, more effective agents targeting FLT3-ITD are needed.
Similarly, therapeutic agents directed against novel targets may augment the response to FLT3 inhibitors and are also needed for treatment of AMLs without a FLT3-ITD mutation. MERTK is a receptor tyrosine kinase that is ectopically expressed in the majority of acute leukemias, and increasing evidence suggests a role for MERTK in multiple solid tumors (12–18). In AML, MERTK is overexpressed on more than 80% of pediatric and adult patient samples relative to normal bone marrow precursor cells (13, 14). Importantly, shRNA-mediated inhibition of MERTK in AML led to decreased signaling through prosurvival pathways, inhibited colony formation, induced apoptosis, and prolonged survival in murine models (13), indicating that MERTK inhibition has therapeutic potential.
We have previously described UNC1666, a first-generation small-molecule inhibitor of both MERTK and FLT3 with potent antileukemia activity (19). Unfortunately, this compound has a limited half-life in animal models and is therefore not appropriate for clinical development. Here, we report preclinical testing of MRX-2843, an orally available small-molecule inhibitor of both MERTK and FLT3 (20). We demonstrate potent antileukemia activity mediated by MRX-2843 in MERTK-dependent and FLT3-ITD models of AML. Moreover, MRX-2843 retains the ability to inhibit activation of both quizartinib-resistant FLT3-ITD D835Y- and F691L-mutant proteins and mediates potent antileukemia activity when either of these clinically relevant FLT3 point mutations is present. Combined, these data form the basis for further exploration of the clinical utility of MRX-2843 in AML.
Results
MRX-2483 is a novel small-molecule inhibitor with selectivity for MERTK and FLT3.
We have previously reported the synthesis and in vivo pharmacokinetic parameters of TKIs of MERTK, including MRX-2843 (Compound 12; ref. 20). MRX-2843 is an orally bioavailable ATP-competitive type 1 TKI with high potency against both MERTK and FLT3 in enzymatic assays (Figure 1A) and with selectivity for these kinases over the other members of the TAM-family, AXL and TYRO-3, and other relevant tyrosine kinases (Supplemental Table 1; supplemental material available online with this article; 10.1172/jci.insight.85630DS1). In contrast, UNC2369 (Compound 20; ref. 20) has a similar structure but much lower potency against MERTK and FLT3 and was used as a negative control TKI in these studies (Figure 1B). In mice, MRX-2843 is 78% orally bioavailable at a dose of 3 mg/kg with a Cmax of 1.3 μM and a t1/2 of 4.4 hours (20).
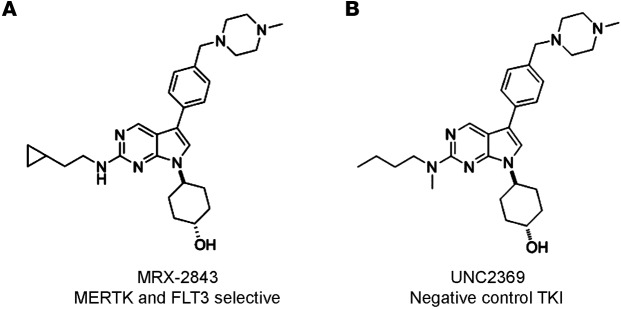
(A) Chemical structure of MRX-2843, with an enzymatic IC50 of 1.3 nM for MERTK and 0.64 nM for FLT3 (B) Chemical structure of UNC2369, which lacks significant activity against MERTK (enzymatic IC50 of 490 nM) or FLT3 (enzymatic IC50 of 360 nM) and was used as a negative control in these studies. FLT3, FMS-like tyrosine kinase 3; TKI, tyrosine kinase inhibitor.
MRX-2843 inhibits MERTK activation and mediates functional antileukemia effects in MERTK-dependent AML models.
Cell lines that express MERTK (19) and have no activating FLT3 mutations (FLT3-WT) were studied to assess the effects of MRX-2843 on MERTK activity and leukemogenic functions. In the Kasumi-1 cell line, treatment with MRX-2843 resulted in dose-dependent inhibition of MERTK phosphorylation (Figure 2A). Decreased phosphorylation was evident at concentrations as low as 10 nM, with near-complete abrogation of MERTK activation at 100 to 300 nM. Similarly, treatment of Kasumi-1 cells with MRX-2843 mediated inhibition of downstream signaling through pathways important for tumor cell survival and proliferation. Phosphorylation inhibition of ERK1/2, AKT, and STAT6, signaling proteins known to be regulated downstream of MERTK (13), was inhibited in a dose-dependent fashion similar to the dose (25–300 nM) required for MERTK phosphorylation inhibition (Figure 2B). In contrast, ERK1/2, AKT, and STAT6 phosphorylation was not affected by treatment with the control TKI (Figure 2, A and B).

(A–D) Kasumi-1 and/or NOMO-1 AML cells were treated with the indicated concentrations of MRX-2843, vehicle (DMSO), or a nontargeting control TKI (Ctrl TKI). (A) After 1 hour, Kasumi-1 cells were treated with pervanadate phosphatase inhibitor for 10 minutes to stabilize p-MERTK, and cell lysates were prepared. MERTK protein was immunoprecipitated, and p-MERTK and total MERTK were detected by immunoblot analysis. (B) After 2 hours, lysates were prepared from Kasumi-1 cells without pervanadate treatment, and phosphorylated and total STAT6, AKT, and ERK1/2 proteins were detected by immunoblotting. Actin was used as a loading control. (C) After 48 hours, MTS reagent was added to cultures for an additional 2 hours, and absorbance was determined as an indicator of the number of viable cells. (D) After 72 hours, cells were stained with Yo-Pro-1 iodide and propidium iodide, and apoptotic and dead cells were detected by flow cytometry. ***P < 0.001, by 1-way ANOVA. (E) Kasumi-1 or NOMO-1 cells were cultured in soft agar overlaid with media containing the indicated concentrations of MRX-2843, vehicle (DMSO), or control TKI. Colonies were stained and counted after 14 to 21 days. Results are shown relative to vehicle-treated controls. Mean values and standard errors were derived from 3 independent experiments. **P < 0.01 and ***P < 0.001, by 1-way ANOVA. (F) Kaplan-Meier curve showing survival of NSGS mice with orthotopic NOMO-1 xenografts that were treated with MRX-2843 or vehicle (saline) beginning on day 21 after transplantation. Median survival was 51 days for MRX-2843–treated mice and 37 days for vehicle-treated control mice (n ≥10 per group). P < 0.001, by log-rank test. AML, acute myeloid leukemia; Ctrl TKI, control tyrosine kinase inhibitor; FLT3, FMS-like tyrosine kinase 3; NSGS, NOD-SCID-γ mice expressing Tg human cytokines; p, phosphorylated.
To determine the effect of treatment with MRX-2843 on clonal expansion, Kasumi-1 cells were cultured in the presence of MRX-2843, and cell numbers relative to vehicle-treated cultures were determined. MRX-2843 treatment resulted in a decrease in relative cell numbers, with an IC50 of 143.5 ± 14.1 nM, indicating that MRX-2843 significantly inhibited tumor cell proliferation and/or survival (Figure 2C).
