Abstract
Free full text

Cancer immunotherapy targeting neoantigens
Abstract
Neoantigens are antigens that are encoded by tumor-specific mutated genes. Studies in the past few years have suggested a key role for neoantigens in cancer immunotherapy. Here we review the discoveries of neoantigens in the past two decades and the current advances in neoantigen identification. We also discuss the potential benefits and obstacles to the development of effective cancer immunotherapies targeting neoantigens.
1. Introduction
The foundation of immunology is based on self-nonself discrimination [1]. Most of the pathogens contain molecular signatures that can be recognized by the host and trigger immune responses [2]. Unlike pathogens, these molecular signatures are not generally expressed by tumor cells, making them more difficult to be distinguished from normal cells. However, T cells can recognize tumor antigens expressed by tumor cells. A class of tumor antigens, named tumor-associated antigens, is expressed in some normal tissues at low levels but is over-expressed in malignant cells. Many of the tumor-associated antigens have been identified as the targets of tumor-reactive T cells, isolated from tumor infiltrating lymphocytes (TILs), from draining lymph nodes or from peripheral blood [3]. However, expression of these antigens in normal cells can trigger central and peripheral tolerance mechanisms that lead to the selection of T cells with low-affinity T cell receptors (TCR). Conversely, attempts to target tumor-associated antigens with high-affinity TCRs can lead to severe toxicities due to normal tissue destruction [4, 5].
Another class of tumor antigens is tumor-specific neoantigens, which arise via mutations that alter amino acid coding sequences (non-synonymous somatic mutations). Some of these mutated peptides can be expressed, processed and presented on the cell surface, and subsequently recognized by T cells. Because normal tissues do not possess these somatic mutations, neoantigen-specific T cells are not subject to central and peripheral tolerance, and also lack the ability to induce normal tissue destruction. As a result, neoantigens appear to represent ideal targets for T cell-based cancer immunotherapy.
2. Approaches to identify T cell neoantigens
2.1. Classical approaches
Some of the initial attempts were focused on common shared mutations that have been well-characterized. Short peptides were synthesized based on the sequences of mutated BRAF [6, 7], KRAS [8-11] and p53 [12] . T cells from patients’ or healthy donors’ blood were stimulated several times by peptide-pulsed target cells, and expanded T cells were studied for their ability to kill tumors carrying these mutations. Alternatively, cells with overexpressed mutated cDNA, such as mutated NRAS cDNA, could serve as target cells to detect and isolate neoantigen-reactive T cells [13].
However, the majority of the neoantigen-reactive T cells recognized unique mutations not shared between cancer patients. Most of the unique neoantigens were identified by cDNA library screening in the past two decades. In this approach, cDNA library and MHC molecules were over-expressed in cell lines, and then co-cultured with T cells to identify antigens that could induce the T cell activation, measured by cytokine secretion or 4-1BB up-regulation. Table 1 is the list of published neoantigens identified by this approach.
Table 1
Human neoantigens discovered by classical approaches.
