Abstract
Purpose
Evolving treatments, disease phenotypes, and biology, together with a changing drug development environment, have created the need to revise castration-resistant prostate cancer (CRPC) clinical trial recommendations to succeed those from prior Prostate Cancer Clinical Trials Working Groups.Methods
An international expert committee of prostate cancer clinical investigators (the Prostate Cancer Clinical Trials Working Group 3 [PCWG3]) was reconvened and expanded and met in 2012-2015 to formulate updated criteria on the basis of emerging trial data and validation studies of the Prostate Cancer Clinical Trials Working Group 2 recommendations.Results
PCWG3 recommends that baseline patient assessment include tumor histology, detailed records of prior systemic treatments and responses, and a detailed reporting of disease subtypes based on an anatomic pattern of metastatic spread. New recommendations for trial outcome measures include the time to event end point of symptomatic skeletal events, as well as time to first metastasis and time to progression for trials in the nonmetastatic CRPC state. PCWG3 introduces the concept of no longer clinically benefiting to underscore the distinction between first evidence of progression and the clinical need to terminate or change treatment, and the importance of documenting progression in existing lesions as distinct from the development of new lesions. Serial biologic profiling using tumor samples from biopsies, blood-based diagnostics, and/or imaging is also recommended to gain insight into mechanisms of resistance and to identify predictive biomarkers of sensitivity for use in prospective trials.Conclusion
PCWG3 moves drug development closer to unmet needs in clinical practice by focusing on disease manifestations most likely to affect prognosis adversely for therapeutics tested in both nonmetastatic and metastatic CRPC populations. Consultation with regulatory authorities is recommended if a trial is intended to seek support for drug approval.Free full text

Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations From the Prostate Cancer Clinical Trials Working Group 3
Abstract
Purpose
Evolving treatments, disease phenotypes, and biology, together with a changing drug development environment, have created the need to revise castration-resistant prostate cancer (CRPC) clinical trial recommendations to succeed those from prior Prostate Cancer Clinical Trials Working Groups.
Methods
An international expert committee of prostate cancer clinical investigators (the Prostate Cancer Clinical Trials Working Group 3 [PCWG3]) was reconvened and expanded and met in 2012-2015 to formulate updated criteria on the basis of emerging trial data and validation studies of the Prostate Cancer Clinical Trials Working Group 2 recommendations.
Results
PCWG3 recommends that baseline patient assessment include tumor histology, detailed records of prior systemic treatments and responses, and a detailed reporting of disease subtypes based on an anatomic pattern of metastatic spread. New recommendations for trial outcome measures include the time to event end point of symptomatic skeletal events, as well as time to first metastasis and time to progression for trials in the nonmetastatic CRPC state. PCWG3 introduces the concept of no longer clinically benefiting to underscore the distinction between first evidence of progression and the clinical need to terminate or change treatment, and the importance of documenting progression in existing lesions as distinct from the development of new lesions. Serial biologic profiling using tumor samples from biopsies, blood-based diagnostics, and/or imaging is also recommended to gain insight into mechanisms of resistance and to identify predictive biomarkers of sensitivity for use in prospective trials.
Conclusion
PCWG3 moves drug development closer to unmet needs in clinical practice by focusing on disease manifestations most likely to affect prognosis adversely for therapeutics tested in both nonmetastatic and metastatic CRPC populations. Consultation with regulatory authorities is recommended if a trial is intended to seek support for drug approval.
INTRODUCTION
In 1999, the Prostate Cancer Clinical Trials Working Group 1 issued recommendations for standardizing prostate-specific antigen (PSA) outcomes in phase II castration-resistant prostate cancer (CRPC) trials.1 In 2008, when docetaxel was the only drug proven to prolong survival in metastatic CRPC (mCRPC),2,3 the successor group, the Prostate Cancer Clinical Trials Working Group 2 (PCWG2), outlined more extensive principles of clinical trial conduct for this disease.4 Incorporating the earlier recommendations on the use of PSA measurements in clinical investigations, PCWG2 applied a clinical states framework in which trial objectives, design, and outcomes were based on (1) early measures of response that represented the control, relief, or elimination of existing disease manifestations and (2) later time-to-event measures that represented the delay or prevention of manifestations that may occur as disease progressed (Appendix, online only).
The Prostate Cancer Clinical Trials Working Group 3 (PCWG3) is an international working group of clinical and translational experts in prostate cancer that convened in June 2012 and worked through February 2015 to update the recommendations of PCWG2 in light of a changing therapeutic landscape5-11 and advances in the understanding of disease biology. The two chairs (H.I.S. and A.J.A.) developed an outline and convened eight meetings that included many of the PCWG2 investigators and 20 additional investigators. A synthesized document was developed and approved by all PCWG3 members. Key principles of trial design and conduct from PCWG3 are shown in Tables 1–5 with comparisons to the prior recommendations of PCWG2.
Table 1.