To more directly assess the impact of treatment with MRX-2843 on cell survival, AML cells were treated with MRX-2843, the control TKI, or vehicle (DMSO), and the percentage of apoptotic and dead cells was determined by flow cytometry. Both Kasumi-1 and NOMO-1 cells exhibited a statistically significant induction of cell death that was dose dependent and correlated with the degree of inhibition of MERTK phosphorylation and downstream signaling in Kasumi-1 cells. At doses of 150 nM and 300 nM in the Kasumi-1 line, the fraction of apoptotic and dead cells was 51.9% ± 12.4% and 71.3% ± 2.3%, respectively, compared with 10.6% ± 2.0% in the vehicle-treated cultures (P < 0.001, Figure 2D). Similarly, there were 34.1% ± 5.6% and 67.1% ± 2.7% apoptotic and dead cells in NOMO-1 cultures treated with 150 nM or 300 nM MRX-2843, respectively, compared with 6.8% ± 0.7% in vehicle-treated cultures (P < 0.001, Figure 2D). In contrast, treatment with control TKI had no significant effect on cell survival.
To further evaluate the functional effects of MRX-2843, colony-forming potential was assessed in soft agar cultures. Kasumi-1 or NOMO-1 cells were cultured in equal numbers in soft agar overlaid with medium containing MRX-2843, the control TKI, or vehicle for 14 days, and the colonies were counted. In both cell lines, treatment with MRX-2843 significantly reduced colony numbers relative to those in the vehicle control in a dose-dependent manner (Figure 2E). Treatment with 50 nM and 100 nM MRX-2843 resulted in 62.3% ± 6.4% and 84.1% ± 7.8% inhibition of colony formation, respectively, in Kasumi-1 cultures (P < 0.01). Similarly, in NOMO-1 cultures, colony formation was inhibited by 54.8% ± 18.1% in response to treatment with 100 nM MRX-2843 (P < 0.001, Figure 2E). Treatment with the control TKI had no significant effect.
To determine whether inhibition of oncogenic phenotypes in MERTK-expressing AML cells translates to animal models, orthotopic murine xenografts were established, and the effects of treatment with MRX-2843 on survival were determined. NOMO-1 cells were injected into the tail veins of NOD-SCID-γ (NSG) mice expressing Tg human cytokines (NSGS), and 65 mg/kg MRX-2843 or an equivalent volume of vehicle (saline) was administered once daily by oral gavage starting 21 days after transplantation. Treatment with MRX-2843 significantly prolonged survival relative to that of vehicle-treated mice, with median survival increasing from 37 days to 51 days (P < 0.001, Figure 2F).
Taken together, these data demonstrate inhibition of MERTK signaling, induction of cell death, and abrogation of oncogenic phenotypes in AML cells treated with MRX-2843. Moreover, MRX-2843 had therapeutic activity in a MERTK-dependent xenograft model, validating the use of MERTK inhibitors as a translational strategy for the treatment of AML.
MRX-2843 inhibits FLT3 activation and mediates functional antileukemic effects in FLT3-ITD AML models.
FLT3-ITD AML cell lines were used to assess the effects of treatment with MRX-2843 on FLT3-ITD activity and leukemogenic functions. The MOLM-14 cell line is FLT3-ITD heterozygous and does not express MERTK (MERTKneg); MV4-11 is FLT3-ITD homozygous and expresses only low levels of MERTK protein (MERTKdim) (19).
In MOLM-14 cells, treatment with MRX-2843 inhibited phosphorylation of FLT3 and downstream signaling through STAT5, ERK1/2, and AKT (Figure 3, A and B). Activation of FLT3 and its signaling pathways was almost completely abrogated by treatment with 50 nM MRX-2843, indicating somewhat higher cellular potency against FLT3 relative to MERTK. This finding was borne out in functional assays, with the 2 FLT3-dependent cell lines being more sensitive to MRX-2843 than the MERTK-dependent cell lines tested (Figure 2, C–E, and Figure 3, C–E). Treatment with MRX-2843 inhibited clonal expansion in MOLM-14 cultures, with an IC50 of 29.5 ± 3.4 nM (Figure 3C), approximately 4-fold lower than that in Kasumi-1 cells. Similarly, significant induction of apoptosis and inhibition of colony formation was observed at lower doses of MRX-2843 (Figure 3, D and E). In MOLM-14 cells, treatment with 50 nM, MRX-2843 induced cell death in 91.0% ± 2.7% cells (P < 0.001), whereas Kasumi-1 and NOMO-1 cells were not affected at this dose. MV4-11 cells were slightly less sensitive, with 45.6% ± 4.2% apoptotic and dead cells after treatment with 50 nM MRX-2843 (P < 0.01) and 59.6% ± 4.1% after treatment with 100 nM MRX-2843 (P < 0.001). Similarly, treatment with MRX-2843 inhibited colony formation in soft agar at concentrations as low as 10 nM, with near-complete abrogation of colonies in both cells lines in response to treatment with 25 nM (Figure 3E). Interestingly, while MOLM-14 cells were more sensitive to MRX-2843 than MV4-11 cells in apoptosis assays, MV4-11 cells were more sensitive in soft agar cultures, where treatment with 10 nM MRX-2843 resulted in a 98.4% ± 0.4% reduction in colony formation relative to that in controls in the MV4-11 line compared with a 66.2% ± 7.9% reduction in colony formation in MOLM-14 cultures.

(A–D) MOLM-14 (MERTKnegFLT3-ITD) and MV4-11 (MERTKdimFLT3-ITD) AML cells were treated with the indicated doses of MRX-2843, vehicle (DMSO), or a nontargeting control TKI. (A) After 1 hour, MOLM-14 cells were treated with pervanadate phosphatase inhibitor for 3 minutes and lysates prepared. FLT3 protein was immunoprecipitated, and phosphorylated and total FLT3 were detected by immunoblot analysis. (B) Lysates were prepared from MOLM-14 cells without pervanadate treatment, and phosphorylated and total STAT5, AKT, and ERK1/2 proteins were detected by immunoblot analysis. Actin was used as a loading control. (C) After 48 hours, MTS reagent was added to cultures for an additional 2 hours ,and absorbance was determined as an indicator of viable cell numbers. (D) After 72 hours of drug treatment, cells were stained with YoPro-1 iodide and propidium iodide dyes, and apoptotic and dead cells were detected by flow cytometry. **P < 0.01 and ***P < 0.001, by 1-way ANOVA. (E) MOLM-14 or MV4-11 cells were cultured in soft agar overlaid with media containing the indicated concentrations of MRX-2843, vehicle (DMSO), or control TKI. Colonies were stained and counted after 14 to 21 days. Results are shown relative to vehicle-treated controls. Mean values and standard errors were derived from 3 independent experiments. **P < 0.01 and ***P < 0.001, by 1-way ANOVA. (F) Kaplan-Meier curve showing survival of NSG mice with orthotopic MOLM-14 xenografts that were treated with MRX-2843 or vehicle (saline) beginning on day 21 after transplantation. Median survival for MRX-2843-treated mice was 126 days compared with 38 days for vehicle-treated controls (n = 8 per group). P < 0.001, by log-rank test. AML, acute myeloid leukemia; Ctrl TKI, control tyrosine kinase inhibitor; FLT3, FMS-like tyrosine kinase 3; ITD, internal tandem duplication; NSG, NOD-SCID-γ; p, phosphorylated.
