Abstract
Free full text

Inducible scAAV2.GRE.MMP1 lowers IOP long-term in a large animal model for steroid-induced glaucoma gene therapy
Abstract
Current treatment of glaucoma relies on administration of daily drops or eye surgery. A gene therapy approach to treat steroid-induced glaucoma would bring a resolution to millions of people worldwide that depend on glucocorticoid therapy for a myriad of inflammatory disorders. Previously, we had characterized a short-term Adh.GRE.MMP1 gene vector for the production of steroid-induced MMP1 in the trabecular meshwork and tested reduction of elevated intraocular pressure (IOP) in a sheep model. Here we conducted a trial transferring the same transgene cassette to a clinically safe vector (scAAV2), and extended the therapeutic outcome to longer periods of times. No evidence of ocular and/or systemic toxicity was observed. Viral genome distributions showed potential re-inducible vector DNAs in the trabecular meshwork (0.4 vg/cell) and negligible copies in six major internal organs (0.00002-0.005 vg/cell). Histological sections confirmed successful transduction of scAAV2.GFP to the trabecular meshwork. Optimization of the sheep steroid–induced hypertensive model revealed that topical ophthalmic drug difluprednate 0.05% (durezol) induced the highest IOP elevation in the shortest time. This is the first efficacy/toxicity study of a feasible gene therapy treatment of steroid-induced hypertension using clinically accepted scAAV vectors in a large animal model.
INTRODUCTION
Glucocorticoids, a class of steroid hormones, are potent immunosuppressants and the preferred treatment for many inflammatory disorders, including ocular inflammation. A wide segment of the worldwide population receives glucocorticoids treatments via various routes of administrations, such as oral, topical, systemic injection, inhalation, etc. Glucocorticoids have in addition, anti-angiogenic and anti-permeability properties and because of that, they are also being widely used in the eye for the treatment of retinal diseases, such as macular edema, age-related macular degeneration and diabetic retinopathy.1 However, glucocorticoids elicit significant secondary effects in the eye, including the development of cataracts and elevated intraocular pressure (IOP).2 For instance, treatment of uveitis with glucocorticoid intravitreal implants results in elevated IOP in 78% of the patients, about half of them requiring IOP-lowering surgeries.3 Topically, ocular treatment with glucocorticoids produces a dose-dependent IOP increase in 30% to 40% of the population,4-6 and in 90% of patients with primary open angle glaucoma (POAG).7,8 Steroid responsive individuals (termed “steroid responders”) are more likely to develop POAG than the non responder counterparts.9 The ocular hypertensive effect of glucocorticoids is also significantly greater in older age groups.10 Although it is reversed when the steroid treatment is stopped,8,10 this adverse effect of glucocorticoids continues to be a major impediment on the clinical management of eye diseases. Because of the essential need to use steroids for serious eye disorders, the search for a treatment to control steroid-induced hypertension is of major importance for the eye.
Glaucoma is a complex optic neuropathy that, if left untreated, results in irreversible blindness. Glaucoma is currently the leading cause of irreversible blindness worldwide. It is estimated that by 2020 there will be 79.6 million cases of glaucoma11 which will increase to 111.8 million by 2040.12 The disease is caused by the death of retinal ganglion cells (RGC) and degeneration of the optic nerve. It is well-established that elevated IOP is the main risk factor associated with the development of the disease.13,14 Physiological and/or elevated IOP is determined by the resistance offered to aqueous humor flow by the trabecular meshwork. The elevated IOP generated in the anterior segment is transmitted to the back of the eye where the sclera senses the pressure fluctuations and exerts a biomechanical strain on the optic nerve, contributing to the death of RGCs.15,16 The trabecular meshwork is a spongiform soft tissue, located at the angle formed by the iris and cornea, and is formed by different types of endothelial-like cells which use a variety of functions to regulate IOP. One of its most relevant functions is the one that controls extracellular matrix (ECM) composition and deposition levels, which has been shown to have a direct correlation with increased aqueous humor flow resistance and glaucoma.17,18
In searching for mechanisms to understand and reduce the hypertensive outcome of glucocorticoids, their effects on the trabecular meshwork have been extensively studied. Dexamethasone decreases trabecular meshwork phagocytosis,19 increases ECM deposition, 20-22 and decreases expression of matrix metalloproteinase 1 (MMP1),23,24 all leading to the obstruction of the aqueous humor outflow pathway and to increased IOP. A treatment addressing these cellular and molecular adverse consequences could counteract the glucocorticoid’s elevated IOP effect. In particular, a long-term, regulated, gene therapy treatment could provide an attractive solution to the high number of people affected by the high IOP steroid response.
MMP1 is an interstitial collagenase and an integral component of the trabecular meshwork scaffold. MMP1 has been the subject of outflow facility studies.25,26 In the trabecular meshwork, MMP1 is downregulated by dexamethasone23,24 and upregulated by latanoprost, a commonly used glaucoma drug.26-28 Recently we have shown that a glucocorticoid inducible MMP1 adenoviral vector Adh.GRE.MMP1 delivered and secreted active MMP1 to the trabecular meshwork of human postmortem eyes placed on perfused anterior segment organ cultures.29 The vector contained a minimal promoter from the Herpes Simplex Virus (HSV) thymidine kinase gene (pTAL) followed by the insertion of three tandem copies of glucocorticoid response elements (GRE) on the 5’ of the MMP1 cDNA.29 Active protein delivery was inducible by dexamethasone and remained silent once the steroid was removed from the cultures.29 Further, when the adenoviral inducible vector was injected into the anterior chamber of the eye in a sheep model of steroid-induced hypertension, it reduced and prevented elevated IOP for approximately 10 days.30
In our quest to develop a potential clinical vector for the treatment of steroid-induced glaucoma, and taking into account the need for an extended on and off glucocorticoid treatment, here we investigate the same steroid-inducible MMP1 cassette under a safer, longer term delivery protocol. We also sought to confirm the effect on different types of sheep on site and to investigate newer glucocorticoids drugs. The vectors used in the first study, replication-deficient adenovirus vectors, have proven to be very efficient for trabecular meshwork delivery studies in vitro and in small and large animals.31-34 However, their short length of expression and their immune response precluded them for gene therapy based eye treatments. Instead, because of their long-term expression, low immunogenicity and success in clinical trials, adeno-associated vectors (AAV) have been the vectors of choice for gene therapy of the eye.35,36 Although AAV vectors are unable to transduce trabecular meshwork cells,37 our laboratory has shown that their lack of transduction was due to the inability of its cells to convert the viral single stranded DNA to double stranded, and as consequence, it was overridden by the use of self-complementary AAV (scAAV).37,38 In monkeys, a single intracameral dose of scAAV2.GFP conferred expression to the trabecular meshwork for over two years, with an early onset and a safe clinical profile.37 Thus, for this study, the same inducible MMP1 cassette was transferred to a scAAV2 viral backbone for the assay in a sheep steroid-induced hypertension model.
Animal models of steroid-induced hypertension have been traditionally difficult. The requirements of eye drops several times daily for several weeks, together with a response in only a given percentage of the animals, posed an important limitation.39 In small animals, such as rats, steroid eye drops to both eyes 4× at day resulted in significant elevation of IOP after 2-4 weeks.40 Likewise, mice dosed 4× a day showed IOP elevation beginning at 2 weeks and continuing up to 7 weeks of continuous drops.41 Also in mice, surgical subcutaneous implantation of osmotic minipumps induced 30% IOP elevation by 3 weeks requiring 20-32 animals to reach significance.42 In large animals, non-human primates trained to receive the steroid 4× a day for 4 weeks showed significant IOP elevation starting at 1 week. Interestingly, the IOP increase response occurred only in 40% of the monkeys, similarly to the response seen in the ophthalmology clinic.43 Instead, sheep exhibit 100% response to steroids and thus have been identified as a good animal species for a steroid-induced ocular hypertension model.44 In addition, because of their docile nature, uncaged upkeep and easy handling, steroid treatment and IOP evaluations can be conducted on awake animals. Their lower cost than monkeys adds to the advantages of the sheep as a large animal model.
Integrating all the past findings together, in this study we investigated the possibility of developing a potential clinical treatment of steroid glaucoma using the sheep model and scAAV2 vectors. No scAAV vectors had been previously tested for a long-term IOP reduction on a steroid-induced hypertensive animal. This is also the first study of a systemic biodistribution of viral particles (VP) after injection of scAAV2 into the anterior chamber of the sheep or any other large animal.