Summary of Major Changes in PCWG3 Recommendations Compared With PCWG2
| Clinical states model |
 1. Considers mCRPC in terms of number of lines of prior therapy rather than in relation to docetaxel treatment (ie, predocetaxel, postdocetaxel; Fig 1) 1. Considers mCRPC in terms of number of lines of prior therapy rather than in relation to docetaxel treatment (ie, predocetaxel, postdocetaxel; Fig 1) |
 2. Advocates recording the specific systemic approaches a patient has received in the order they were administered, including start and stop dates and response if available 2. Advocates recording the specific systemic approaches a patient has received in the order they were administered, including start and stop dates and response if available |
 3. Distinguishes other histologies (ie, small-cell carcinomas) from adenocarcinomas 3. Distinguishes other histologies (ie, small-cell carcinomas) from adenocarcinomas |
 4. Emphasizes the importance of serial biologic profiling of the disease at the start of a new therapy and time of progression 4. Emphasizes the importance of serial biologic profiling of the disease at the start of a new therapy and time of progression |
| Principles of trial conduct |
 1. Emphasizes discovering and qualifying post-treatment outcomes that reflect patient benefit or can serve as surrogates of that benefit for use in regulatory submissions, to accelerate drug approvals 1. Emphasizes discovering and qualifying post-treatment outcomes that reflect patient benefit or can serve as surrogates of that benefit for use in regulatory submissions, to accelerate drug approvals |
 2. Highlights the distinction between the need to consistently report measures of progression in a trial v the clinical need to continue a particular therapy beyond progression as long as the patient is benefiting from the treatment (Fig 3) 2. Highlights the distinction between the need to consistently report measures of progression in a trial v the clinical need to continue a particular therapy beyond progression as long as the patient is benefiting from the treatment (Fig 3) |
| Eligibility for enrollment |
 1. Defines eligibility criteria using clinical and biologic parameters intended to homogenize the prognosis of the patients enrolled while enriching for tumor biomarker profiles most likely to respond to a particular therapy 1. Defines eligibility criteria using clinical and biologic parameters intended to homogenize the prognosis of the patients enrolled while enriching for tumor biomarker profiles most likely to respond to a particular therapy |
 2. Encourages the use of testosterone assays that accurately measure levels in the 1-2 ng/dL range performed in a central laboratory 2. Encourages the use of testosterone assays that accurately measure levels in the 1-2 ng/dL range performed in a central laboratory |
 3. Assesses lymph node size on the basis of the short axis and eliminates the requirement for a lymph node to be ≥ 2 cm in size to be considered measurable 3. Assesses lymph node size on the basis of the short axis and eliminates the requirement for a lymph node to be ≥ 2 cm in size to be considered measurable |
 4. Encourages designing specific trials that are based on different clinical phenotypes defined by the location and distribution of radiographic metastases for which specific therapies have formal indications or exclusions 4. Encourages designing specific trials that are based on different clinical phenotypes defined by the location and distribution of radiographic metastases for which specific therapies have formal indications or exclusions |
| Treatment: defining dose, schedule, and pharmacodynamic markers |
 1. Encourages the use of pharmacodynamic outcome measures that confirm the mechanism of action and determine a dose and schedule specific to the effect of a particular agent on the malignant process 1. Encourages the use of pharmacodynamic outcome measures that confirm the mechanism of action and determine a dose and schedule specific to the effect of a particular agent on the malignant process |
 2. Places a greater focus on pharmacodynamic biomarkers that establish proof of mechanism and can also be used to determine dose and schedule on the basis of biology (and safety) rather than safety alone 2. Places a greater focus on pharmacodynamic biomarkers that establish proof of mechanism and can also be used to determine dose and schedule on the basis of biology (and safety) rather than safety alone |
 3. Advises that the post-treatment biomarker measurements used to assess antitumor activity be tailored to each agent’s mechanism of action and that these measurements be performed at fixed intervals (detailed in Table 4) 3. Advises that the post-treatment biomarker measurements used to assess antitumor activity be tailored to each agent’s mechanism of action and that these measurements be performed at fixed intervals (detailed in Table 4) |
| Baseline disease assessments |
 1. Expands baseline assessments to include tumor histology; the timing, duration, and response (if available) for all prior systemic treatments; a standardized assessment of blood-based, PRO-based, and imaging-based biomarkers; and the molecular characterization of the tumor (detailed in Table 2) 1. Expands baseline assessments to include tumor histology; the timing, duration, and response (if available) for all prior systemic treatments; a standardized assessment of blood-based, PRO-based, and imaging-based biomarkers; and the molecular characterization of the tumor (detailed in Table 2) |
 2. Emphasizes molecular/biologic subtypes of CRPC in addition to the five clinical subtypes (defined by extent and location of metastases) 2. Emphasizes molecular/biologic subtypes of CRPC in addition to the five clinical subtypes (defined by extent and location of metastases) |