To assess the effects of MRX-2843 treatment on FLT3-dependent leukemogenesis in vivo, orthotopic murine xenografts were generated by injecting MOLM-14 cells into the tail veins of NSG mice. Bone marrow engraftment was monitored by flow cytometry to determine the presence of human leukemia cells (hCD45+). Once-daily treatment with 50 mg/kg MRX-2843 or an equivalent volume of vehicle (saline) was initiated 21 days after transplantation, when mice had an average of 10.7% ± 1.4% bone marrow blasts (data not shown), and therapy was continued until 140 days after transplantation. Mice treated with MRX-2843 had significantly prolonged survival compared with that of mice treated with vehicle, with median survival of 136 days and 38 days, respectively (P < 0.0001, Figure 3F). Taken together, these data support the potential utility of MRX-2843 for treatment of FLT3-ITD AML.
MRX-2843 selectively inhibits colony formation in primary AML patient samples and prolongs survival in patient-derived xenograft models of AML.
To determine whether MRX-2843 has activity against patient-derived myeloblasts, primary apheresis samples were obtained from 4 adult patients with AML at diagnosis. All of these patient samples expressed MERTK protein (MERTK+), consistent with our previously reported findings demonstrating MERTK expression in 80% to 90% of pediatric and adult AMLs (13). In addition, 2 samples had FLT3-ITD mutations. Mononuclear cells were isolated and cultured in methylcellulose medium containing MRX-2843 or vehicle for 10 days before colonies were counted. Primary human MERTK-expressing leukemic blasts with and without FLT3-ITD mutations were very sensitive to treatment with MRX-2843, with decreased colony formation evident at concentrations as low as 5 nM and near-complete abrogation of colony formation in response to treatment with 50 nM MRX-2843 in 3 of the 4 samples (Figure 4, A and B). In contrast, treatment with MRX-2843 at doses up to 500 nM had no significant effect on colony-forming potential in methylcellulose cultures of normal hematopoietic cells isolated from human cord blood, indicating a substantial therapeutic window (Figure 4C). In addition, MRX-2843 retained activity in the presence of human plasma, with a minimal shift in the dose needed to inhibit FLT3 activation in a plasma inhibitory assay (ref. 21 and Supplemental Figure 1), suggesting that clinical activity would not be significantly compromised by plasma protein binding.

(A and B) Primary AML patient samples were cultured in methylcellulose medium with the indicated concentrations of MRX-2843 or vehicle (DMSO). Colonies were stained and counted after 10 days. Each sample was plated in triplicate, and mean values and standard errors are shown. (C) Human cord blood collected from healthy donors was cultured in methylcellulose, and colonies were counted as above. Mean values and standard errors were derived from 3 independent experiments and are relative to vehicle-treated controls. **P < 0.01 and ***P < 0.001, by 1-way ANOVA. AML, acute myeloid leukemia; FLT3, FMS-like tyrosine kinase 3; ITD, internal tandem duplication.
While cell line–based xenograft models have been used successfully for preclinical evaluation of therapeutic agents, establishment in culture may select for specific characteristics not representative of the spectrum of biology exhibited in patients. For this reason, patient-derived xenograft models may more accurately recapitulate the biology of leukemia in humans. Therefore, orthotopic xenografts were generated from primary (Figure 5, A and B) or xenograft-passaged (Figure 5, C and D) samples from patients with AML by injection into the tail veins of NSGS mice. Peripheral disease burden was monitored by flow cytometry, and daily treatment was initiated when leukemic blasts (human CD45+ [hCD45+]) were detected. Mice transplanted with a MERTK+FLT3-WT sample were used to determine the effects mediated by MERTK inhibition. In this study, once-daily treatment with 75 mg/kg MRX-2843 or an equivalent volume of vehicle was initiated 53 days after transplantation, when the average peripheral disease burden was 2.9% ± 0.3%. Treatment with MRX-2843 prolonged median survival from 28.5 days after treatment in vehicle-treated controls to 42 days after treatment (P < 0.01, Figure 5A). Additionally, MRX-2843 resulted in a significant decrease in peripheral disease burden relative to that seen in vehicle-treated mice (P < 0.001, Figure 5B). A similar model was developed using a MERTK+FLT3-ITD patient’s sample. Because FLT3-ITD cell lines and patient samples were generally more sensitive to MRX-2843 in cell culture assays, lower doses of 30 mg/kg and 50 mg/kg MRX-2843 were used for treatment in this study. Once-daily treatment with MRX-2843 was initiated 42 days after transplantation, with an average peripheral disease burden of 5.0% ± 0.5%, and continued for 48 days. Median survival was significantly increased from 23 days after treatment in the control group to 68 and 89 days in mice treated with 30 mg/kg or 50 mg/kg MRX-2843, respectively (P < 0.001, Figure 5C). As before, treatment with either dose of MRX-2843 resulted in significantly decreased peripheral disease burden relative to that seen with treatment with vehicle (P < 0.001, Figure 5D). Interestingly, although there was no significant difference in the percentage of peripheral blasts in mice treated with 30 mg/kg or 50 mg/kg MRX-2843 on the last day of treatment (day 48), the time to disease progression was significantly prolonged in mice treated with 50 mg/kg relative to that for mice treated with the lower dose (P < 0.05, Figure 5C).

(A and C) Kaplan-Meier curves showing survival of NSGS mice xenografted with the indicated patient sample and treated with MRX-2843 or vehicle (saline). (B and D) Peripheral disease burden (percentage of hCD45+ peripheral blood mononuclear cells) was determined by flow cytometry 3 days prior to initiation of treatment (Pre-rx) and on the indicated days after treatment. Mean values and standard errors are shown. (A and B) Mice were started on therapy 53 days after transplantation with leukemia cells (day 0). Median survival of MRX-2843–treated mice after treatment was 42 days compared with 28.5 days for vehicle-treated controls (n = 12 per group). P < 0.005, by log-rank test (A); ***P < 0.001 versus saline, by 1-way ANOVA (B). (C and D) Mice were started on therapy 42 days after transplantation (day 0). Median survival of MRX-2843–treated mice was 68 days (30 mg/kg MRX-2843) and 89 days (50 mg/kg MRX-2843) after treatment compared with 12 days for vehicle-treated controls (n ≥8 per group). P < 0.001 and P < 0.05, by log-rank test (C); ***P < 0.001 versus saline, by 1-way ANOVA (D). AML, acute myeloid leukemia; FLT3, FMS-like tyrosine kinase 3; hCD45+, human CD45+; NSGS, NOD-SCID-γ mice expressing Tg human cytokines. NOD-SCID-γ mice expressing Tg human cytokines
MRX-2843 retains activity against quizartinib-resistant FLT3-mutant proteins.