Results
Large animal clinical model of steroid-induced glaucoma
Eyes (n=20) in a total of 10 sheep, ages 7 months to 3 years, breeds Katahdin and Corriedale were used in this study. As a first approach to evaluate the therapeutic effect of MMP1 on steroid-induced elevated IOP, we searched for the optimal steroid-induced model in sheep. We compared three types of steroids and three routes of administration, all of which are currently used in the clinic for the treatment of ocular diseases. Difluprednate 0.05% (Durezol) and Prednisolone acetate 1% (Pred Forte) were administered topically as 0.05 ml eye drops three times daily during working hours (8:00 am, 1:00 pm and 4:00 pm) for the duration of the experiment. Triamcinolone acetonide 4% (Kenalog-40) was administered as single 1 ml injections either to the periocular sub-tenon or intraocular vitreous compartments. Pressures were taken one to two times per week. All measurement values from each week were averaged and reported as IOP average per week. Eyes used for controls were treated with PBS and IOPs assessed at the same time points. Comparison of the effects of steroid types and routes of administration on the sheep IOP with time are summarized in Figure 1.
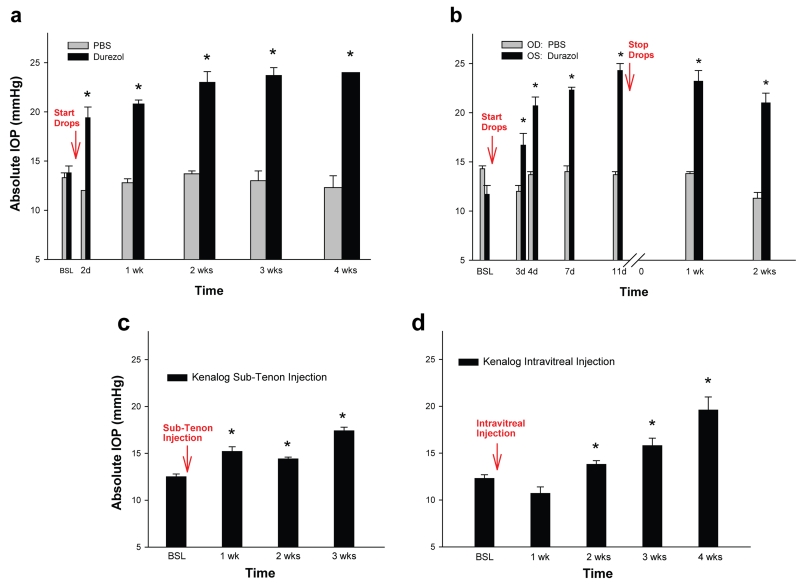
Sheep model of steroid-induced elevated Intraocular Pressure (IOP). Different steroid drugs and routes of administration. (a) Durezol (difluprednate 0.05%) and PBS control were administered as eye drops 3× daily (n=11 and n=4 eyes respectively). IOP was elevated in all durezol-treated eyes and reached 40.6% at 2 days, and 73.9% at 4 weeks (last point). IOP from PBS-treated eyes stayed at baseline. (b) a representative sheep was administered with durezol in the OS and with PBS in the contralateral OD, same regimen as in A. IOP was elevated only in the steroid-treated OS. (c) Kenalog (triamcinolone acetonide 4%) was administered by Sub-Tenon injection (n=2). IOP was elevated in both treated eyes and reached 21.6% at 1 week and 39.2% at 3 weeks (last point). (d) Kenalog was administered by intravitreal injection (n=4). IOP was elevated in two eyes and reached 28.0% at 2 weeks and 59.3% at 4 weeks (last point). *: P < 0.0004. OD: right eye, OS: left eye. Eye topical administration of Durezol induces the fastest and higher ocular hypertensive response in sheep.
The durezol treatment was conducted in a group of n=11 eyes belonging to seven sheep. The average baseline value on these eyes was 13.8 ± 0.7 mmHg. We found that the IOP elevation effect of durezol occurred very fast and was significantly different from baseline at all time points. Two days after the initial dose, IOPs raised 5.6 mmHg, to an average of 19.4 ± 1.1 mmHg (P = 0.0004). After one week, pressures raised to 20.6 ± 0.4 mmHg (P = 2 × 10−7) and further reached 23.0 ± 1.1 mmHg at the end of the second week (P = 5 × 10−7). Three and four weeks from the first topical administration, IOP values continued to show a slight increase and achieved 23.7 ± 0.8 mmHg (P = 7 × 10−7) and 24.0 ± 0.0 mmHg (P = 5 × 10−7) respectively (Figure 1a). At the same time points, eyes treated with PBS drops (n = 4 eyes) had IOPs of 13.3 ± 0.5 mmHg (baseline), 12.0 ± 0.0 mmHg (day 2), 12.8 ± 0.4 mmHg (week 1), 13.7 ± 0.3 mmHg (week 2), 13.0 ± 1.0 mmHg (week 3) and 12.3 ± 1.2 mmHg (week 4) (Figure 1a). At every time point, PBS IOP values were not significantly different than those of their own baseline (P = 0.5 to 0.8). Figure 1b shows similar IOP results from a representative single sheep whose right eye (OD) was treated with PBS while the left eye (OS) was treated with durezol for almost 2 weeks and then stopped. Comparing IOPs from both eyes of the same sheep (n=3) also revealed that the steroid did not have any contralateral effect.
Although with a positive response, three other steroids’ administration resulted in a lower IOP elevation and somewhat more variable response among each of the sheep. Thus, treatment with 1% prednisolone (n=6 eyes) was elevated to 15.5 ± 0.6 mmHg at 4 days over a baseline of 13.8 ± 0.5 mmHg and (P = 0.09). Eye drops were stopped at 16 days and values returned to baseline 24 hours after that time. Administration of kenalog via sub-tenon injection (n=2) induced a moderate, albeit significant elevation of IOP at the end of 3 weeks, reaching 17.4 ± 0.4 mmHg over 12.5 ± 0.3 mmHg of their own baseline (P = 2 × 10−9) (Figure 1c). When kenalog was injected intravitreally (n = 4) the IOP response was higher, reaching 15.8 ± 0.8 mmHg at 3 weeks and 19.6 ± 1.4 mmHg at 4 weeks over their own baseline of 12.3 ± 0.4 mmHg (P = 2 × 10−4 and 2 × 10−6 respectively) (Figure 1d).
Altogether, there results indicate that administration of durezol induces the fastest, higher and uniformly sustainable steroid-induced elevated IOP and would thus serve as a good model for this type of glaucoma in a large animal. Response to the steroid was 100%, with highly significant elevation of 6.8 mmHg after one week and 10.2 mmHg after four weeks. IOP values after durezol administration exhibited less variability among the eyes. Neither age nor breed had a significant effect on the IOP response to durezol. For example, at the end of the first week, old Katahdin sheep (n = 5 eyes) exhibited an IOP increase of 21.0 ± 0.6 mmHg over a baseline of 15.2 ± 1.0, while young Katahdin (n = 3) exhibited an IOP increase of 19.3 ± 1.2 mmHg over a baseline of 11.7 ± 0.3 (P between old and young = 0.16). Similarly, old Corriedale sheep (n = 3 eyes) exhibited an IOP increase of 19.8 ± 0.8 mmHg over a baseline of 14.0 ± 1.2, while old Katahdin exhibited an IOP increase of 21.0 ± 0.6 mmHg over a baseline of 15.2 ± 1.0 (P between Corriedale and Katahdin = 0.2).