 3. Defines the type of progression at trial entry as PSA-only progression, radiographic progression by site of disease spread, or both; for radiographic progression, records whether progression was caused by growth of existing lesions, appearance of new lesions, or both 3. Defines the type of progression at trial entry as PSA-only progression, radiographic progression by site of disease spread, or both; for radiographic progression, records whether progression was caused by growth of existing lesions, appearance of new lesions, or both |
| Measuring outcomes and reporting: blood-based and molecular measures |
 1. When there are progressing lesions, recommends rebiopsy of the progressing metastatic site for histology and biomarker assessment 1. When there are progressing lesions, recommends rebiopsy of the progressing metastatic site for histology and biomarker assessment |
 2. Suggests that PSA outcomes should be interpreted within the context of a drug’s mechanism of action, and the anticipated timing of a potential favorable/unfavorable effect on PSA should be considered 2. Suggests that PSA outcomes should be interpreted within the context of a drug’s mechanism of action, and the anticipated timing of a potential favorable/unfavorable effect on PSA should be considered |
 3. Includes suggestions on how to define and report outcomes related to CTC enumeration (using CellSearch platform) 3. Includes suggestions on how to define and report outcomes related to CTC enumeration (using CellSearch platform) |
| Measuring outcomes and reporting: PROs |
 1. Recognizes the importance of the patient perspective in prostate cancer clinical trials and the need to further optimize the assessment, collection, analysis, and presentation of PRO data 1. Recognizes the importance of the patient perspective in prostate cancer clinical trials and the need to further optimize the assessment, collection, analysis, and presentation of PRO data |
 2. Recommends measuring disease-related symptoms including pain intensity and interference, and physical functioning, using validated instruments 2. Recommends measuring disease-related symptoms including pain intensity and interference, and physical functioning, using validated instruments |
 3. Recommends collecting patient-reported adverse events using the NCI’s PRO-CTCAE 3. Recommends collecting patient-reported adverse events using the NCI’s PRO-CTCAE |
| Measuring outcomes and reporting: imaging and clinical measures |
 1. Reconsiders the mixed response designation, which may be a manifestation of disease heterogeneity 1. Reconsiders the mixed response designation, which may be a manifestation of disease heterogeneity |
 2. Advises recording whether disease progression represents growth of pre-existing lesions, development of new lesions, or both, and separately recording whether progression is occurring in a single organ or disease site v multiple sites 2. Advises recording whether disease progression represents growth of pre-existing lesions, development of new lesions, or both, and separately recording whether progression is occurring in a single organ or disease site v multiple sites |
 3. Suggests that the first post-treatment bone scan be used as the baseline scan with which all future bone scans are compared (Fig 2); also emphasizes the notion of response in bone, caused by the advent of novel bone-targeting agents 3. Suggests that the first post-treatment bone scan be used as the baseline scan with which all future bone scans are compared (Fig 2); also emphasizes the notion of response in bone, caused by the advent of novel bone-targeting agents |
 4. Advises recording the location of nodal disease (pelvic v extrapelvic) and visceral disease (lung/liver/adrenal/CNS) separately, because these sites have separate prognostic implications 4. Advises recording the location of nodal disease (pelvic v extrapelvic) and visceral disease (lung/liver/adrenal/CNS) separately, because these sites have separate prognostic implications |
 5. Also advises monitoring up to five individual lesions per site of spread (eg, nodes, lung, liver as separate sites) to address disease heterogeneity 5. Also advises monitoring up to five individual lesions per site of spread (eg, nodes, lung, liver as separate sites) to address disease heterogeneity |
 6. Proposes new criteria to define the first occurrence of metastatic disease in men with nmCRPC at enrollment 6. Proposes new criteria to define the first occurrence of metastatic disease in men with nmCRPC at enrollment |
 7. Highlights and defines the bone-related outcomes, SREs and SSEs, but suggests focusing on SSEs, which represent a more direct clinical benefit to patients 7. Highlights and defines the bone-related outcomes, SREs and SSEs, but suggests focusing on SSEs, which represent a more direct clinical benefit to patients |
 8. Introduces the concept of treatment beyond progression where clinical benefit by one or more disease manifestations is being observed, thus defining an objective of NLCB 8. Introduces the concept of treatment beyond progression where clinical benefit by one or more disease manifestations is being observed, thus defining an objective of NLCB |
Abbreviations: CRPC, castration-resistant prostate cancer; CTC, circulating tumor cell; mCRPC, metastatic castration-resistant prostate cancer; nmCRPC, nonmetastatic castration-resistant prostate cancer; NCI, National Cancer Institute; NLCB, no longer clinically benefiting; PRO, patient-reported outcome; PRO-CTCAE, Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events; PSA, prostate-specific antigen; SRE, skeletal-related event; SSE, symptomatic skeletal event.
Table 5.
Suggested Outcome Measures for Clinical Trials in Metastatic Prostate Cancer: Report by Disease Manifestation
| Variable | PCWG2 (2008) | PCWG3 (2015) |
|---|---|---|
| Histology | Not addressed | Encourage rebiopsy of metastatic sites or local recurrence at progression to evaluate for histologic (ie, neuroendocrine/small cell) transformation; in the context of clinical trials, encourage rebiopsy for biomarker assessment |
| Blood-based markers | ||
 PSA PSA | Recognize that a favorable effect on PSA may be delayed for ≥ 12 weeks, even for a cytotoxic drug | Retained |