| Cancer type | Year | Mutated gene name | Approach | Source of T cells | Reference |
|---|---|---|---|---|---|
| Melanoma | 1995 | CDK4 | cDNA library | PBL | [14] |
| Melanoma | 1995 | MUM1 | cDNA library | PBL | [57] |
| Melanoma | 1996 | CTNNB1 | cDNA library | TIL | [15] |
| Melanoma | 1999 | CDC27 | cDNA library | TIL | [58] |
| Melanoma | 1999 | TRAPPC1 | cDNA library | PBL | [59] |
| Melanoma | 1999 | TPI | Chromatographic purification | TIL | [60] |
| Melanoma | 2000 | ASCC3 | cDNA library | PBL | [61] |
| Melanoma | 2001 | HHAT | cDNA library | TIL | [62] |
| Melanoma | 2002 | FN1 | cDNA library | TIL | [63] |
| Melanoma | 2002 | OS-9 | cDNA library | PBL | [64] |
| Melanoma | 2003 | PTPRK | cDNA library | TIL | [65] |
| Melanoma | 2004 | CDKN2A** , HLA-A11 | cDNA library | TIL | [18] |
| Melanoma | 2005 | GAS7, GAPDH | cDNA library | TIL | [66] |
| Melanoma | 2005 | SIRT2, GPNMB, SNRP116, RBAF600, SNRPD1 | cDNA library | PBL | [67] |
| Melanoma | 2005 | Prdx5 | cDNA library | PBL | [68] |
| Melanoma | 2011 | CLPP | cDNA library | TIL | [69] |
| Melanoma | 2013 | PPP1R3B | cDNA library | TIL | [46] |
| Lung cancer | 1998 | EF2 | Chromatographic purification | PBL | [20] |
| Lung cancer | 2001 | ACTN4 | cDNA library | TIL | [70] |
| Lung cancer | 2001 | ME1 | cDNA library | PBL | [71] |
| Lung Cancer | 2006 | NF-YC | cDNA library | draining lymph node | [72] |
| Renal cancer | 1996 | HLA-A2 | cDNA library | PBL | [17] |
| Renal cancer | 1999 | HSP70-2 | cDNA library | TIL | [73] |
| Renal cancer | 2005 | KIAA1440 | cDNA library | PBL | [74] |
| Head and neck squamous cell carcinoma | 1997 | CASP8 | cDNA library | PBL | [16] |
TIL: tumor infiltrating lymphocytes; PBL: peripheral blood lymphocytes.
Neoantigens have been identified predominantly in melanoma, likely due to the relatively high mutation rate in this tumor type. Nonetheless, neoepitopes have also been identified in multiple tumor types including lung and renal cancers. The majority of neoantigens were encoded by point-mutated gene products, although frameshift deletion and insertions have also been found to generate neoepitopes. Some mutated gene products recognized by T cells appear to be driver mutation products and play a role in tumorigenesis. These include CDK4, β-catenin (CTNNB1) and Caspase-8 (CASP8) proteins [14-16]. Interestingly, HLA-A2 and A11 containing point-mutations have also been identified as tumor-specific T cell antigens, suggesting that somatic mutations in HLA molecules can be a source of neoantigens [17-19].
In another approach, a mutated (EF2) and non-mutated (gp100:154) T cell epitopes have been identified by using mass spectrometry to sequence peptides that were eluted from HLA molecules isolated from the surface of tumor cells [20]. This approach has been limited by the sensitivity of these methods. Nevertheless, recent advances in the sensitivity and throughput of these techniques may facilitate the application of this approach to identifying tumor antigens.
2.2. Approaches utilizing next-generation sequencing techniques
Although the classical cDNA library screening approach led to the discovery of multiple neoantigens, this approach is labor-intensive and low-throughput. In addition, some large transcripts, GC-rich transcripts and low-expression transcripts cannot be cloned easily, leading to the failure of identifying some mutated antigens. Recently, several investigators have taken advantage of whole-exome sequencing technologies to identify non-synonymous mutations in tumors. These mutated genes were subjected to in silico analysis to predict potential high-affinity epitopes that bind to MHC molecules [21-23]. Additional filters could be applied to eliminate (1) epitopes predicted to be poorly processed by the immunoproteasome and (2) epitopes with lower binding affinity than the corresponding wild-type sequences [24]. Candidate mutated peptides are synthesized and screened to identify T cell neoantigens. This approach can be very efficient, and can identify neoantigens where previous cDNA library screening failed to discover [25].
This peptide-based screening approach, while effective in identifying many epitopes, may nevertheless be limited by the accuracy of MHC/HLA-binding prediction algorithms, which have not been thoroughly examined for MHC class II alleles and infrequent HLA alleles. In addition, the expression of potential T cell epitopes on the cell surface is influenced by a variety of mechanisms, especially the expression of multiple forms of the proteasome, which limit the number of peptides that are truly processed and presented [26, 27]. An alternative approach is pulsing antigen presenting cells with relatively long synthetic peptides that encompass minimal T cell epitopes [28]. In a recent report, nonsynonymous mutated epitopes were identified in three melanoma lesions by evaluating the response of CD4+ TIL to autologous B cells that were pulsed with 31 amino-acid long peptides encompassing individual mutations. Use of this approach resulted in the identification of mutated CIRH1A, GART, ASAP1, RND3, TNIK, RPS12, ZC3H18 and LEMD2 T cell epitopes. Furthermore, in a recent report, a peptide screening was carried out based on the combination of two peptide libraries: (1) 15-mer overlapping long-peptides (2) peptides based on MHC-binding prediction. This screening led to the identification of mutated HSDL1-reactive T cells isolated from an ovarian tumor [29].