Several approaches were used to determine whether MRX-2843 has activity against FLT3-ITD–mutant proteins that are resistant to previously described FLT3 TKIs (10). First, BA/F3 cell lines stably expressing FLT3-ITD or FLT3-ITD with a resistance-conferring mutation were generated, and the impact of treatment with MRX-2843 on clonal expansion was assessed in the absence of IL-3 (8). In this system, cytokine-independent expansion is driven by FLT3-ITD activity. An IC50 of 3.0 ± 0.5 nM MRX-2843 was observed in BA/F3 cells expressing FLT3-ITD alone, indicating potent inhibition of FLT3-ITD (Figure 6A and Supplemental Table 2). MRX-2843 retained inhibitory activity against clinically relevant point mutations at the D835 and F691 loci, with IC50 values of 7.2 ± 1.3 nM and 20.4 ± 4.3 nM in BA/F3 lines expressing FLT3-ITD D835Y and FLT3-ITD F691L, respectively (Figure 6A and Supplemental Table 2). In contrast, point mutations at these loci rendered resistance to the FLT3 inhibitor quizartinib (AC220; Supplemental Figure 3). MRX-2843 retained activity against additional FLT3-ITD D835, F691, and Y842 locus mutants that were identified using a mutagenesis screen to determine changes causing resistance to quizartinib (ref. 8, Supplemental Figure 3, and Supplemental Table 2). Two derivatives of the human FLT3-ITD AML cell line MOLM-14 that acquired either a D835Y or F691L mutation after selection in escalating doses of quizartinib (11) were used to confirm and extend these findings. In this model, treatment with MRX-2843 decreased expansion of the parental MOLM-14 cells with an IC50 of 19.1 ± 4.7 nM and demonstrated minimally reduced activity against both MOLM-14:D835Y and MOLM-14:F691L derivative cells with IC50 values of 21.7 ± 4.3 nM and 34.8 ± 7.9 nM, respectively (Figure 6B and Supplemental Table 2). In biochemical assays, FLT3 phosphorylation and downstream signaling through STAT5, AKT, and ERK1/2 were abrogated in the MOLM-14 parental cell line treated with either quizartinib or MRX-2843 at similar doses of 10 to 25 nM (Figure 6C and Supplemental Figure 2). In MOLM-14:D835Y and MOLM-14:F691L cells, MRX-2843 retained potent activity with similar (D835Y) or slightly increased (F691L) doses mediating inhibition of FLT3 and downstream signaling. In contrast, significantly higher doses of up to 100 nM quizartinib were required to affect the activation of these pathways, particularly in the F691L cell line. To better understand the ability of MRX-2843 to retain activity against aa substitutions at these loci, a structural model of the MRX-2843:FLT3 complex was developed. The binding mode of MRX-2843 to FLT3 was derived from a crystal structure featuring UNC569, a close MRX-2843 analog, bound to MERTK (PDB:3TCP; ref. 22). The overlaid MRX-2843 and quizartinib bound to F691L mutant and WT FLT3, respectively, are shown in Figure 6D. The binding model clearly suggests that MRX-2843 and quizartinib adopt substantially different inhibition mechanisms. In particular, quizartinib features a “tail” (including the isoxazole group) that penetrates into the back pocket formed by the c-helix and the activation loop. Consequently, it can only bind the so-called “DFG-out,” catalytically inactive, conformation and is therefore a type II kinase inhibitor (23). In contrast, the bound MRX-2843 is confined within the adenine-binding pocket, such that it has equal potencies with respect to both the “DFG-in” and “DFG-out” enzyme conformations and is therefore a type I kinase inhibitor. This binding mode makes MRX-2843 much more active against the F691L FLT3–mutant protein than quizartinib. For instance, the flexible cyclopropylethyl group in MRX-2843 forms favorable van der Waals interactions equally well with either the leucine or phenylalanine side chains, while quizartinib binding is dependent on a stacking interaction with the F691 side chain. Moreover, phenylalanine is also likely to favor a metastable conformation required for quizartinib engagement (24).

(A) BA/F3 cells expressing FLT3-ITD or an FLT3-ITD mutant (D835Y or F691L) were cultured in the absence of IL-3 and treated with the indicated concentrations of MRX-2843. After 48 hours, CellTiter-Glo reagent was added to cultures, and absorbance was determined as an indicator of viable cell numbers relative to vehicle-treated controls. Cultures grown in the presence of IL-3 are shown for reference (Parental BA/F3 + IL-3). Each condition was assessed in triplicate, and mean values and standard errors were derived from 3 independent experiments. (B) WT MOLM-14 and derivative cell lines expressing a D835Y- or F691L-mutant FLT3-ITD were cultured in the presence of MRX-2843, and relative numbers of viable cells were determined as above. (C) MOLM-14 and derivative cell lines were cultured in the presence of MRX-2843, quizartinib (AC220), vehicle (DMSO), or a nontargeting control TKI for 1 hour, and signaling effectors downstream of FLT3 were detected by immunoblot analysis as described in Figure 3B. (D) Structural model of MRX-2843 (thick bars) and quizartinib (thin bars) bound to the FLT3 F691L mutant (magenta) and FLT3 WT (cyan). Positions of F691 (the gatekeeper) and F830 (part of the DFG-out motif) are indicated. Ctrl TKI, control tyrosine kinase inhibitor; FLT3, FMS-like tyrosine kinase 3; ITD, internal tandem duplication; p, phosphorylated.
MRX-2843 mediates functional antileukemic effects in quizartinib-resistant FLT3-ITD cell lines.
Comparison of MRX-2843 and quizartinib in apoptosis and colony-forming assays was used to determine the functional effects of MRX-2843 in quizartinib-resistant cell lines. As expected, the parental MOLM-14 cell line was sensitive to both MRX-2843 and quizartinib at similar doses (Figure 7, A and B). Specifically, treatment with 50 nM MRX-2843 or quizartinib induced cell death in 78.8% ± 12.3% or 91.5% ± 3.8% of cells, respectively, and a dose of 10 nM inhibited colony formation by 48.1% ± 20.3% or 98.3% ± 0.7%. As predicted based on the biochemical assays, MRX-2843 retained the ability to induce apoptosis (Figure 7A) and inhibit colony formation (Figure 7B) in both the MOLM-14:D835Y and MOLM-14:F691L mutant cell lines, with similar (D835Y) or slightly reduced (F691L) potency relative to that of parental MOLM-14 cells. In contrast, quizartinib did not significantly induce apoptosis or alter colony formation in MOLM-14:D835Y or MOLM-14:F691L cell lines.