| Monitor PSA by cycle but plan to continue through early rises for a minimum of 12 weeks unless other evidence of progression | Retained | |
| Ignore early rises (before 12 weeks) in determining PSA response | Retained | |
| For control/relieve/eliminate end points: | For control/relieve/eliminate end points: | |
 Record the percent change from baseline (rise or fall) at 12 weeks, and separately, the maximal change (rise or fall) at any time using a waterfall plot Record the percent change from baseline (rise or fall) at 12 weeks, and separately, the maximal change (rise or fall) at any time using a waterfall plot |  Retained, except with timing (8-9 or 12 weeks) depending on trial design Retained, except with timing (8-9 or 12 weeks) depending on trial design | |
 Separately report the proportion of patients who have undergone radical prostatectomy and achieved a nadir less than 0.2 ng/mL v primary radiation therapy–treated patients who achieved a nadir less than 0.5 ng/mL Separately report the proportion of patients who have undergone radical prostatectomy and achieved a nadir less than 0.2 ng/mL v primary radiation therapy–treated patients who achieved a nadir less than 0.5 ng/mL | ||
 Describe absolute changes in PSA over time from baseline to best response Describe absolute changes in PSA over time from baseline to best response | ||
| For delay/prevent end points (progression): | For delay/prevent end points (progression): | |
 After decline from baseline: record time from start of therapy to first PSA increase that is ≥ 25% and ≥ 2 ng/mL above the nadir, and which is confirmed by a second value ≥ 3 weeks later (ie, a confirmed rising trend); the requirement for an increase of 5 ng/mL was decreased to 2 ng/mL, and the requirement for a 50% increase was reduced to 25% After decline from baseline: record time from start of therapy to first PSA increase that is ≥ 25% and ≥ 2 ng/mL above the nadir, and which is confirmed by a second value ≥ 3 weeks later (ie, a confirmed rising trend); the requirement for an increase of 5 ng/mL was decreased to 2 ng/mL, and the requirement for a 50% increase was reduced to 25% |  Retained (standards for reporting PSA progression date may not indicate a need to stop treatment) Retained (standards for reporting PSA progression date may not indicate a need to stop treatment) | |
 Recording the duration of PSA decline of little value Recording the duration of PSA decline of little value | Retained | |
| No decline from baseline: PSA progression ≥ 25% increase and ≥ 2 ng/mL increase from baseline beyond 12 weeks | Relate to mechanism of drug and anticipated timing of potential favorable/unfavorable effects on PSA, if present | |
 CTC CTC | Not addressed | Enumerate at the start of treatment: Record as favorable (four or fewer cells per 7.5 mL of blood) or unfavorable (five or more cells per 7.5 mL) |
| If unfavorable, monitor for changes after treatment | ||
| For control/relieve/eliminate end points: | ||
 Report as change from unfavorable (five or more cells per 7.5 mL of blood) to favorable (four or fewer cells per 7.5 mL) and separately, the percent change from baseline using a waterfall plot Report as change from unfavorable (five or more cells per 7.5 mL of blood) to favorable (four or fewer cells per 7.5 mL) and separately, the percent change from baseline using a waterfall plot | ||
| For delay/prevent end points: no validated definition exists (however, rising CTC counts are associated with a poor prognosis) | ||
 LDH, total alkaline phosphatase, bone-specific alkaline phosphatase, urine N-telopeptide, hemoglobin, NLR LDH, total alkaline phosphatase, bone-specific alkaline phosphatase, urine N-telopeptide, hemoglobin, NLR | Not addressed | Descriptively report changes over time, may include the proportion showing normalization of a given biomarker and/or waterfall plots of percent change from baseline in a given biomarker |
| Report institutional normal ranges to determine normalization of a given biomarker | ||
| Imaging biomarkers: nodal and visceral | ||
 For control/relieve/eliminate end points For control/relieve/eliminate end points | ||
  General General | Record changes in nodal sites separately from visceral sites | Record changes in lymph nodes, lung, liver, adrenal, and CNS sites separately |
| Use RECIST with caveats: | Record up to five lesions per site of disease | |
 Record changes in size using waterfall plot Record changes in size using waterfall plot | Use RECIST 1.1 with caveats: | |
 Confirm favorable change with second scan Confirm favorable change with second scan |  Record changes in size using waterfall plot Record changes in size using waterfall plot | |
 Record complete elimination of disease at any site separately Record complete elimination of disease at any site separately |  Confirm favorable change with second scan Confirm favorable change with second scan | |
 Record complete elimination of disease at any site separately Record complete elimination of disease at any site separately | ||
  Nodes Nodes | Only report changes in lymph nodes that were ≥ 2 cm in the long axis at baseline | Only report changes in lymph nodes that were ≥1.5 cm in the short axis |
| Record changes in pelvic (regional) nodes v extrapelvic (distant/metastatic) nodes separately | ||
  Visceral Visceral | Use RECIST with caveats above | Use RECIST 1.1 with caveats: |
 Record changes in liver, lung, adrenal, and CNS separately Record changes in liver, lung, adrenal, and CNS separately | ||
 Only report changes in lesions ≥ 1.0 cm in the longest dimension Only report changes in lesions ≥ 1.0 cm in the longest dimension | ||
 For delay/prevent end points For delay/prevent end points | ||
  Nodal and visceral Nodal and visceral | Use RECIST criteria for progression, with additional requirement that progression be confirmed by a second scan ≥ 6 weeks later (the second scan is particularly important when anticipated effect on PSA is delayed, or for biologic therapies) | General: |