Selection of viral vector and combinatory elements
We generated a glucocorticoid inducible scAAV2 vector where the full human MMP1 cDNA with Kozak sequences was driven by a 148 bp TATA-like region from the HSV thymidine kinase promoter (PTAL),45 commonly used for the assay of transcriptional enhancers. The 45 bp glucocorticoid response element (GRE) used here contained two directs and one inverse repeat in between (repeat sequence: GGTACATTTTGTTCT) inserted in a BglII site upstream of the PTAL element. Upstream of the GRE, the vector contains a 153 bp synthetic transcription blocker composed of polyA and transcription pause sites for reducing background.46 The cassette contained a 33 bp MCS in between the transcription blocker and the GRE, and a 247 bp SV40 polyA signal at the 3’ end. This cassette was isolated from an adenovirus shuttle vector (pMG17)29 and inserted into a the pHpa-trs-SK plasmid47 containing the adeno-associated vector (AAV) wild-type and mutated- terminal repeats and an additional 196 bp bovine growth hormone (BGH) polyA signal (pMG21). This vector comprising an entire transgene expression cassette of 2,249 bp (Figure 2) was used for the generation of the self-complementary vector serotype 2 (scAAV2.GRE.MMP1). Characterization of the steroid inducibility of the enzyme MMP1 was conducted in vitro by infecting primary human trabecular meshwork (HTM) cells with the viral vector and analysis of MMP1 expression by TaqMan real time RT-PCR. Addition of 10−7 M dexamethasone to the HTM cells induced expression of MMP1 478-fold after 4 days.
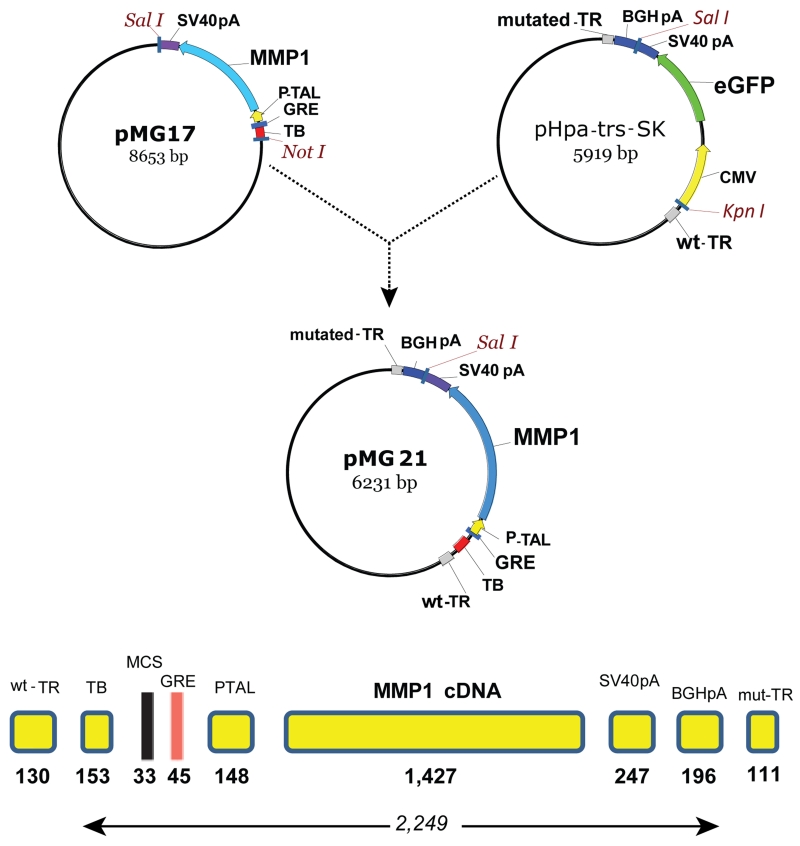
Simplified cloning strategy and regulatory elements of plasmid pMG21 used to generate the scAAV2.GRE.MMP1 virus. TR, terminal repeat; TB, transcription blocker; MCS, multiple cloning site; GRE, glucocorticoid response element; PTAL, HSV thymidine kinase promoter; MMP1, human matrix metalloproteinase cDNA; SV40pA & BGHpA, termination signals, pMG17, adenovirus shuttle29 and pHpa-trs-SK, terminal repeat containing plasmid.47 pMG21 was co-transfected with pXX6-80 and pXX262 to generate the therapeutic virus.
Single dose of scAAV2.GRE.MMP1 reduces steroid-induced elevated IOP long-term
To investigate whether a single dose of the inducible MMP1 viral vector was able to specifically reduce the steroid-induced elevated IOP long-term, a series of complementary experiments were performed in a total of 10 sheep (7-36 months of age) with scAAV2 viruses, which are known to transduce the trabecular meshwork in vivo for at least 2 years.38 At different times after steroid-induced elevation (from 4 to 17 days), sheep eyes were injected into the anterior chamber with 0.3-1 ×1011 viral genomes (vg) (maximum 100 µl volume) of either scAAV2.GRE.MMP1 (treated group), scAAV2.GFP, or left uninjected (control groups). One sheep received adenoviral vectors. Reduction of steroid-induced IOP of the treated versus the control was observed in all sheep that received the MMP1-carrying vectors. Results are summarized in Figures 3 and and44.
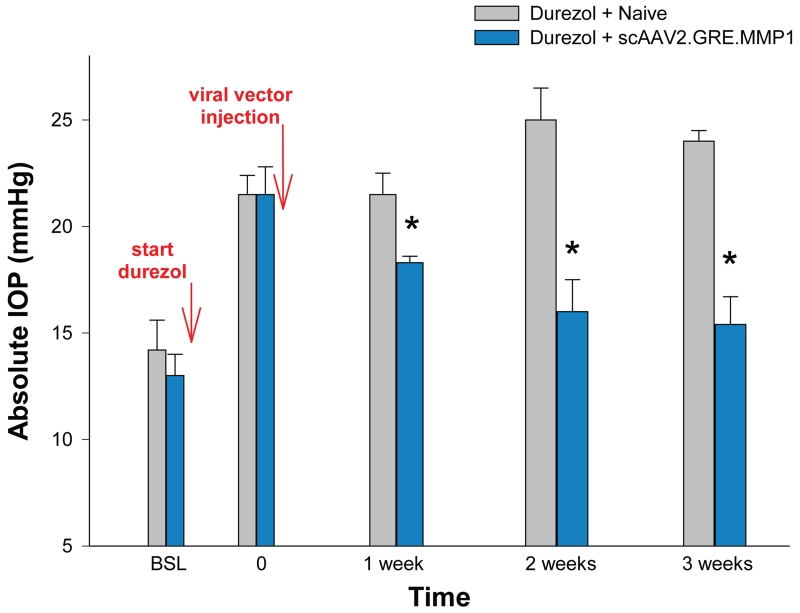
Single intracameral dose of scAAV2.GRE.MMP1 reduced durezol-induced hypertension in sheep. After baseline readings, sheep eyes (n=8) were treated topically with durezol 3× a day to induce elevated IOP (T=0). Four of the eyes were intracamerally injected with 0.3-1 × 1011 scAAV2.GRE.MMP1 viral particles (blue bars). The other four eyes were left uninjected (grey bars). Durezol administration continued after T0 in all eyes for 3 weeks. The ΔP-IOP of viral-treated eyes was reduced from 8.5 mmHg (durezol-T0 minus baseline) to 5.3 mmHg (durezol-virus minus baseline) (37.6%) at 1 week, and from 8.5 mmHg (durezol T0 minus baseline) to 2.4 mmHg (durezol-virus minus baseline) (71.8%) at 3 weeks. *: P < 0.0005. Viral injected eyes showed reduction of elevated IOP and continued to prevent hypertension for the 3 weeks duration of the experiment.