To solve all the potential issues mentioned above, we have developed a tandem minigene screening approach [30]. A tandem minigene construct comprises 6 to 24 minigenes that encode polypeptides containing a mutated amino acid residue flanked on their N- and C-termini by 12 amino acids. Tandem minigene constructs are synthesized and used to transfect autologous APCs or cell lines co-expressing autologous HLA molecules. Using this approach, mutated KIF2C and POLA2 epitopes were identified in two melanoma patients [30]. In addition, a mutated ERBB2IP epitope was identified in a patient with cholangiocarcinoma [31]. Recent studies using this approach have led to the identification of mutated antigens express on gastrointestinal, breast and ovarian cancers [32] (and unpublished data). Notably, the neoantigen reactivity could be identified from TILs isolated from patients with cholangiocarcinoma or gastrointestinal cancer, which has a relatively low number of mutations.
A recent approach combined whole-exome/transcriptome sequencing analysis, MHC binding prediction, as well as mass spectrometric technique to detect peptides eluted from HLA molecules [33]. Interestingly, only a small fraction of predicted high-binding peptides were confirmed by mass spectrometry. The relative small number of mutated peptides identified by mass spectrometry might be due to the sensitivity of the peptide purification and mass spectrometry, but it could also suggest that natural antigen process and presentation in cells could be very inefficient. Among 7 neo-epitopes identified by this approach, mutated Adpgk, Reps1 and Dpagt1 epitopes were confirmed to be immunogenic [33].
3. The correlation between neoantigens and tumor regressions
It has been established that T cells recognizing non-mutated tumor antigens could induce tumor regression [34]. This has been demonstrated by clinical trials involving adoptive transfer of T cells targeting HLA-A*0201-restricted NY-ESO-1. Objective responses were observed in 61% of patients with synovial cell sarcoma, 55% of patients with melanoma and 80% of patients with myeloma [35-37]. However, it remains unclear whether T cells recognizing neoantigens can also induce tumor regression in human.
In the past few years, there have been significant advances in checkpoint blockade cancer immunotherapy. Tumor regressions were observed in a portion of cancer patients who underwent CTLA-4, PD-1 or PD-L1 blockade clinical trials [38]. Currently, the widely acceptable hypothesis is that tumors with more mutations likely generate more neoepitopes, which can be recognized by tumor infiltrating T cells. Checkpoint blocking antibodies reactivate these T cells in vivo and induce tumor regressions. As a result, cancers with high mutation rates, such as melanoma and lung cancer, are more susceptible to checkpoint blockade therapies. (One notable exception for this hypothesis is renal cell carcinoma, which is susceptible to checkpoint blockade therapies but with low mutation load). A recent study in a murine model has provided some evidence for this hypothesis. In an anti-PD-1 and/or anti-CTLA-4 immunotherapy model, two dominant neoantigens asparagine-linked glycosylation 8 (Alg8) (A506T) and laminin alpha subunit 4 (Lama4) (G1254V) were identified from a sarcoma cell line d42m1-T3. Mutated Lama4 and Alg8 long peptide vaccines could induce tumor rejection comparable to checkpoint blockade immunotherapy. In addition, the checkpoint blockade immunotherapy increased the number and enhanced the activity of neoantigen-specific CD8+ T cells [24]. These results suggested that neoantigens and neoantigen-specific T cells were strongly associated with tumor regressions after checkpoint blockade therapy.