 Record changes in nodal and visceral (lung, liver, adrenal, and CNS) disease separately Record changes in nodal and visceral (lung, liver, adrenal, and CNS) disease separately | ||
 Use RECIST 1.1 but clearly record type of progression (growth of existing lesions v development of new lesions) separately by site Use RECIST 1.1 but clearly record type of progression (growth of existing lesions v development of new lesions) separately by site | ||
 The recommendations apply to both nmCRPC and mCRPC The recommendations apply to both nmCRPC and mCRPC | ||
 Record up to five lesions per site of spread Record up to five lesions per site of spread | ||
 Report the proportion who have not progressed at fixed time points (6 or 12 months) Report the proportion who have not progressed at fixed time points (6 or 12 months) | ||
| Note that for some treatments, a lesion may increase in size before it decreases | Retained | |
  Nodal Nodal | As above | Previously normal (< 1.0-cm) lymph nodes must have grown by ≥ 5 mm in the short axis from baseline or nadir and be ≥ 1.0 cm in the short axis to be considered to have progressed |
| Nodes that have progressed to 1.0 to less than 1.5 cm are pathologic, subject to clinical discretion, and nonmeasurable | ||
| For existing pathologic adenopathy (≥ 1.5 cm), progression is defined per RECIST 1.1 | ||
| Imaging biomarkers: bone | ||
 Metastatic Metastatic | For control/relieve/eliminate end points: | For control/relieve/eliminate end points: |
 Record changes as improved or stable (no new lesions) or worse (new lesions) Record changes as improved or stable (no new lesions) or worse (new lesions) |  Retained with addition of resolved bone lesion Retained with addition of resolved bone lesion | |
 Changes in intensity of uptake alone do not constitute progression or regression Changes in intensity of uptake alone do not constitute progression or regression |  Retained Retained | |
 No new lesions: continue therapy in absence of other signs of progression No new lesions: continue therapy in absence of other signs of progression |  Retained Retained | |
 New lesions (See Progression below) New lesions (See Progression below) |  Retained Retained | |
| For delay/prevent end points (progression): | For delay/prevent end points (progression): | |
 Progression: Progression: |  Progression: Progression: | |
  Exclude pseudoprogression in the absence of symptoms or other signs of progression Exclude pseudoprogression in the absence of symptoms or other signs of progression |   Retained Retained | |
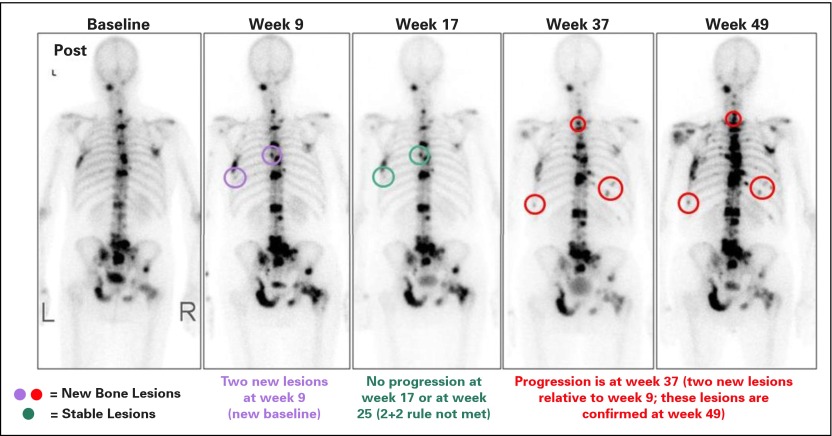
  At least two new lesions on first post-treatment scan, with at least two additional lesions on the next scan (2+2 rule) At least two new lesions on first post-treatment scan, with at least two additional lesions on the next scan (2+2 rule) | ||
  If at least two additional new lesions are seen on the next (confirmatory) scan, the date of progression is the date of the first post-treatment scan, when the first two new lesions were documented If at least two additional new lesions are seen on the next (confirmatory) scan, the date of progression is the date of the first post-treatment scan, when the first two new lesions were documented | Retained | |
  For all scans after the first post-treatment scan, at least two new lesions For all scans after the first post-treatment scan, at least two new lesions | For scans after the first post-treatment scan, at least two new lesions relative to the first post-treatment scan confirmed on a subsequent scan | |
  Date of progression is the date of the scan that first documents the second lesion Date of progression is the date of the scan that first documents the second lesion | Retained | |
  Changes in intensity of uptake alone do not constitute either progression or regression Changes in intensity of uptake alone do not constitute either progression or regression | Retained | |
| Report the proportion of patients who have not progressed at fixed time intervals (6 and 12 months) | ||
 nmCRPC nmCRPC | Not addressed | Nonmetastatic to metastatic progression: |
 Any new unequivocal bone lesion, except if that lesion appears in the first post-treatment scan; in that case, document the event, continue treatment until 2 additional new lesions appear, and record both events Any new unequivocal bone lesion, except if that lesion appears in the first post-treatment scan; in that case, document the event, continue treatment until 2 additional new lesions appear, and record both events | ||
| Patient-reported outcomes | Consider independently of other outcome measures | Pain palliation assessment requires a patient population with clinically meaningful pain at baseline (eg, ≥ 4 on a 10-point pain intensity scale) and response defined as a clinically meaningful score improvement at a subsequent time point (eg, a 30% relative or 2-point absolute improvement from baseline at 12 weeks, confirmed at least 2 weeks later, without an overall increase in opiate use) |
| For control/relieve/eliminate end points: | For control/relieve/eliminate end points: | |
 Document pain and analgesia at entry with a lead-in period and measure repeatedly at 3- to 4-week intervals Document pain and analgesia at entry with a lead-in period and measure repeatedly at 3- to 4-week intervals |  Serial (eg, daily × 7 days) assessments at each time point can improve the stability of values Serial (eg, daily × 7 days) assessments at each time point can improve the stability of values | |
| Perform serial assessments of global changes in HRQoL, urinary or bowel compromise, pain management, additional anticancer therapy | Principles may be extended for any PRO for which a clinically meaningful baseline PRO score has been determined together with a responder definition that is based on a sustained clinically meaningful score improvement | |
 Ignore early changes (≤ 12 weeks) in pain or HRQoL in absence of compelling evidence of disease progression Ignore early changes (≤ 12 weeks) in pain or HRQoL in absence of compelling evidence of disease progression | ||