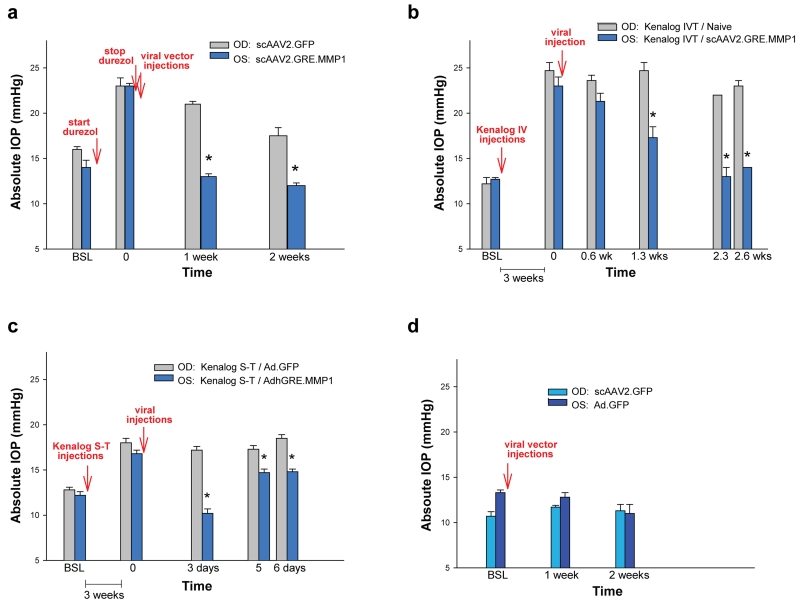
scAAV2.GRE.MMP1’s reduction of IOP is extended to other regimens. (a) when using topical durezol to raise the IOP and removing the steroid at post-viral injection, the MMP1-virus lowered the ΔP surge from 8.7 mmHg (durezol-T0 minus baseline) to less than baseline levels (MMP1-virus minus baseline = −0.7 mmHg) in 1 week. At the same time-point, the ΔP on the control GFP-virus went from 7.4 mmHg to 4.4 mmHg. The effect of the durezol slowly declined and began to disappear at two weeks. (b) when using intravitreal (IVT) kenalog injection to raise the IOP, the MMP1-virus reduced the ΔP surge from 10.3 mmHg (kenalog IVT-T0 minus baseline) to 4.6 mmHg (MMP1-virus minus baseline) (55%) in 1.3 weeks. At the same time-point, the ΔP on the control non-virus eyes stayed the same (ΔP 12.5 mmHg) (0%). At 2.6 weeks, the ΔP in the MMP-1 virus was reduced to 1.8 mmHg (82.5% reduction) while that of the control was reduced just to 10.8 mmHg (13.6%). (c) when using Sub-Tenon (S-T) kenalog injection, an AdhGRE.MMP1 virus reduction of ΔP was short-lived. At 3 days, it went from ΔP 4.6 mmHg to lower than baseline (ΔP −2.0 mmHg) but the lowering effect started to wear off at 6 days. The control, Ad.GFP did not result in any ΔP reduction. (d) both control viruses, (scAAV2.GFP and Ad.GFP) were innocuous in normal eyes. *: P < 0.008. OD: right eye, OS: left eye. MMP-1 carrying viruses reduced the steroid-induced pressure under all steroid and route of administrations tried.
After baselines measurements, the first set, pre-control eyes (n=4) and pre-viral treated (n=4) received topical durezol 3× a day to obtain elevation of IOP, which was assigned as time zero (T=0). All eyes exhibited IOP increases. In the pre-control group IOP rise was from 14.2 ± 1.4 mmHg (baseline) to 21.5 ± 0.9 (P = 0.005) and in the pre-viral treated group it went from 13.0 ± 1.0 mmHg (baseline) to 21.5 ± 1.3 (P = 0.005). At this time (T=0), eyes from one group were injected with the scAAV2.GRE.MMP1 therapeutic vector (treated) while the eyes from the second group were left naïve (control). Durezol dosing continued in the eyes of both groups to the end of the experiment. Pressures in the control group stayed high with values of 21.5 ± 1.0, 25.0 ± 1.5 and 24.0 ± 0.5 mmHg after 1, 2 and 3 weeks respectively. Pressures in the viral-treated group were significantly reduced with values of 18.3 ± 0.3, 16.0 ± 1.5 and 15.4 ± 1.3 mmHg at the same time points (P = 0.002, 5 × 10−8, and 1 × 10−10 respectively) (Figure 3).
Additional experiments under different steroids and routes of administration confirmed the finding. In one sheep, where topical durezol was stopped after attaining the high response and after viral injections (Figure 4a), the scAAV2.GFP-injected control eye IOPs went from 23.3 ± 0.9 mmHg at T=0 to 20.7 ± 0.3 mmHg after 1 week. In contrast, the therapeutic MMP1-treated contralateral eyes IOPs went from 22.7 ± 0.3 mmHg at T=0 to 13.3 ± 0.3 mmHg at 1 week (viral-treated vs control P = 0.0001) (Figure 4a). In this sheep, 2 weeks after stopping the durezol, eyes returned to near baseline in the control eye (17.5 mmHg from 16.3 mmHg baseline) or to lower than baseline in the MMP1-treated eye (12.0 mmHg from 14.0 mmHg baseline) (Figure 4a). This result indicates that, while in the control eye the durezol effect was lasting over two weeks, the MMP1 transgene was able to cause a rapid decrease to baseline as soon as one week, and probably earlier.
In another sheep, where pressure elevation in both eyes was induced by intravitreal injections of kenalog followed by a viral injection in one eye (Figure 4b), the non viral-treated, control eye maintained the elevated IOP (24.7 ± 0.9 mmHg) at 2.6 weeks post-steroid elevation (23.0 ± 0.6 mmHg). At the same time points, the IOP of the contralateral, scAAV2.GRE.MMP1-injected eye, was reduced from 23.0 ± 0.9 to 14.0 ± 0.6 mmHg (treated vs control P = 0.0001) (Figure 4b). In this animal, IOP reduction was observed at 0.6 weeks and become significant at 1.2 weeks (treated vs control P = 0.008) (Figure 4b).
The effect of the therapeutic vector in reducing the elevated IOP induced by sub-tenon injection of kenalog was tested in an additional sheep with the short-term adenoviral vectors, AdhGRE.MMP1 therapeutic and Ad.GFP control (Figure 4c). The post-steroid IOP elevation by this route of administration was lower, reaching 18.0 ± 0.5 and 16.8 ± 0.4 mmHg in the pre-control and pre-viral treated. In the control eye, IOP was maintained to 18.5 ± 0.4 mmHg at 6 days post-Ad.GFP injection, while in the contralateral MMP1-treated eye (AdhGRE.MMP1), IOP was reduced from 16.8 ± 0.4 mm Hg to 14.8 ± 0.3 mmHg at the same time period (treated vs control P =4E-5) (Figure 4c). The IOP reduction effect was more pronounced at three days post-viral injection but started to wear off after that time (Figure 4c).
Effects on IOP of the control vectors, both the long- and the short-term ones were tested in one sheep (Figure 4d). Neither scAAV2.GFP (OD, right eye) nor Ad.GFP (OS, left eye) induced IOP changes from baseline at 1 and 2 weeks post-injection. The IOPs of the scAAV2.GFP-injected eye were 10.7 ± 0.5 mmHg at baseline and 11.7 ± 0.2 and 11.3 ± 0.7 mmHg at 1 and 2 weeks respectively (P = 0.1 and 0.7 respectively). The IOPs of the Ad.GFP-injected eye were 13.3 ± 0.3 mmHg at baseline and 12.8 ± 0.5 and 11.0 ± 1.0 mmHg at 1 and 2 weeks respectively (P = 0.5 and 0.2 respectively). (Figure 4d).
All together, these studies demonstrate that the transgene human MMP1 cDNA, delivered by scAAV2 gene therapy vectors, is able to counteract the elevated pressure induced by steroids in the sheep eyes. Single treatment with the virus also prevented IOP elevation despite continuing steroid administration. This efficacy is extended to all steroids and all routes of administrations studied. Likewise, the effect is observed in young and older animals, and in all the breeds studied. The effect of a single dose lasted for at least 3 weeks, which was the last time point studied.
Toxicity effects: clinical signs and histopathology
No adverse clinical signs were observed in the eyes of any of the sheep during the experiments. No redness or tearing occurred, and cornea and lens remained clear. No formation of cataracts was observed. The morphology of the outflow pathway area was assessed by light microscopy in paraffin embedded quadrants of the anterior segment sectioned on the meridional plane. Five µm sections from a total of 15 blocks (naïve, durezol and durezol/ MMP1-treated eyes) were analyzed. We first observed that the outflow pathway area of the sheep eye exhibited a unique shape. The trabecular meshwork tissue is meridionally wider than in most described species. It crosses the angle and extends inferiorly deep into the iris root, which is thinner to make space for this part of the meshwork. The tissue appears to have a more reticular configuration than the higher defined beam structure observed in humans, but areas reminiscent of the classical three layers of this tissue are distinguishable. The area underneath the Schlemm’s canal appears to be compact, while the corresponding trabecular meshwork area shows the appearance of some beams and wide spaces in between which become more prominent in the corresponding uveal layer. The Schlemm’s canal is present and occasionally looked fragmented (Figure 5, top row). In contrast, the outflow pathway of the sheep eye administered topically with durezol looked very different. The angle remained open but the intercellular spaces in all layers of the trabecular meshwork appeared obstructed due to the expected deposition of excessive extracellular material. The whole area looked significantly more compact (Figure 5, middle row). After intracameral injections of the therapeutic vector scAAV2.GRE.MMP1, the outflow pathway reverted to a morphology which was closer to the naïve animal trabecular meshwork. The intercellular spaces became more open as in what could be an indication of clearing of the ECM by the action of MMP1. The overall architecture, and the opened Schlemm’s canal was preserved. Results from this morphology study provide an explanation, and nicely agree with the physiological IOP results obtained above. After the administration of the steroid, an obstructed outflow pathway would lead to an increase aqueous humor flow resistance and elevated IOP (Figures 5 and and1),1), while clearance of the extracellular material by the extra levels of delivered MMP1 will allow aqueous humor to flow freely and the eye to return to physiological pressure.