In human, recent studies have established the correlation between the number of mutations/neoantigens and clinical outcomes. In a PD-1 blockade clinical trial comparing colorectal cancer patients with or without mismatch-repair deficiency, prolonged progression-free survival was associated with high somatic mutations, which were found in tumors with mismatch repair-deficiency [39]. Other studies utilized in silico analyses to predict potential high-affinity neoepitopes for calculating the numbers of neoantigens. In two anti-CTLA-4 melanoma immunotherapy studies, the number of neoantigens was significantly associated with clinical benefits after CTLA-4 blockade therapies [40, 41]. However, it’s unclear whether the presence of “tetrapeptide” signatures within predicted T cell epitopes could be employed to predict the clinical outcomes for the anti-CTLA-4 immunotherapy [40, 41]. Similar to CTLA-4 blockade studies, higher neoantigen burden was correlated with clinical benefit and progression-free survival in PD-1 blockade immunotherapy for patients with non-small cell lung cancer [42]. These studies suggested that the number of neoantigens was positively associated with clinical benefit after immune checkpoint blockade therapies. Lastly, adoptive transfer of mixed TILs could induce tumor regressions in melanoma patients [43]. In a recent study from our group, objective tumor regressions in three melanoma patients were associated with adoptive transferring of TILs all recognizing neoantigens [25]. In a subsequent study, two TIL products were associated with complete tumor regressions observed in two melanoma patients, and each TIL could recognize one unique neoantigen [30]. Taken together, these correlative studies suggested that neoantigen-reactive T cells were likely the dominant player inducing tumor regressions in patients.
4. Current evidence of cancer immunotherapy targeting neoantigens
The first approach to target neoantigens is via cancer vaccine. In an initial mouse study, candidate mutated epitopes were identified by whole-exome sequencing of the B16F10 murine melanoma. Fifty selected mutation-coding long peptides were injected into mice to elicit immune responses, and 11 out of 50 peptides induced immune responses preferentially recognizing the mutated epitopes. Among these 11 peptides, mutated Kif18b (K739N) was found to be the dominant mutated antigens, and mice immunized with mutated Kif18b peptide could slow tumor growth and improve survival [22]. In another study, MC-38 tumor-bearing mice vaccinated with mutated peptides (Adpgk, Reps1 and Dpagt1) showed sustainable inhibition of tumor growth [33]. Recently, a synthetic RNA “pentatope” vaccine was developed. Each pentatope contains five 27-mer minigenes with the mutated amino acids in the center, and each fused to each other by 10-mer glycine-serine linker (pentatopes). The immunization of this RNA pentatope conferred disease control and survival benefit in a murine CT26 tumor model. Notably, mutated MHC class II epitopes were more immunogenic than class I epitopes in this study [44]. A recent study has brought a neoantigen cancer vaccine to a clinical trial in which whole-exome sequencing was carried out to identify somatic mutations in tumors from 3 patients with melanoma. Candidate HLA-A*02:01 epitopes containing residues arising from mutations were initially filtered using an HLA binding prediction algorithm, and then evaluated using competitive binding assays. Three patients were vaccinated with autologous dendritic cells that had been pulsed with the top 7 highest binding peptides identified from each tumor. The breadth and diversity of neoantigen-specific T cells were increased in all 3 patients after the vaccination. In addition, the results indicated that T cells generated by vaccination with 7 out of the 21 epitopes could recognize target cells transfected with the corresponding tandem minigene constructs, indicating that these neoantigens could be endogenously processed and presented [45]. While these results demonstrated that neoantigen cancer vaccines could elicit neoantigen-specific T cells in cancer patients, no clinical benefits to these patients were shown in this study. It remains unclear whether current cancer vaccine therapies are potent enough to provide therapeutic benefits in cancer patients with bulky disease.