(A and B) Parental MOLM-14 cells and derivatives harboring mutations conferring quizartinib resistance (D835Y and F691L) were treated with MRX-2843, quizartinib (AC220), vehicle (DMSO), or a nontargeting control TKI, and apoptotic and dead cell numbers (A) and colony formation (B) were determined as described in Figure 2. Mean values and standard errors were derived from 3 independent experiments and are shown relative to vehicle-treated cells. *P < 0.05, **P < 0.01, and ***P < 0.001, by 1-way ANOVA. Ctrl TKI, control tyrosine kinase inhibitor; FLT3, FMS-like tyrosine kinase 3; ITD, internal tandem duplication.
MRX-2843 prolongs survival in quizartinib-resistant FLT3-ITD xenograft models.
To determine the therapeutic effects mediated by MRX-2843 in quizartinib-resistant FLT3-ITD AML models, the MOLM-14 parental and derivative cell lines were used to generate orthotopic xenografts in NSG mice, and the impact of treatment with MRX-2843 or quizartinib was determined. Mice were treated with 50 mg/kg MRX-2843, 10 mg/kg quizartinib (the maximum published dose; refs. 25, 26), or an equivalent volume of vehicle (saline or 50 mg/kg hydroxybutenyl-β-cyclodextrin in saline, respectively) administered once daily by oral gavage. In MOLM-14 parental xenografts, both quizartinib and MRX-2843 increased median survival compared with that of vehicle-treated mice (172.5 days versus 40 days and 121 days versus 36 days, respectively, P < 0.001, Figure 8A). In this model, quizartinib was more effective than MRX-2843 (P < 0.005), although higher doses of MRX-2843 were not evaluated. In MOLM-14:D835Y xenografts, quizartinib prolonged survival compared with that of vehicle-treated mice, but the effect was minimal (median survival 45 days vs. 36 days, P < 0.001). In contrast, MRX-2843 resulted in a greater than 2-fold increase in median survival in MOLM-14:D835Y xenografts (median survival 94 days vs. 35.5 days, P < 0.001; Figure 8B). In MOLM-14:F691L xenografts, treatment with MRX-2843 prolonged survival by almost 2-fold in NSG (Figure 8C) and NSGS (Figure 8D) mice (median survival 87 vs. 44.5 days and 87 vs. 48 days, respectively, P < 0.005). Treatment with quizartinib also increased median survival in NSG (57 vs. 44.5 days) and NSGS (67 vs. 55.5 days, P < 0.05) mice, although this difference was not significant in NSG mice. Similarly, increased survival was observed in response to treatment with MRX-2843 versus quizartinib, but the difference was only significant in NSG mice.

(A–D) Kaplan-Meier curves showing survival of NSG (A, B, and D) or NSGS (C) mice with orthotopic xenografts of MOLM-14 parental (A), MOLM-14:D835Y (B), or MOLM-14:F691L (C and D) cells, treated with MRX-2843, quizartinib (AC220), MRX-2843 vehicle (saline), or quizartinib vehicle (CD). (A) Disease burden was monitored in cohorts of mice, and treatment was initiated on day 22 after transplantation, when 7.2% ± 1.0% blasts were detected in the bone marrow. Treatment continued until day 145 after transplantation. Median survival was 121 days and 172.5 days for mice treated with MRX-2843 or quizartinib, respectively, compared with 36 days and 40 days for mice treated with MRX-2843 or quizartinib vehicle (n = 5 per group). (B) Disease burden was monitored in cohorts of mice, and treatment was initiated on day 26 after transplantation, when 6.8% ± 1.2% blasts were detected in the bone marrow. Treatment continued until conclusion of the study. Median survival was 94 days and 45 days after transplantation for mice treated with MRX-2843 or quizartinib, respectively, compared with 35.5 days and 36 days for mice treated with MRX-2843 or quizartinib vehicle (n ≥5 per group). (C) Treatment was initiated on day 28 after transplantation and continued until the conclusion of the study. Median survival was 87 days and 67 days after transplantation for mice treated with MRX-2843 or quizartinib, respectively, compared with 48 days and 55.5 days for mice treated with MRX-2843 or quizartinib vehicle (n ≥8 per group). (D) Disease burden was monitored in cohorts of mice, and treatment was initiated on day 38 after transplantation, when 49% ± 11% blasts were detected in the bone marrow. Treatment continued until 120 days after transplantation. Median survival was 87 days and 57 days for mice treated with MRX-2843 or quizartinib, respectively, compared with 44.5 days for mice treated with saline vehicle (n ≥4 per group). All P values in A–D were determined by log-rank test. CD, hydroxybutenyl-β-cyclodextrin; FLT3, FMS-like tyrosine kinase 3; ITD, internal tandem duplication; NSG, NOD-SCID-γ; NSGS, NOD-SCID-γ mice expressing Tg human cytokines.
Discussion
Here, we describe the preclinical characterization of MRX-2843, a small-molecule TKI of MERTK and FLT3, 2 attractive targets in AML. MRX-2843 inhibits phosphorylation of MERTK and FLT3 and activation of their downstream effectors, leading to induction of apoptosis and decreased clonal expansion and/or colony formation in AML cell lines and patient samples. In addition, we demonstrate that daily oral treatment with MRX-2843 prolongs survival in cell line– and patient-derived orthotopic mouse xenograft models of MERTK-positive, FLT3-ITD–positive, and dual-positive AML. The effects of treatment with MRX-2843 in MERTK-dependent models recapitulate the phenotypes observed in response to shRNA-mediated MERTK inhibition, providing evidence that MRX-2843 antileukemic activity is a consequence of MERTK inhibition in this context (13). Similarly, treatment with MRX-2843 mediates antileukemic activity in FLT3-dependent models with a potency similar to that of quizartinib. MRX-2843 retains activity against FLT3 proteins, with aa changes at D835 or F691, two loci where mutations have been identified in patients resistant to treatment with other FLT3 inhibitors. Taken together, these data validate MRX-2843 for translational application as a dual MERTK and FLT3 inhibitor. Given the near-ubiquitous expression of MERTK and the 20%–30% incidence of activating mutations in FLT3, MRX-2843 could be a widely applicable therapeutic in patients with AML.
In addition, MRX-2843 is an ATP-competitive type 1 inhibitor, and, unlike quizartinib and other type 2 inhibitors that bind target proteins in their inactive conformation (24), acquisition of mutations that stabilize the active form of the target protein is unlikely to be a mechanism of acquired resistance. Indeed, MRX-2843 retains activity against FLT3-mutant proteins containing activating aa changes at D835.