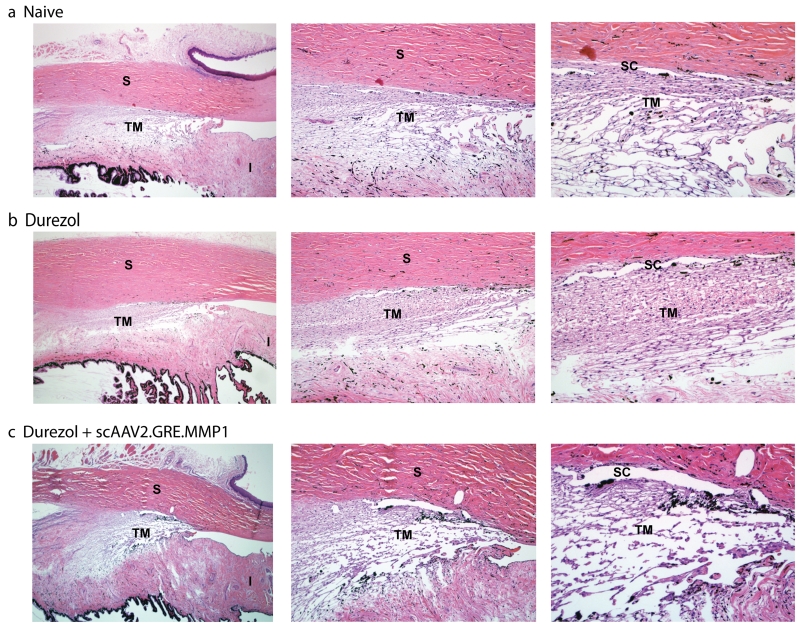
Morphology of the sheep’s trabecular meshwork after steroid and viral transgene treatments. Representative 5 µm light microscopy meridional sections from paraffin embedded sheep eye angle tissue, stained with hematoxylin and eosin. (a. Naive) sheep eye dosed 3× a day with PBS for 28 days. The trabecular meshwork in sheep is meridionally wider than in other animal species and extends inferiorly to the iris root. Although with some reticular appearance, traditional layers are distinguishable. Schlemm’s canal is present and occasionally looks fragmented. (b. Durezol) sheep eye dosed 3× a day with durezol for 25 days. Angle is opened and there is a considerable increase of extracellular material deposition in all layers. (c. Durezol + scAAV2.GRE.MMP1) sheep eye dosed with durezol 3× a day for 30 days. Viral vector was injected at day 9. Architecture is preserved, Schlemm’s canal is open, and intercellular spaces are cleared from the increased ECM deposition. Original magnification 40× (left), 100× (middle), 200× (right). TM: trabecular meshwork, SC: Schlemm’s canal, S: sclera, I: iris. Morphology of the steroid-treated trabecular meshwork reverts to that of PBS treated after therapeutic vector injection.
Viral genome distribution
Distribution of human MMP1 viral DNA vg in the sheep internal organ tissues after the single intracameral injection was analyzed by quantitative TaqMan PCR on six major organs (brain, heart, kidney, liver, lung and spleen) from three sheep, where one of the two eyes was injected with 8 ×1010 VP of scAAV2.GRE.MMP1 while the contralateral eye was naïve or injected with PBS. Tissues were also collected from the different compartments of the eye: trabecular meshwork, iris/ciliary body, cornea, and retina. Tissues were collected at necropsy at 12, 22 and 30 days post-injection respectively, and stored at −80ºC until use. For the genomic DNA (gDNA) determination, standard curves were obtained with sheep commercial gDNA (Zyagen, San Diego, CAL, USA) and the single copy human RPPH1 (Applied Biosystems / Thermo Fisher Scientific, Waltham, MA, USA) probe. Because of the reduced homology between the sheep and human genomes, sheep DNA concentrations (NanoDrop ND-100 spectrophotometer, Thermo Fisher) were previously adjusted to obtain CT values between 26 and 36, which corresponded to concentrations between 180-0.3 ng/µl (R square ≥ 0.98). All CT values from each of the sheep gDNA experimental samples fell inside the standard curve. For the determination of the number of vg in the sample, the same volume from the same tube used for the gDNA determination was hybridized to the transgene human MMP1 cDNA TaqMan probe (Applied Biosystems/ Thermo Fisher). The standard curve for the vg was obtained with the purified plasmid vector DNA (pMG21) adjusted to detect from 68,000 to 6.8 plasmid copies which yielded CT values between 22 to 37 (R square ≥ 0.98). Results were expressed in vg/ cell. The results of a representative sheep shown in Figure 6a-left indicate that negligible to no viral genomes per cell were found in the major organs of the three sheep at all time-points after viral injection.
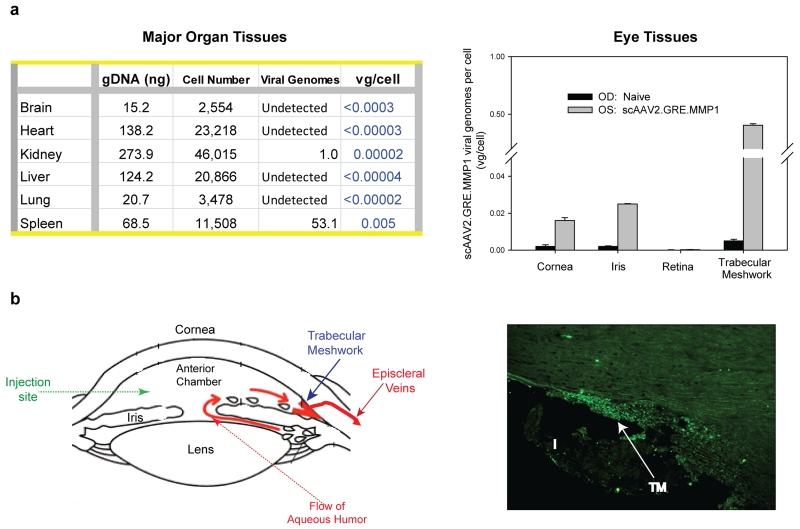
Distribution of the MMP1 vector genomes per cell (vg/cell) in tissues collected at the termination of the study. (a) major organs (left) and eye tissues (right) DNA from a representative sheep injected intracamerally in one eye with 8 × 1010 VP of the MMP1-vvirus. Taqman probes were single-exon human RPPH (gDNA) and exon-spanning human MMP1 cDNA (viral genomes). Lower limit of the vg/cell is calculated based on the minimal detection of the TaqMan run (0.8 vg) (b) (left): diagram of the anterior segment of the eye showing delivery site and flow direction and drainage of the aqueous humor (right): 10 µm meridional cryosection of the trabecular meshwork from a sheep eye injected intracamerally with 1 ×1011 VP scAAV2.GFP. TM: trabecular meshwork; I: iris
Determination of vg in four main eye tissues was conducted in four sheep also at harvesting. Figure 6a-right shows eye tissues results from the same sheep shown in Figure 6a-left. Since eyes were injected intracamerally (diagram in Figure 6b-left), the posterior segment (retina) showed negligible vg copy numbers/ cell. Likewise, as expected, the anterior segment tissues showed detectable number of vg. The highest number was observed in the target outflow tissue, the trabecular meshwork, and ranged from 0.4 ± 0.01 vg/ cell (Figure 6a right) to 5.2 ± 0.10 /cell. The iris and cornea tissue values were about ten times lower with ranges of 0.025 ± 0.0002 (Figure 6a right) to 0.85 ± 0.02 and 0.016 ± 0.002 (Figure 6a-right) to 0.03 ± 0.002 vg/ cell, respectively. Together these results indicate that no detectable viral DNA spread to unwanted parts of the body after intracameral injection, while a small amount of these genomes are able to persist in the targeted trabecular meshwork tissue.