The second approach to target neoantigens is by transferring neoantigen-specific T cells directly into host. Although so far no evidence in mouse models has shown that such T cells can induce tumor regressions in vivo, some evidence has linked neoantigen-reactive T cells to tumor regressions in human. A clue came from a report studying a unique melanoma TIL product, with no reactivity against non-mutated antigens. Instead, ~50% of this TIL product showed predominant reactivity against HLA-A*01-restricted mutated PPP1R3B. Following adoptive transfer of this TIL product, the patient experienced a complete response ongoing beyond 10 years. In addition, mutated PPP1R3B-reactive T cells could be still detected in the patient’s peripheral blood 5 years after the immunotherapy [46]. The most direct clinical evidence came from an adoptive cell transfer immunotherapy using nearly pure neoantigen-reactive T cells. A patient with metastatic cholangiocarcinoma was treated with one billion mutated ERBB2IP-reactive CD4+ T cells, and she experienced a partial response ongoing for more than 2 years since treatment [31]. This suggested that neoantigen-reactive T cells could induce long-term tumor regressions in cancer patients, and it also brought up the possibility of applying immunotherapy to a variety of cancer types other than melanoma.
5. Conclusions and future perspectives
Based on current knowledge, neoantigens are ideal targets for cancer immunotherapy. Because neoantigens are specifically expressed in the tumor, it is less likely to induce tolerance and nearly impossible to induce normal tissue toxicity. However, it remains challenging to reduce the cost of such highly personalized therapy.
Many outstanding questions remain unanswered (Table 2). How can we apply to cancer immunotherapy to a variety of cancer types by targeting neoantigens? Does the average number of neoantigens discovered per tumor correlate with the mutation rate in each cancer type? If yes, how can we apply immunotherapy to cancers with low mutation rates? Does the tumor microenvironments in different cancer types influence the efficacy of immunotherapy? Can the fitness or the number of T cells overcome the potentially harsh tumor microenvironment in different cancer types?
Table 2
Outstanding questions for cancer immunotherapy targeting neoantigens.
| 1. | How does this approach overcome the heterogeneity of tumor? |
| 2. | Does the number of neoantigens correlate with the mutation rate in each tumor specimen? |
| 3. | Can this approach apply to a variety types of cancers, especially cancers with low mutation rate? |
| 4. | Can neoantigens induce tolerance? Can Treg specifically recognize neoantigens and induce tolerance? |
| 5. | Are the number and fitness of neoantigen-specific T cells important for the efficacy of cancer immunotherapy? |
One major concern is the heterogeneity of tumor. Neoantigens may be expressed in some tumor cells, but not all tumor cells in an individual patient, leading to tumor escape from immunotherapy. Conversely, we and others have observed complete tumor regressions in dozens of patients after immunotherapy. Some potential approaches may address this concern. One is to target multiple neoantigens at the same time, so all tumor cells expressing at least one neoantigen can be destroyed. Another approach is to target a single neoantigen, which is ideally expressed in all tumor cells within a patient.
Targeting driver mutations can be an effective strategy [30]. Aggressive tumor cells are actively expressing driver mutations, which are essential for carcinogenesis or metastasis. Although some tumor cells may not express driver mutations and may escape from immunotherapy, those tumor cells likely lose the metastatic potential and cannot grow aggressively. Nonetheless, defining driver mutations versus passenger mutations remains a challenging issue. The most definitive way to identify driver mutations is based on the functional studies found in the literature. From the neoantigens reviewed in this article, CDK4, β-catenin and Caspase-8 are likely driver mutations. The majority of neoantigens are likely random mutations (passenger mutations) recognized by T cells, but it is also possible that some of the mutations are not well-characterized. The Cancer Genome Atlas attempted to address this issue by sequencing and analyzing hundreds of tumor specimens. The recurrent mutations were identified as the potential driver mutations [47-49].
Recent checkpoint blockade immunotherapies have shown some efficacies in lung cancer, bladder cancer and renal cancer [50-53]. Based on the assumption that neoantigen-reactive T cells are responsible for tumor regressions in checkpoint blockade immunotherapies, directly transferring neoantigen-reactive T cells into cancer patients may achieve a much higher response rate. Additionally, neoantigen cancer vaccines may enhance the strength and persistence of T cells, and ultimately improve the efficacy of immunotherapy. With the recent advances in technology, we can identify neoantigens and isolate neoantigen-specific TCRs from individual patients in a timely and cost effective manner [54-56] (and unpublished data). It is possible that patients with a wide variety of cancer types, including cancers with very few mutations, can receive the proposed cancer immunotherapy targeting neoantigens (Figure 1).