Furthermore, MRX-2843 retains activity against both of the clinically relevant FLT3 mutants that have been identified in patients resistant to therapy with FLT3 TKIs. Point mutations at 2 loci, D835 (activation loop) and F691 (gatekeeper), emerge in patients who relapse while on therapy with quizartinib, and approximately half of the recurrent tumors contain polyclonal mutations (8). Mutations at both loci confer significant cross-resistance to sorafenib in vitro, and acquisition of a D835Y point mutation has been detected in patients who relapsed while receiving sorafenib therapy (9). Crenolanib is a FLT3 inhibitor with activity against the D835-mutant protein and is currently in clinical trials for treatment of AML with FLT3-activating mutations (10, 27). However, gatekeeper mutations render relative resistance to crenolanib, such that patients remain susceptible to the development of resistance via acquisition of mutations at the F691 loci or selection of existing F691-mutant clones. This is of particular concern, given the high number of patients who develop polyclonal FLT3 mutations. Similarly, the multi-kinase inhibitor ponatinib has activity in FLT3-ITD AMLs with F691L mutation, but is not active against activation loop mutants (11). MRX-2843 is a FLT3 TKI that retains activity against both D835Y- and F691L-mutant proteins and may thereby provide a therapeutic option for patients with tumors that have acquired point mutations mediating resistance to current FLT3 inhibitors without the potential to select additional mutations. Clearly, the emergence of point mutations in FLT3 is not the only mechanism of resistance in FLT3-ITD AMLs, but it appears to be the most common one (28). A similar mechanism mediates resistance to TKIs targeting BCR-ABL in chronic myeloid leukemia (CML) (29) and acute lymphocytic leukemia (30), indicating the importance of this mechanism. While treatment with MRX-2843 was sufficient to delay leukemia progression and doubled or even tripled survival in multiple xenograft models of AML, including patient-derived xenografts, all of the mice eventually succumbed to disease. The reason for the lack of curative growth suppression in MRX-2843–treated models remains to be elucidated.
Several lines of evidence suggest that dual inhibition of MERTK and FLT3 will provide greater therapeutic benefit than inhibition of either target alone. MERTK signaling has a variety of functional consequences in cancer cells, but its ability to promote tumor cell survival is particularly important (15). For instance, in acute lymphoblastic leukemia models, stimulation with GAS6, a MERTK ligand, promotes chemoresistance (31), and shRNA-mediated MERTK knockdown enhances sensitivity to cytotoxic chemotherapeutics (12, 14). Studies investigating the role of MERTK in resistance to targeted therapeutics, such as those directed at FLT3, have not been reported. However, another member of the TAM family, AXL, has been implicated in acquired resistance to a variety of TKIs and in multiple tumor types. Notably, in AML cell lines, AXL knockdown via shRNA restored sensitivity to the FLT3 inhibitor midostaurin (32). Given the known role of MERTK in promoting survival and resistance to traditional chemotherapies and its close relationship to AXL, it is likely that MERTK also has a role in mediating resistance to targeted therapies, and thus dual inhibition of MERTK and FLT3 may decrease the development of resistance relative to inhibition of FLT3 alone.
In addition to delaying or preventing development of resistance, coinhibition of MERTK and FLT3 may improve therapeutic efficacy by other mechanisms. Overexpression of the TAM family kinase ligand GAS6 is a poor prognostic indicator in cytogenetically normal AML, and depletion of GAS6 results in decreased FLT3 phosphorylation and clonal expansion of FLT3-ITD cell lines and primary patient samples (33, 34). While this effect is mediated, at least in part, by inhibition of AXL, introduction of AXL siRNA only partially reduced FLT3 phosphorylation, and depletion of GAS6 had a much more substantial impact, suggesting that MERTK and/or TYRO-3 activity also play a role in promoting FLT3 activity.
Enzymatic assays reveal very close IC50 values of MRX-2843 for MERTK (1.3 nM) and FLT3 (0.64 nM). The heightened cell-based assay potency of MRX-2843 in cell lines expressing FLT3-ITD is approximately 3- to 4-fold greater than that in MERTK+FLT3-WT cells. This may simply reflect unique cell line attributes, but it may also reflect the presumed action of FLT3-ITD and MERTK, with the former being a classic oncogenic driver and the latter being a survival factor providing nononcogenic addiction.
One goal of targeted therapies with selectivity for tumor cells is to minimize toxicities. Daily treatment with MRX-2843 was well tolerated in mice for up to 120 days. In addition, MRX-2843 had a 10-fold differential effect on myeloblasts derived from patients with AML and normal mononuclear cells derived from human cord blood samples, indicating a large therapeutic window. The primary physiologic roles of MERTK include immune regulation and clearance of apoptotic debris (15). Complete loss of function of MERTK protein has been identified in humans, with the primary clinical effect of retinitis pigmentosa, likely due to defects in apoptotic clearance in the retina (35). In a rat model, the phenotype was reversible with exogenous MERTK expression (36), indicating that short-term pharmacologic inhibition of MERTK is unlikely to lead to permanent adverse effects. With regard to FLT3 inhibition, the effects of inhibitors such as quizartinib have been well described, with myelosuppression being one of the most prominent toxicities, likely due to the combined inhibition of both FLT3 and c-KIT (37, 38). In comparison with current FLT3 inhibitors, MRX-2843 has reduced activity against c-KIT, and we do not anticipate combined inhibition of FLT3 with MERTK to exacerbate myelosuppression, as MERTK is not expressed on normal hematopoietic cells (14).
In summary, MRX-2843 is a potent small-molecule inhibitor of both MERTK and FLT3 that has antileukemic activity in preclinical assays and robust therapeutic activity in vivo. MRX-2843 is selective for leukemia cells compared with normal hematopoietic progenitors, suggesting a wide therapeutic window. Moreover, MRX-2843 is active against FLT3 proteins containing clinically relevant activation loop or gatekeeper mutations and is one of the only FLT3 TKIs reported to effectively target both mutant proteins. These observations suggest that treatment with MRX-2843 will provide more durable responses compared with those elicited by current FLT3 inhibitors and indicate the potential utility of MRX-2843 for the treatment of patients with acquired resistance to these agents. In addition, the high frequency of FLT3-ITD mutations and nearly ubiquitous overexpression of MERTK in AML make dual targeting a particularly attractive therapeutic strategy. Together, these observations validate MRX-2843 as a translational agent and support its clinical development for the treatment of AML.
Methods
Inhibitors.
MRX-2843 and the control TKI were synthesized as previously described (20). The amount of kinase inhibitor required for 90% inhibition in vivo was estimated using Michaelis-Menton kinetic equations as previously described (20). Quizartinib (AC220) was obtained from the Colorado Clinical and Translational Sciences Institute Medicinal Chemistry core facility as a 1:4 formulation with hydroxybutenyl-β-cyclodextrin (CD). For in vitro studies, stock solutions were prepared in DMSO (Sigma-Aldrich), and the DMSO vehicle control concentration was equivalent to the highest dose of test agent for the experiment. For in vivo studies, test agents were either dissolved (MRX-2843) or prepared in a homogeneous suspension (quizartinib and CD) in saline.
Patient samples and cell culture.
Deidentified cord blood from healthy donors and peripheral blood apheresis samples from patients with AML at the time of diagnosis were obtained from the University of Colorado. Samples from patients with AML were cultured as previously described (39).