Fluorescent microscopy of one sheep eye injected with 1×1011 vg of scAAV2.GFP revealed intense fluorescence only in the cells of the trabecular meshwork region, confirming the targeting of the virus (Figure 6b-right).
Discussion
Clinical management of steroid induced hypertension requires close monitoring of IOP elevations and the concomitant use of glaucoma drugs and/or surgery.48 In some cases, it requires cessation of the treatment.48 Although all steroid administrations have been shown to lead to steroid-induced glaucoma, the recent popular use of triamcinolone acetonide intravitreal injections for macular edema and choroidal neovascularization has resulted in a higher incidence of steroid-induced hypertensive cases,49,50 all of them with limited treatment possibilities.51 A gene therapeutic approach, based on molecular findings of the recent years would offer new options for the management of the disease.
Of the several gene targets that could have been selected for the treatment of steroid-induced glaucoma, the gene encoding the MMP1 enzyme proved to be a successful one. In our original studies, we showed that MMP1 was highly efficient in reducing steroid-induced ECM accumulation and the subsequent obstruction of the trabecular meshwork outflow pathway leading to elevated IOP.29 In there, adenoviral MMP1 vectors, designed to be inducible only in the presence of the steroid, demonstrated their molecular and functional properties in vitro and in perfused human organ cultures,29 and reduced IOP in a steroid sheep model at the University of Corrientes (Argentina).30,44 To continue towards the development of a potential gene therapy treatment in humans, here we investigated the feasibility of establishing the sheep model on site, the induction of elevated IOP by different steroids and routes, the efficacy of the inducible MMP1 cassette long-term in a scAAV2 vector, and the systemic distribution of the viral particle after delivery to the eye.
The use of small animals such as rodents to study steroid glaucoma can provide useful molecular information about mechanisms. However the development of a human treatment for steroid glaucoma would require large animals whose size, structures, and pharmacokinetics would mimic better that of humans. Of the current large animals available for eye research, only cow and sheep appeared to be amenable for the study of steroid-induced hypertension. There was just some moderate IOP response in cats,52 and dogs, and in these, only in those dogs with inherited glaucomatous53 (reviewed in39). In the non-human primate, which is the favorite species for glaucoma studies, the glucocorticoid response occurs in just 40% of the animals,43 while in the cow and sheep the response is 100%.44,54 Of the two, sheep are logistically the easiest animal to keep, because of their docile nature, easy training and because of being able to conduct IOP evaluations in non-sedated animals. The current study using sheep from local North Carolina farmers showed no significant difference among the breed or the age of the sheep. The ten sheep used here were from the Katahdin and Corriedale breeds and range from 7 months to 3 years of age. We did find a difference, though, on the response to different type of steroids and among the route of administrations used.
The majority of steroid induced studies in the literature have used dexamethasone (either 0.1% topical 40,41,43,51 or 4% systemic 42,55) or prednisolone at 0.5%.44 In our studies, 1% prednisolone induced elevated IOP at a later time and lower level than it did in the Argentina sheep model.30,44 Instead, the newer topical steroid drug durezol resulted in a rapid and consistently higher response, with significant average IOP elevation of 5.6 mmHg after 2-4 days. Neither topical prednisolone, nor periocular sub-tenon, nor intravitreal kenalog injections induced a similarly high IOPs at an early time. The durezol findings in sheep appear to correlate with results reported in an uveitis treatment clinical trial where durezol IOP elevation response was high and early even in patients considered to be no responders to prednisolone.56 In such study, response was higher in children than in adults, while we saw no difference between the young and older sheep in our study. The durezol induced IOP response increased with time, reaching average elevations of 9.2 mmHg at 2 weeks and 10.2 mmHg at 4 weeks, when the experiment was terminated. In our sheep, kenalog intraocular injections induced also elevated IOP albeit at a slower rate, and to a lower end point. The sub-tenon route, in contrast to the quick and high response of the Argentine model,30 induced an elevation of only 5 mmHg after 3 weeks. The intravitreal route exhibited a significant, although small elevation of 1.5 mmHg at 2 weeks and reached 7.3 mmHg at 4 weeks. Results from a recent 4-years clinical trial at 75 sites on intravitreal triamcinolone have reported a mean 34-52 days from time of injection to IOP increases larger than 10 mmHg,57 which coincides quite well with the result in sheep. In addition, and consistent with what it has been reported for prednisolone administrations on the sheep,44 and with a chart’s review study on steroid treated patients,58 we did not observe any steroid effect in the contralateral eye using durezol. Altogether, these findings plus logistics associated with the experimental procedure, including daily maintenance costs, place the sheep durezol model as the preferable translational model for steroid-induced gene therapy experiments.
We previously reported in vivo short efficacy of the inducible MMP1 cassette carried in adenoviral vectors.30 While adenoviruses contribute to demonstrate proof of principle, they do not provide the same stable transduction and lack of toxicity than AAVs. Here, the inducible MMP1 transgene inserted in the scAAV2 vector resulted in a reduction of the steroid-induced IOP in every sheep treated. After a single dose injection of the therapeutic vector, the reduction occurred very quickly and was maintained for at least four weeks, when the experiment was terminated. Although we showed efficacy, in this study we did not examine amounts of transgene MMP1 RNA in the trabecular meshwork. However, a qualitative positive transduction was indirectly assessed by the presence of the GFP fluorescence in the sheep trabecular meshwork of an eye injected with the same viral serotype carrying the reporter protein. At the present time we don’t know for how much longer we could obtain efficacy with a single dose, but based on our published reports looking at a reporter GFP in monkeys,38 one would expect to last for at least two years. For this study, we used the higher dose allowed by the titer of the virus, that is, we injected the maximum volume allowed into the small anterior chamber compartment. That translated into using 0.3-1 × 1011 vg per dose. Having seen efficacy, dosing studies will follow to determine the minimal effective dose.
Together these results suggest that a gene therapy approach for the treatment of this crippling secondary effect would result in a much more effective treatment than the use of daily conventional glaucoma drugs, which suffers from the serious compliance issue of the older population. The on and off switch inserted in the MMP1 cassette will additionally protect the trabecular meshwork from an excessive thinning of the ECM when the steroid is not present and ECM build up is not occurring. At the same time, the presence of the switch would allow a sole administration to protect the individual from future high IOP responses resulting from future steroid treatments. We have shown that this on and off cycle occurs in vitro.29 Experiments that would test the latency in the sheep are in progress.
A literature review of the steroid-induced elevated IOP shows that the IOP elevation seen in the clinic appears to peak quite higher than the peak ΔIOP observed on the sheep. In patients, ΔIOP ranges have been reported to be from 10 mmHg to up to greater than 30 mmHg57 while the maximum ΔIOP observed here in sheep was 13 mmHg. We do not know whether, when encountered with a higher clinical IOP response, MMP1 would be able to reduce the same absolute mmHg values observed here or, as also seen in the sheep, to reduce the pressure to closer to baseline. In any case, when the treatment is safe, a 20-30% reduction target is quite desirable and the possibility of higher reduction would be an added benefit.
Existing and completed clinical trials using AAV viral vectors have established the long-term safety of ocular gene therapy approach.36 In this study we performed gross clinical examination of the eye. Eyes were monitored for signs of inflammation, redness, tearing, visual cues and formation of cataracts. No adverse signs were observed and lenses appeared clear at euthanization. Neither the topical administration of the steroid, the intracameral injection or the therapeutic viral vector caused apparent toxicity. After the steroid treatment, the histopathology of the trabecular meshwork showed a thickening of the ECM compared to the naïve eye, which agrees with the numerous reports stating increase of ECM deposition after exposure to glucocorticoids.20-22 We then observed a less dense ECM after the delivery of the MMP1 enzyme, a morphology which appeared to practically revert to the naïve state. We also observed a more reticular morphology of the trabecular meshwork region than the more beam-like structure seen in humans and primates and which is similar to the one observed in bovine.21,59 We see a Schlemm’s canal, which appears to be more elongated than the outflow loops described as a Schlemm’s canal substitute in the bovine.21 Interestingly, we observed that the trabecular meshwork of sheep extends inferiorly and seems to occupy an area of the iris root. This morphology had been also observed, albeit not described, in angle photographs of the fresh bovine eyes,60 and by personal communication (Lütjen-Drecoll). The aqueous humor route of outflow does not seem though to be using the iris-extended trabecular meshwork region. Our data showing fluorescence of a scAAV2.GFP reporter virus injected in the anterior chamber in vivo, which would be indicative of the aqueous humor outflow pathway, shows that the lower trabecular meshwork-iris region is not transduced. This could be an indication that this region of the trabecular meshwork has no part on the filtering function of the tissue and that all outflow occurs only through the “classical” trabecular meshwork site, located at the angle above the iris. Additional experiments would need to be conducted though, to further elucidate whether this observation could be due to other reasons such as that the virus is not able to transduce the lower region.