| For delay/prevent end points: | For delay/prevent end points: | |
 Confirm response or progression of pain or HRQoL end points ≥ 3 weeks later Confirm response or progression of pain or HRQoL end points ≥ 3 weeks later |  Patients with any level of baseline pain, including no pain, are eligible to be evaluated for prevent/delay end points; those without pain are followed for development of pain, whereas those with baseline pain are followed for progression (eg, a 2-point increase without an overall decrease in opiate use) Patients with any level of baseline pain, including no pain, are eligible to be evaluated for prevent/delay end points; those without pain are followed for development of pain, whereas those with baseline pain are followed for progression (eg, a 2-point increase without an overall decrease in opiate use) | |
| Pain assessment should be administered at treatment discontinuation and once again if feasible (eg, 2 to 4 weeks later) | ||
 Time to deterioration of physical function and/or HRQoL scores should also be included, with a priori thresholds defining clinically meaningful deterioration score changes that are based on prior published data for the selected questionnaire Time to deterioration of physical function and/or HRQoL scores should also be included, with a priori thresholds defining clinically meaningful deterioration score changes that are based on prior published data for the selected questionnaire |
Abbreviations: CTC, circulating tumor cell; HRQoL, health-related quality of life; LDH, lactate dehydrogenase; mCRPC, metastatic castration-resistant prostate cancer; NLR, neutrophil/ lymphocyte ratio; nmCRPC, nonmetastatic castration-resistant prostate cancer; PCWG2, Prostate Cancer Clinical Trials Working Group 2; PCWG3, Prostate Cancer Clinical Trials Working Group 3; PRO, patient-reported outcome; PSA, prostate-specific antigen; RECIST, Response Evaluation Criteria in Solid Tumors.
CONCEPTUALIZING THE DISEASE
The decision milestones in PCWG2 were based on disease states defined by the status of the primary tumor, the presence or absence of distant disease on imaging (metastatic versus nonmetastatic), testosterone levels, and prior chemotherapy exposure.
PCWG3 presents a revised disease states model that, like its predecessor, defines trial objectives on the basis of state-specific clinical needs (Fig 1). Emphasis is placed on designing clinical trials within a biomarker context, and focusing on how biomarkers can be developed to predict outcome, guide management, and influence clinical decision making. The revised model aligns with the indications and uses of the currently approved drugs (see Appendix Table A1, online only) and provides the framework for a decision tree that closely follows contemporary clinical practice. Key new recommendations of the decision tree include (1) distinguishing adenocarcinomas from other histologies including pure small-cell carcinomas and variants with neuroendocrine differentiation; (2) replacing the pre- versus postchemotherapy distinction with a dynamic classification that considers the lines of therapy a patient has received independent of the mechanism of action, the order in which they were administered, and the sensitivity of the tumor to each; (3) designing dedicated trials for the different CRPC phenotypes defined by the pattern of spread; (4) adding recommendations for trials in the nonmetastatic CRPC (nmCRPC) state, formerly defined as the rising PSA castrate state; (5) emphasizing the importance of serial biologic profiling of the disease using minimally invasive blood-based assays of tumor material, imaging, or biopsy of a metastatic tumor site to identify and target mechanisms of primary or adaptive resistance and to better enable treatment selection to be based on disease biology; (6) including clinically relevant time-to-event end points such as symptomatic skeletal events (SSEs); and (7) with our increased recognition of disease heterogeneity and emerging resistance, focusing more on determining when a treatment should be discontinued when the patient is no longer clinically benefiting (NLCB) rather than strictly at the first evidence of progression.
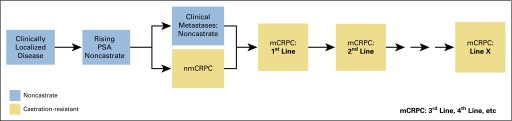
Prostate cancer clinical states model, a framework for patient treatment and drug development, updated for the Prostate Cancer Clinical Trials Working Group 3. Combination therapy is considered one line of therapy. mCRPC, metastatic castration-resistant prostate cancer; nmCRPC, nonmetastatic castration-resistant prostate cancer; PSA, prostate-specific antigen.
OBJECTIVES
The objectives of the PCWG3 recommendations are to identify subsets of patients for whom specific agents are most (or least) appropriate, ensure that the determination of dose and schedule for noncytotoxic treatments is mechanism specific, develop intermediate end points to support regulatory submissions, assess pre- and post-treatment disease biology, demonstrate the best use of agents to maximize patient benefit, and introduce the concept of a new time-to-event measure, NLCB, as the indicator that a change in therapy is necessary.
ELIGIBILITY FOR ENROLLMENT
Eligibility criteria define the population to be considered for enrollment as one that has a high likelihood of demonstrating the hypothesized effect of a treatment. The criteria may be based on clinical features and laboratory measures that are prognostic, predictive, or important from a safety perspective.
PATIENT DEMOGRAPHICS AND PATHOLOGY
Baseline Assessment
The baseline disease assessment recommendations of PCWG2 are expanded in PCWG3 (Table 2) to include histology; prior radiation therapy; the timing and duration of, and (if available) response to all prior systemic treatment(s); a standardized assessment of blood-based biomarkers; patient-reported outcomes (PROs); imaging; profiling disease by a repeat biopsy that also determines changes in histology or through blood-based assays for molecular markers/determinants of prognosis and drug sensitivity.