The cell lines MV4-11 and Kasumi-1 were purchased from the American Type Culture Collection (ATCC) in 2008 and last authenticated in June 2013 and April 2014, respectively. NOMO-1 was obtained from the DSMZ (German Collection of Microorganisms and Cell Culture) in 2006 and last authenticated in April 2014. MOLM-14 cells were obtained from the laboratory of Scott Kogan (Department of Laboratory Medicine, UCSF, San Francisco, California, USA) in 2008 and authenticated in November 2013. Cell-line identities were confirmed using short tandem repeat microsatellite loci analysis. Frozen stocks of all cell lines were generated at the time of authentication, and stocks were thawed for experiments no more than 3 months before use. MOLM-14:D835Y, MOLM-14:F691L, and Ba/F3 cells expressing FLT3-ITD were previously described (8, 11). All cell lines were cultured in RPMI medium (HyClone) supplemented with 10% FBS (or 20% for Kasumi-1) and penicillin-streptomycin (complete RPMI [cRMPI]). All cell lines were free of Mycoplasma.
Immunoblot analysis of downstream signaling pathways.
Three million exponentially growing cells were cultured in cRPMI supplemented with vehicle, control TKI, or the indicated concentration of kinase inhibitor for 1.5 hours. Cell lysates were prepared in lysis buffer (50 mM HEPES, pH 7.5, 150 mM NaCl, 10 nM EDTA, 10% glycerol, 1% Triton X-100) supplemented with protease and phosphatase inhibitors. Proteins were quantitated, resolved on 8% Tris-Glycine SDS-PAGE gels (Invitrogen), and transferred onto nitrocellulose membranes. Membranes were blocked with 5% milk in tris-buffered saline with 0.1% Tween-20 and then incubated with Abs specific to the proteins of interest. Proteins were visualized on x-ray film using HRP for chemiluminescence detection (PerkinElmer). Phosphorylated proteins were first detected, then membranes were stripped and probed for total protein or actin, which was used as a protein concentration control. Anti-actin Ab (sc-1616) and donkey anti-goat IgG-HRP (sc-2020) were from Santa Cruz Biotechnology Inc. Abs against phosphorylated STAT5 (p-STAT5) (Tyr694, #9359); p-STAT6 (Tyr641, #9364); p-AKT (Ser473, #9271L); p-ERK1/2 (Thr202/Tyr204, #9106); STAT5 (#9358); STAT6 (#9362); AKT (#9272); and ERK1/2 (#9102) were from Cell Signaling Technology. Goat anti-mouse IgG-HRP and goat anti-rabbit IgG-HRP were from Bio-Rad. Proteins were quantitated by densitometry using ImageJ software (NIH). The data shown are representative of at least 3 independent experiments.
Immunoblot analysis of FLT3 and MERTK.
For analysis of p-FLT3 and p-MERTK only, cells were treated as above, but 0.12 mM pervanadate (an irreversible phosphatase inhibitor) was added to the cultures for 3 minutes (FLT3) or 10 minutes (MERTK) prior to preparation of the cell lysates to stabilize the phosphorylated proteins. Lysates were incubated with anti-MERTK (MAB8912; R&D Systems) or anti-FLT3 S18 (sc-480; Santa Cruz Biotechnology Inc.) Abs overnight on a rotator, and recombinant protein G sepharose beads (Invitrogen) were added for an additional 2 hours. Beads were washed 3 times with lysis buffer, and bound proteins were eluted by boiling in 2× Laemmli sample buffer (Bio-Rad). Proteins were detected by immunoblotting as described above using p-MERTK (Y749, Y753, Y754; Phosphosolutions), p-FLT3 (#3461; Cell Signaling Technology), MERTK (ab52968; AbCam), or FLT3 (sc-480; Santa Cruz Biotechnology Inc.) Abs. Data shown are representative of at least 3 independent experiments.
Clonal expansion assay.
Exponentially growing cells (BA/F3: 5 × 103 cells/well; others: 1 × 104 cells/well) were cultured in triplicate for 48 hours in 0.1 ml cRPMI containing the appropriate concentration of test agent or vehicle. ATP levels were determined as an indicator of viable cell numbers using CellTiter-Glo reagent (Promega) according to the manufacturer’s recommendation. Luminescence was detected and quantitated using a SpectraMax M3 microplate reader and SpectraMax Pro Software (Molecular Devices). Luminescence intensities relative to the mean intensity in vehicle-treated samples were calculated, and IC50 values were determined by nonlinear best-fit regression analysis using Prism 5 software, version 5.01 (GraphPad Software).
Apoptosis assay.
Cells (3 × 105 per sample) were cultured in cRPMI with vehicle, control TKI, or kinase inhibitor at the indicated concentration. After 72 hours, cells were harvested and resuspended in PBS with 1 μM YO-PRO-1 iodide (Invitrogen) and 1.5 μM propidium iodide (Invitrogen) for 15 to 30 minutes prior to assessment of dye uptake by flow cytometry using an FC500 cytometer and CXP analysis software (both from Beckman Coulter).
Colony-forming assays.
Cell lines were cultured (10,000 cells/sample) in 0.35% Noble agar on a 0.5% Noble agar base layer and overlaid with cRPMI containing kinase inhibitor or vehicle. The overlying medium was replaced 2–3 times per week, and vehicle treatment was assessed in duplicate. After 14 days or 21 days (Kasumi-1 cells only), colonies were stained with 1 mg/ml nitrotetrazolium blue (Sigma-Aldrich) for 4 hours and counted using a GelCount colony counter (Oxford Optronix). Mononuclear cells were isolated from human cord blood and samples from AML patients using Ficoll-Paque PLUS (GE Healthcare Life Sciences). Patient samples were cultured in triplicate at a density of 1 × 106 cells/ml in MethoCult H4434 Classic Methylcellulose-Based Medium with Recombinant Cytokines for Human Cells (STEMCELL Technologies) containing MRX-2843 or vehicle. Colonies were counted after 10 days using the GelCount colony counter (Oxford Optronix). Cord blood cells were incubated for 1 hour in serum-free Iscove’s modified Dulbecco’s medium (IMDM) (HyClone) supplemented with BIT 9500 Serum Substitute (STEMCELL Technologies), low-density lipoproteins (EMD Millipore), and 2-ME (Sigma-Aldrich), and then cultured in triplicate at a density of 2 × 106 cells/ml in Methocult H4434 methylcellulose containing MRX-2843 or vehicle. Colonies were manually counted in a blinded manner after 14 days.
Murine xenograft models.