The systemic biodistribution of the viral particles which were injected into the anterior chamber is a key toxicity parameter to determine the safety of the system and thus the feasibility of taking the treatment to the clinic setting. The aqueous humor leaves the eye through the trabecular meshwork/ Schlemm’s canal and drains into the systemic circulation going through, the aqueous veins, episcleral veins, superior ophthalmic vein and superior vena cava, to the heart. We conducted biodistribution studies not only in the heart but in lung, liver, spleen, kidney and brain. To calculate the number of viral genomes per cell, we developed a protocol using a human MMP1 cDNA probe to quantify the number of viral genomes, and a conserved human single copy gene RPPH probe to quantify the number of sheep cells in the analyzed sample. Validations were carefully conducted, selecting exon-spanning (MMP1) and single-exon (RPPH) probes, performing standards curves with the MMP1 original plasmid vector, with commercial sheep DNA, and running all reactions at the same CT thresholds. Results showed negligible to none viral genomes within the major internal six organs collected at euthanasia 22 days after intracameral injection. Inside the eye, that is, at the local site of injection, the individually analyzed anterior segment tissues were able to retain small number of viral molecules, in particular, the trabecular meshwork. This finding would be of great advantage for the planned steroid treatment proposed here. Since the MMP1 is only transcribed in the presence of the steroid, a number of silent particles remaining inside the trabecular meshwork would be innocuous under standard conditions, and could re-start producing the therapeutic transgene in the event of a next occurrence of steroid treatment. Our studies thus corroborated the safety of the intraocular AAV vectors,61 and reinforce the concept that the availability of a clinically safe proven steroid-inducible expression vector would meet requirements to address the adverse ocular effects of steroid treatment of patients
MATERIALS AND METHODS
Experimental Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of North Carolina and were conducted in accordance with the tenets of the declaration of Helsinki and the Association for Research in Vision and Ophthalmology (ARVO) statement on the Use of Animals in Ophthalmic Research. Ten healthy (female) sheep (Katahdin and Corriedale breed) between 7 and 36 months of age, and weighing 35 to 80 kg each, were selected from local farms in the eastern region of USA, for this study. The sheep and their eyes were examined to be in good health and tested negative for Q-fever upon arrival. Sheep were tagged for individual identification on their ear lobes and kept uncaged, allowing them to room freely in a temperature-controlled 140 square feet animal room, under 12 h light cycling (7 am to 7 pm). Groups of 2 or 3 sheep were used at all times and provided with food and water at libitum. The room was equipped with a showing rail containing a head rest (Vittetoe, Inc, Keota, IA) and custom-adjusted to have just one animal at a time (Figure 7). The room also counted with enrichment toys to enhance the life of the animals. Sheep were adjusted during the first week of arrival with daily petting and training to enter the show rail. Animals were very gentle and non-threatening. After each non-invasive procedure (drops instillation or IOP measurements), sheep were rewarded with raisins which they eat out of the operators hand.
Measurement of IOP on Conscious Sheep with the Handheld Tonometer
For the IOP measurements, sheep were guided to the rail one at a time, heads placed on the head rest and gently restrained by two loose chains around the neck and snout. This arrangement allowed one person to secured the sheep from behind and gently hold the eye lids opened, while the second operator measured the IOP from both sides outside the rail (Figure 7). IOPs measurements were conducted by the use of the TonoVet rebound tonometer (Colonial Medical Supply, Franconia, NH) positioning the visual axis horizontal to the probe and following manufacturer’s recommendations. Before the IOP measurement, 2 drops of topical 0.5% tetracaine (Bausch & Lomb, Tampa, FL) were instilled. Three sets of five measurements were taken on each eye. Only mean values with a SD (expressed as percentage of the mean) less than 5% were accepted. All IOP measurements were taken between 1 pm and 3 pm every 2-3 days. The whole operation took between 5 to 10 min. Baseline IOPs from each eye were taken at one week post-arrival, and two to three times at several days before any treatment. Values were averaged and taken as related to the baseline of the given eye.
Administration of Steroids
Steroids drops were topically applied by a single operator after guiding the sheep to the rail or to the rail corner of the room, secure them from behind and gently opening their eye lids. Two to three days after determination of baseline IOPs, two drops of 1% prednisolone acetate ophthalmic suspension (Pred Forte, Alcon Laboratories Fort Worth, TX) or two drops of 0.05% difluprednate ophthalmic emulsion (Durezol, Alcon Laboratories) were topically instilled three times daily at 8 am, 12 pm and 5 pm Monday to Friday and for the durations indicated in each of the experiments. In some experiments, the steroid was administered to both eyes; in others, only one eye received the steroid.
Ocular hypertension was also induced by periocular injection into the sub-tenon space. After topical instillation of two drops of tetracaine 0.5% (Bausch and Lomb), a single 1-ml injection of sterile triamcinolone acetonide (Kenalog, 40 mg/mL or 4%; Bristol-Myers Squibb, Princeton, NJ) was injected into the sub-tenon space, 5 mm from the limbus using a 30-G, ½ inch needle.
For intravitreal injections of the triamcinolone, sheep were anesthetized with an intramuscular injection of a ketamine/xylazine (Butler Schein, Columbus, OH) cocktail to achieve a concentration of 3.5 mg/kg (ketamine) 0.6 mg/kg (xylazine) respectively. Occasionally, sheep received a buster of 2 mg/ kg ketamine. A 100 µl of the steroid suspension was loaded into a 100 µl 710LT Hamilton syringe (Hamilton, Reno, NV, #80601) equipped with a 30 G, ½ inch needle. The needle was inserted at 5 mm posterior from limbus at an 80ºC angle towards the posterior pole. Once inside, an assistant pushed slowly the plunger to deliver the steroid into the vitreous compartment of the eye.
Construction of the viral vector scAAV2.GRE.MMP1
The three recombinant plasmids used for the generation of the scAAV2.GRE.MMP1 virus were: pXX6-80, carrying the adenoviral helper function genes 62, pXX2, carrying the AAV virus rep and cap genes to confer serotype 2 62, and pMG21, containing the AAV terminal repeats and the transgene expression cassette encoding a steroid-inducible human matrix metalloproteinase 1 (MMP1). Plasmids pXX6-80 and pXX2 were obtained from the University of North Carolina (UNC) Vector Core facility (http://genetherapy.unc.edu/services.htm) and pMG21 was generated in our laboratory.
Plasmid pMG21 was derived from pMG17, which has been described.29 Plasmid pMG17, containing a transcription blocker, GRE sequences, a TATA-like PTAL minimal promoter, a human MMP1 cDNA and a SV40 polyA, was linearized with NotI, blunted and further digested with SalI (Figure 2). The plasmid vector backbone was pHpa-trs-SK,47 which contains one wild-type and one-deleted AAV terminal repeat to allow the generation of a double stranded AAV (scAAV) upon co-infection of HEK293 cells with pXX2 and pXX6-80. This plasmid was provided by the UNC Gene Therapy center.
To make pMG21, the 2,054 bp gel purified 5’-Not1.blunt/ Sal1-3’ fragment from pMG17 was inserted into the pHpa-trs-SK vector backbone fragment obtained by gel purification of a 4,177 bp 5’-KpnI.blunt/ Sal1-3’ fragment DNA. Thus, the resulting pMG21 (6,231 bp) contains one wild type and one deleted terminal repeats, the inducible MMP1 expression cassette and an additional polyA site from the cloning vector (Figure 2). Triple transfection with pXX6-80, pXX2 and pMG21 for the generation of the recombinant virus serotype 2, scAAV2.GRE.MMP1, was conducted at the UNC Vector Core as previously described.62 Viral preparations used in this study had, for controls: 1×1012 vg/ml (scAAV2.GFP) and 1.6×1012 vg/ml (Ad.GFP), and for the transgene: 2.4×1011 vg/ml (AdhGRE.MMP1) and 2.5×1011 vg/ml and 7.6×1011 vg/ml (scAAV2.GRE.MMP1).