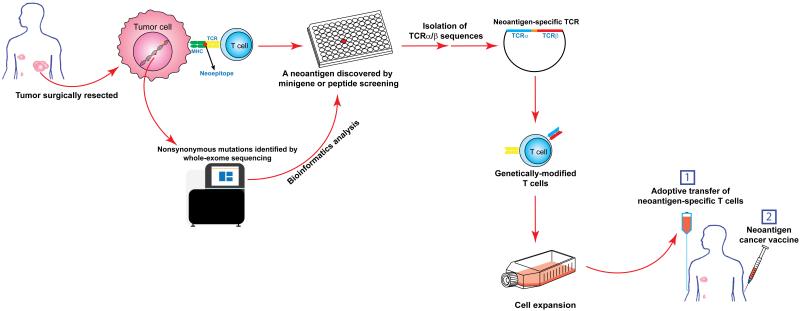
Cancer immunotherapy targeting neoantigens by the adoptive transfer of genetically-modified T cells. In this proposed study, a tumor will be surgically resected from a cancer patient. Part of the tumor specimen will be subjected to whole-exome sequencing to identify nonsynonymous mutations. The rest of the tumor specimen will be used to grow TILs. Potential neoantigens will be identified by minigene or peptide screening approach described in the text. Neoantigen-specific TCRs will be isolated and introduced into patient’s peripheral blood T cells. These genetically-modified T cells will be adoptively transferred back to the patient to fight cancer. A neoantigen cancer vaccines will be injected to enhance the strength and persistence of T cells.
Acknowledgements
The authors thank Todd Prickett and Lucas McDuffie for suggestions and discussions. This work was supported by the Intramural Research Program of National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Citations & impact
Impact metrics
Article citations
Tumor neoantigens and tumor immunotherapies.
Aging Med (Milton), 7(2):224-230, 12 Apr 2024
Cited by: 0 articles | PMID: 38725698 | PMCID: PMC11077340
Review Free full text in Europe PMC
DeepHLApan: A Deep Learning Approach for the Prediction of Peptide-HLA Binding and Immunogenicity.
Methods Mol Biol, 2809:237-244, 01 Jan 2024
Cited by: 0 articles | PMID: 38907901
Tumor Vaccines: Unleashing the Power of the Immune System to Fight Cancer.
Pharmaceuticals (Basel), 16(10):1384, 29 Sep 2023
Cited by: 7 articles | PMID: 37895855 | PMCID: PMC10610367
Review Free full text in Europe PMC
The Value of Microbes in Cancer Neoantigen Immunotherapy.
Pharmaceutics, 15(8):2138, 14 Aug 2023
Cited by: 1 article | PMID: 37631352 | PMCID: PMC10459105
Review Free full text in Europe PMC
Biomarkers of immune checkpoint inhibitor response and toxicity: Challenges and opportunities.
Immunol Rev, 318(1):157-166, 20 Jul 2023
Cited by: 6 articles | PMID: 37470280 | PMCID: PMC10528475
Review Free full text in Europe PMC
Go to all (137) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Neoantigen identification strategies enable personalized immunotherapy in refractory solid tumors.
J Clin Invest, 129(5):2056-2070, 05 Mar 2019
Cited by: 116 articles | PMID: 30835255 | PMCID: PMC6486339
Targeting neoantigens to augment antitumour immunity.
Nat Rev Cancer, 17(4):209-222, 24 Feb 2017
Cited by: 495 articles | PMID: 28233802 | PMCID: PMC5575801
Review Free full text in Europe PMC
The role of neoantigens in response to immune checkpoint blockade.
Int Immunol, 28(8):411-419, 05 Apr 2016
Cited by: 101 articles | PMID: 27048318 | PMCID: PMC4986233
Review Free full text in Europe PMC
Immunopharmacogenomics towards personalized cancer immunotherapy targeting neoantigens.
Cancer Sci, 109(3):542-549, 14 Feb 2018
Cited by: 27 articles | PMID: 29288513 | PMCID: PMC5834780
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Intramural NIH HHS (1)
Grant ID: Z99 CA999999