Table 2.
Standard Baseline Disease Assessments Recommended by PCWG3 in Comparison With PCWG2 Recommendations
| Assessment | PCWG2 (2008) | PCWG3 (2015) |
|---|---|---|
| Histology | Not addressed | Adenocarcinoma |
| Adenocarcinoma with small-cell or neuroendocrine features | ||
| Small-cell carcinoma | ||
| Report Gleason sum for primary | ||
| Consider rebiopsy of metastatic disease | ||
| Clinical | History and physical examination | Age, pain, analgesic consumption, performance status, comorbidity assessment, history, and physical examination; prior local therapy; TNM stage at diagnosis; and PSA |
| Prior systemic treatment | Pre- and postchemotherapy | Record each line of systemic therapy (single agent or combination) in order of administration, including start and stop dates, dose(s), and schedule(s), the disease state in which it was administered, and response (resistant v sensitive) on the basis of PSA if appropriate |
| Record type of progression on prior therapy (PSA, radiographic [bone, nodal, visceral], clinical [eg, pain escalation]) | ||
| Prior radiation therapy | Not addressed | Site, administered dose per fraction and treatment duration |
| Blood-based biomarkers | PSA Testosterone | Host: CBC with differential, ALK, kidney/liver function, albumin, LDH, testosterone* |
| Tumor: PSA and cPSA kinetics | ||
| Optional: CEA, chromogranin A, neuron-specific enolase, CTC enumeration | ||
| Imaging | ||
 Prostate/ prostate bed Prostate/ prostate bed | Endorectal MRI | Retained, cross-sectional imaging of prostate region if applicable |
 Nodal Nodal | CT: Only nodes ≥ 2 cm were assessed for change in size | CT or MRI: |
| Nodes ≥ 1.5 cm in the short axis are considered measurable; nodes ≥ 1.0 and less than 1.5 cm in the short axis are considered pathologic according to clinical discretion, and nontarget; nodes less than 1.0 cm in the short axis are nonpathologic | ||
| Record pelvic and extrapelvic (retroperitoneal, mediastinal, thoracic, other) nodal disease separately; up to five nodes in total | ||
| Record new lesions v growth of pre-existing lesions, and sites of new lesions | ||
 Visceral Visceral | CT: reported as visceral per RECIST | CT or MRI: |
| Record individual sites of spread (lung, liver, adrenal, CNS) separately; up to five lesions per site | ||
| Lesions ≥ 1.0 cm in the longest dimension are considered measurable | ||
| Record new lesions v growth of pre-existing lesions, and sites of new lesions | ||
 Bone Bone | 99mTc MDP | Record new lesions and sites of new lesions |
| Tumor profiling for determinants of prognostic, predictive, and resistance biomarkers | Not addressed | Consider rebiopsy of metastatic or locally recurrent lesion(s) for biologic characterization |
| Patient-reported outcomes | None | Pain assessment, opiate analgesia consumption, physical functioning (functional status), health-related quality of life; consider fatigue and PRO-CTCAE. Validated PRO instruments strongly recommended |
Abbreviations: ALK, alkaline phosphatase; CBC, complete blood count; CEA, carcinoembryonic antigen; CT, computed tomography; CTCs, circulating tumor cells; CTCAE, Common Terminology Criteria for Adverse Events; LDH, lactate dehydrogenase; MRI, magnetic resonance imaging; PCWG2, Prostate Cancer Clinical Trials Working Group 2; PCWG3, Prostate Cancer Clinical Trials Working Group 3; PRO, patient-reported outcome; PRO-CTCAE, Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events; PSA, prostate-specific antigen; RECIST, Response Evaluation Criteria in Solid Tumors; 99mTc MDP, 99mTc methylene diphosphonate.
Prior Systemic Treatment
To better define and characterize a patient’s disease at the time of trial enrollment and to explore potential favorable or unfavorable interactions among therapies, PCWG3 recommends recording all lines of therapy (represented as a single agent or combination) in the order they were administered. Ideally, reporting should include the start and stop dates, or at least the duration, for each prior line of therapy; the dose and schedule of the agent(s); the disease state in which it was administered; and the type of progression (biochemical [ie, a rising PSA], radiographic, and/or symptomatic). Response (resistant versus sensitive) should be categorized on the basis of the post-therapy PSA or radiographic change pattern for agents that reduce tumor burden. For example, Appendix Fig A1 (online only) shows that post-therapy PSA change patterns with agents that effect cell kill allow one to categorize response as resistant versus sensitive. Similar response patterns to prior therapy that were based on other clinical biomarkers such as imaging or pain may be considered and reported, recognizing the challenges in obtaining such historical data.
The definition of a prior hormonal intervention is changed to include only the addition of a hormonal therapy that had the intent of treating the cancer. Assessing for a withdrawal response to an antiandrogen is no longer considered an intervention and is not required, but should be considered for 4 to 6 weeks for patients who have been on long-term antiandrogen therapy with apparent benefit. A response, if it was observed on discontinuation, should be recorded.
Prior Radiation Treatment
Details of the dates, portals, number of fractions, dose per fraction, and total administered dose by portal should be recorded for all courses of radiation therapy, including those directed at the primary and metastatic site(s). Details of prior radioisotope therapy should also be recorded.