Intraocular Administration of Recombinant Viral Vectors
Sheep were anesthetized with a ketamine/ xylazine cocktail as indicated above for the intravitreal injection of kenalog. While resting on its side on the surgical table, the correspondent sheep eye was facing upward and placed under a KL 1500 LCD light source (Zeiss, Peabody, MA). The corneas were anesthetized with one drop of 0.5% tetracaine (Bausch and Lomb). Up to 100 µl of the viral vector solution was loaded into a 710LT Hamilton syringe with a 30-G, ½ inch needle. For the intracameral vector delivery, the needle was inserted through the superior cornea a few mms anterior to the limbus, with the bevel up to gently reach the center of the anterior chamber. Once inside, the sample was delivered by the assistant over 30 seconds and fluid entrance monitored by direct visualization. Because of the size of the eye, the injections were performed with the use of GL4, 2.5 magnification × 340 mm working distance loupes (Ted Pella, Redding CA) without the need of the ophthalmic microscope. The needle was left in place for 1 to 2 min and gradually withdrawn to minimize leaking. Topical antibiotic ointment (neomycin 3.5 mg/g, polymyxin B 10,000U/g, and bacitracin 400 U/g; Akorn, Lake Forest, IL) was placed on the eye, and animals were returned to the pen and kept with their head propped up until recovery (between 15 to 30 min).
Histology and Fluorescence Histochemistry
Eyes were enucleated immediately after euthanasia and immersed in fresh 4% paraformaldehyde (PFA) for 30 min. Eyes were subsequently dissected above the equator, the lens removed and wedge shape specimens containing the trabecular meshwork were post-fixed to assess morphology and green fluorescent protein (GFP). For morphology, tissue wedges were immersed in 4% PFA/ 2.5% glutaraldehyde/ 0.1M cacodylic acid, pH7.3 at 4ºC overnight. Specimens were then rinsed in distilled water for 10 min and transferred to 70% ethanol for delivery to the UNC Histology Core facility for paraffin embedding. Meridional sections 5 μm sections were mounted on microscope slides (Superfrost Plus, Thermo Fisher Scientific) and stained with hematoxylin and eosin.
To evaluate GFP fluorescence, tissue wedges were post-immersed in fresh PFA for 4-18 h, then consecutively immerse in 10% sucrose (6 hours) and 30% sucrose overnight and embedded in Optimal Cutting Temperature (OCT) compound (Tissue-Tek; Sakura Finetek, Torrance, Cal). Frozen meridional 10 μm sections were mounted on Superfrost Plus microscope slides with Fluoromount G (SouthernBiotech, Birmingham, AL) and GFP visualized on a fluorescence microscope (model 1X71, Olympus, Center Valley, PA). Images were captured with a digital camera (DP70, Olympus) and accompanying software.
Tissue harvesting, DNA extraction, Quantitative TaqMan PCR and Biodistribution
Animals were anesthetized with a ketamine/ xylazine cocktail (3.5 mg/kg and 0.6 mg/kg respectively) and euthanized by overdose of sodium pentobarbital. To assess biodistribution of the human MMP1 viral particles, major organ tissue samples (brain, heart, kidney, liver, lung and spleen) were collected from three sheep. Likewise, relevant eye tissues (cornea, iris, retina, and trabecular meshwork) were dissected from both eyes of four sheep. Tissue DNA was purified using a DNeasy tissue kit (Qiagen, Valencia, CAL). Equal aliquots from each extracted sample were used to quantify gDNA and vg. Taqman PCR reactions were performed in duplicate in a total of 20 μl using 10 μl of either Taqman or Taqman Fast 2× universal PCR master mixes (Applied Biosystems/ Thermo Fisher), 1 µl of the corresponding probe and 1 to 9 μl aliquots of template experimental sample. Non-template controls were run in parallel. Quantifications were performed by TaqMan real time PCR in a StepOnePlus instrument (Applied Biosystems/ Thermo Fisher). For the gDNA, samples were hybridized to a single copy human RPPH1 single-exon probe (AB, Hs03297761_s1) and quantified against a standard curve of RPPH1 with commercial sheep DNA (Zyagen). For the vg, samples were hybridized to a human exon-spanning MMP1 cDNA probe (Applied Biosystems, Hs0023958_m1) and quantified against a standard curve of hMMP1 with the plasmid DNA which carried the transgene (pMG17, Figure 2) and had been used for the generation of the viral vectors. To allow comparisons among runs, all RPPH1 runs were analyzed at a CT at threshold of 0.4 and all MMP1 runs were analyzed at a CT at threshold of 0.18. Data is reported as the number of double-stranded vector DNA molecules per diploid cell. Amount of sheep genomic DNA (1.7 × 1012 MW) is calculated to be of 168 ng/ cell.
Data Analysis
Averages values are expressed as mean ± SE. The significance of experimental changes was analyzed using Student’s t-test as either paired or unpaired data. For the calculation of P values, all technical replicates from all biological replicates were used. The difference was considered statistically significant when P-value was < 0.05.
Acknowledgements
This study was supported by National Institutes of Health Grants EY11906 (TB) and by an unrestricted grant from the Research to Prevent Blindness to the Department of Ophthalmology at the University of North Carolina at Chapel Hill. The authors thank Dr. Donald L. Budenz for suggestions and advise, Dr. Scott D. Lawrence for performing the sub-tenon injections; and members of the laboratory Dr. Juan Carabana, Dr. Brandon Lane, and Renekia Elliot, for their help managing the sheep.
Footnotes
CONFLICT OF INTERESTS
None
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1038/gt.2016.14
Read article for free, from open access legal sources, via Unpaywall:
https://www.nature.com/articles/gt201614.pdf
Citations & impact
Impact metrics
Article citations
Engineered sensor actuator modulator as aqueous humor outflow actuator for gene therapy of primary open-angle glaucoma.
J Transl Med, 22(1):791, 28 Aug 2024
Cited by: 0 articles | PMID: 39198903 | PMCID: PMC11350963
Steroid-Induced Ocular Hypertension in Mice Is Differentially Reduced by Selective EP2, EP3, EP4, and IP Prostanoid Receptor Agonists.
Int J Mol Sci, 25(6):3328, 15 Mar 2024
Cited by: 0 articles | PMID: 38542305 | PMCID: PMC10970031
Therapeutic strategies for glaucoma and optic neuropathies.
Mol Aspects Med, 94:101219, 13 Oct 2023
Cited by: 1 article | PMID: 37839232
Review
The concept of gene therapy for glaucoma: the dream that has not come true yet.
Neural Regen Res, 19(1):92-99, 01 Jan 2024
Cited by: 7 articles | PMID: 37488850 | PMCID: PMC10479832
Review Free full text in Europe PMC
Role of Glucocorticoids and Glucocorticoid Receptors in Glaucoma Pathogenesis.
Cells, 12(20):2452, 14 Oct 2023
Cited by: 6 articles | PMID: 37887296 | PMCID: PMC10605158
Review Free full text in Europe PMC
Go to all (24) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Mechanisms of AAV transduction in glaucoma-associated human trabecular meshwork cells.
J Gene Med, 8(5):589-602, 01 May 2006
Cited by: 39 articles | PMID: 16506246
Development of a gene therapy virus with a glucocorticoid-inducible MMP1 for the treatment of steroid glaucoma.
Invest Ophthalmol Vis Sci, 51(6):3029-3041, 20 Jan 2010
Cited by: 36 articles | PMID: 20089870 | PMCID: PMC2891462
Treatment of sheep steroid-induced ocular hypertension with a glucocorticoid-inducible MMP1 gene therapy virus.
Invest Ophthalmol Vis Sci, 51(6):3042-3048, 20 Jan 2010
Cited by: 55 articles | PMID: 20089869 | PMCID: PMC2891463
Elevation of intraocular pressure in rodents using viral vectors targeting the trabecular meshwork.
Exp Eye Res, 141:33-41, 27 May 2015
Cited by: 26 articles | PMID: 26025608 | PMCID: PMC4628881
Review Free full text in Europe PMC