NOD.Cg-PrkdcscidIl2rgtm1WjlTg(CMV-IL3,CSF2,KITLG)1Eav/MloySzJ (NSGS) mice and NOD.Cg-PrkdcscidIl2rgtm1Wj1/SzJ (NSG) mice were purchased from The Jackson Laboratory or bred in-house and maintained under sterile conditions. Established leukemia cell lines or mononuclear cells isolated from samples from patients with AML (1 × 106 to 2.5 × 106 per mouse) were suspended in PBS and injected into the tail veins of NSG or NSGS mice to establish xenografts. All mice were 4–6 months of age at the time of injection and were male, with the exception of the NOMO-1, MOLM-14:D835Y, and MOLM-14:F691L NSG xenografts, which were established in female mice. Myeloblasts were detected in peripheral blood (patient-derived xenografts) or bone marrow (MOLM-14 xenografts) samples after staining with a FITC-conjugated anti-human CD45 Ab (BD Biosciences). Samples were analyzed by flow cytometry using a Gallios flow cytometer and Kaluza software (both from Beckman Coulter). After engraftment, the mice were weighed and treated once daily with MRX-2843, quizartinib, or vehicle administered by oral gavage in a volume of 10 ml/kg. When mice appeared ill or lost more than 20% of their body weight, they were euthanized.
Structural modeling.
A high-resolution crystal structure of the MERTK protein kinase domain in complex with UNC569 (PDB:3TCP; ref 22) was used as a structural template for modeling the MRX-2843:FLT3 complex. To this end, the 3TCP MERTK kinase structure was aligned with the 4XUF FLT3 structure and UNC569 was transferred into 4XUF and “alchemically” transformed into MRX-2843. All modeling and calculations were performed using the Maestro 10.3 Software Suite (Schrödinger). The resulting MRX-2843:FLT3 complex was refined in implicit solvent by means of Monte Carlo sampling and the OPLS-2005 force field using the Prime module. All protein structures were preprocessed using the protein preparation workflow available in Maestro.
Statistics.
Data were analyzed by 1-way ANOVA with Bonferroni’s test for multiple comparisons. Data are presented as the mean ± SEM. P values of less than 0.05 were considered statistically significant. IC50 values were determined by nonlinear best-fit regression analysis. Kaplan-Meier survival curves were compared using the log-rank test. All statistical analyses were performed using Prism 5 software, version 5.01 (GraphPad Software).
Study approval.
All animal experiments were conducted in accordance with the relevant regulatory standards set forth and were approved by the IACUC of the University of Colorado. Patient samples were obtained from the University of Colorado after written informed consent was received, in accordance with the principles of the Declaration of Helsinki. All experiments conformed to the regulatory standards approved by the Colorado Multiple Institutional Review Board.
Author contributions
KAM, CCS, DD, CL, ABLS, MGH, EAL, GDK, MAS, WZ, DK, and XW designed and performed the experiments and analyzed the data. CTJ, SVF, HSE, NPS, and DKG analyzed the data. KAM, CCS, DD, ABLS, DK, HSE, and DKG wrote the manuscript.
Acknowledgments
The authors thank the University of Colorado Cancer Center Flow Cytometry Core for technical assistance (P30CA046934); the University of Colorado Diabetes and Endocrinology Research Center Molecular Biology Core Facility (NIH P30DK57516) for cell line authentication services; and Lauren S. Page for her technical assistance. This work was supported by grants from the NIH (2T32CA082086-11, to K.A. Minson and 1R01CA137078, to D.K. Graham); the Leukemia and Lymphoma Society Translation Research Program (to D.K. Graham); the University of North Carolina Cancer Research Fund; and federal funds from the National Cancer Institute’s Experimental Therapeutics (NExT) program, NIH, under contract HHSN261200800001E. C.C. Smith is an American Society of Hematology Faculty Scholar. C. Libbrecht received funding from L’Institut Servier.
Footnotes
Conflict of interest: D.K. Graham, D. DeRyckere, and H.S. Earp have filed patents on targeting of the MERTK tyrosine kinase as cancer therapy. D. Kireev, X. Wang, W. Zhang, and S.V. Frye have filed patents on MRX-2843. Additionally, D. DeRyckere, D. Kireev, X. Wang, S.V. Frye, H.S. Earp, and D.K. Graham hold stock in Meryx Inc. (a company developing novel therapeutics against MERTK).
Reference information:JCI Insight. 2016;1(3):e85630. 10.1172/jci.insight.85630.
References
Articles from JCI Insight are provided here courtesy of American Society for Clinical Investigation
Full text links
Read article at publisher's site: https://doi.org/10.1172/jci.insight.85630
Read article for free, from open access legal sources, via Unpaywall:
http://insight.jci.org/articles/view/85630/files/pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1172/jci.insight.85630
Article citations
MERTK inhibition selectively activates a DC - T-cell axis to provide anti-leukemia immunity.
Leukemia, 25 Sep 2024
Cited by: 0 articles | PMID: 39322710
MERTK Is a Potential Therapeutic Target in Ewing Sarcoma.
Cancers (Basel), 16(16):2831, 12 Aug 2024
Cited by: 0 articles | PMID: 39199601 | PMCID: PMC11352666
Could Gas6/TAM Axis Provide Valuable Insights into the Pathogenesis of Systemic Sclerosis?
Curr Issues Mol Biol, 46(7):7486-7504, 15 Jul 2024
Cited by: 0 articles | PMID: 39057085 | PMCID: PMC11275301
Review Free full text in Europe PMC
MERTK Inhibition as a Targeted Novel Cancer Therapy.
Int J Mol Sci, 25(14):7660, 12 Jul 2024
Cited by: 0 articles | PMID: 39062902 | PMCID: PMC11277220
Review Free full text in Europe PMC
Co-targeting JAK1/STAT6/GAS6/TAM signaling improves chemotherapy efficacy in Ewing sarcoma.
Nat Commun, 15(1):5292, 21 Jun 2024
Cited by: 0 articles | PMID: 38906855 | PMCID: PMC11192891
Go to all (47) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Protein structures in PDBe (2)
-
(2 citations)
PDBe - 3TCPView structure
-
(2 citations)
PDBe - 4XUFView structure
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A dual inhibitor overcomes drug-resistant FLT3-ITD acute myeloid leukemia.
J Hematol Oncol, 14(1):105, 03 Jul 2021
Cited by: 10 articles | PMID: 34217323 | PMCID: PMC8255005
A novel irreversible FLT3 inhibitor, FF-10101, shows excellent efficacy against AML cells with FLT3 mutations.
Blood, 131(4):426-438, 29 Nov 2017
Cited by: 57 articles | PMID: 29187377
Novel AXL-targeted agents overcome FLT3 inhibitor resistance in FLT3-ITD+ acute myeloid leukemia cells.
Oncol Lett, 21(5):397, 18 Mar 2021
Cited by: 6 articles | PMID: 33777220 | PMCID: PMC7988696
Quizartinib (AC220): a promising option for acute myeloid leukemia.
Drug Des Devel Ther, 13:1117-1125, 08 Apr 2019
Cited by: 26 articles | PMID: 31114157 | PMCID: PMC6497874
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NCI NIH HHS (7)
Grant ID: T32 CA082086
Grant ID: HHSN261200800001E
Grant ID: P30 CA046934
Grant ID: HHSN261200800001C
Grant ID: P30 CA016086
Grant ID: K08 CA187577
Grant ID: R01 CA137078
NIDDK NIH HHS (1)
Grant ID: P30 DK057516
NIGMS NIH HHS (1)
Grant ID: T32 GM008497