Histology and Pathologic Subtype
To better inform future decision making, PCWG3 encourages the direct biologic characterization of the tumor at the time a new treatment is being considered by performing a biopsy of a metastatic site or with blood-based diagnostics such as circulating tumor cells (CTCs), circulating nucleic acids, or host factors (eg, markers of bone turnover or immune function).
Although a number of prostate cancer histologic subtypes have been described, only the pure small-cell phenotype is a distinct entity that is diagnosed consistently.12,13 It represents less than 1% of newly diagnosed prostate cancers but leads directly to treatment with cytotoxic regimens similar to those used to treat small-cell lung carcinomas of the lung and should thus be noted specifically. Treatment-emergent histologic variants that contain nonadenocarcinomatous elements are still being defined but are anticipated to differ from the de novo small-cell phenotype.13
BLOOD-BASED BIOMARKERS
Baseline Measures
A baseline assessment of hematologic, hepatic, and renal function is essential to assess prognosis, to predict response to therapy, and for safety. Multivariable analyses suggest that hemoglobin, alkaline phosphatase, albumin, and lactate dehydrogenase are established prognostic factors for survival14 and should be included in all studies.
Testosterone.
PCWG3 retains the definition of castrate as ≤ 50 ng/dL of testosterone (or ≤ 0.50 ng/mL or 1.73 nmol/L) but encourages investigators to consider using ultrasensitive testosterone assays with a sensitivity to 1 to 2 ng/dL when evaluating a drug known or suspected to change androgen levels.
Bone biomarkers.
Biochemical markers of osteoblast and osteoclast activity are often reported but rarely used for eligibility in most clinical trials.15 Although not required, they may be measured as a prognostic and pharmacodynamic marker in certain contexts.16
Immune function.
Assays of immune function such as serum cytokines, antibody response, T-cell subsets, function and antigen specificity are increasingly included in trials. Most have not achieved the necessary level of analytical validity, with the exceptions of the absolute lymphocyte count and neutrophil-to-lymphocyte ratio, which are obtained from a complete blood count.17,18
PSA and other tumor markers.
A rising PSA is typically the first sign of tumor regrowth, followed later by worsening of disease by imaging and the development of clinical symptoms.19 PSA should be assessed in all trials, and in particular for those with PSA change as a primary end point, in a central laboratory to minimize interassay variability. PCWG3 retains the PCWG2 criteria for PSA progression.5,6,10,11 For nmCRPC, PCWG3 advises recording PSA doubling times (PSADTs) and focusing drug development efforts on patients with shorter times who have the greatest risk of developing detectable metastatic disease or symptoms, and dying of their cancers.20,21 The preferred method for calculating PSADT is described in the Appendix. Other markers in the blood, such as chromogranin A, neuron-specific enolase, and carcinoembryonic antigen, have not been validated in prospective studies (Table 2).
CTC number.
The usefulness of CTC analyses at baseline and during treatment is an area of active research. Although multiple platforms are under development,22 CellSearch (Janssen Diagnostics, Raritan, NJ), which reports the number of cells captured from whole blood with an EpCAM-positive immunomagnetic ferrofluid, is the only US Food and Drug Administration–cleared CTC assay at the present time.22 Baseline CTC count, reported as favorable or unfavorable (< 5 versus ≥ 5 CTC/7.5 mL of blood, respectively), has been shown to be an independent prognostic factor for survival in CRPC both before and after treatment.22-24 Although the strengths and limitations of CTC enumeration as an indicator of antitumor effects require further study, CTCs may be considered for use as a screening end point for activity in patients with unfavorable counts at baseline.25 CTCs can also serve as a source of tissue for biologic profiling.26-28
PROs
PCWG3 recognizes the importance of patient-centered drug development and reporting the patient experience on study.29 Pain is the most established PRO in this population and is associated with inferior survival and diminished quality of life.30-33 When pain is an important component or primary end point of a study, PCWG3 recommends a baseline assessment using serial measurements, including pain intensity, pain interference with activities, and opiate intake, over several days before starting treatment, using methods described by the US Food and Drug Administration.34 The average score over those days is used as the baseline pain value.35 Pain questions using numerical rating scales (0 to 10) can be used, eliciting worst pain intensity and interference over the previous 24 hours.34
Physical functioning should also be assessed and can be measured at baseline and during treatment using an established multi-item questionnaire such as the physical function measure of the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 or Patient-Reported Outcomes Measurement Information System (PROMIS) instruments. Collection of patient-reported adverse events should be considered at baseline and during treatment using the National Cancer Institute’s Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE).36
IMAGING
Imaging provides critical information on disease distribution, prognosis, extent, biology, and host reaction to the tumor.
Baseline by Site of Disease
PCWG3 retains the PCWG2 recommendations with modifications that include developing, recording, and validating measures of disease burden. Imaging of the chest, abdomen, and pelvis using a contrast-enhanced computed tomography (CT) scan with ≤ 5-mm axial slices is advised for all patients. For those intolerant of contrast, a cross-sectional magnetic resonance imaging (MRI) scan of the abdomen and pelvis, with a noncontrast CT scan of the chest, may be considered. In phase I and II trials, recognizing that individual lesions may be biologically distinct, PCWG3 recommends reporting whether progression on entry was in the growth of pre-existing lesions, the development of new lesions, or both. PCWG3 advises following Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 for extraskeletal disease but recommends that up to five lesions per site of metastatic spread (eg, lung, liver, lymph nodes as separate sites) be recorded to address disease heterogeneity and to track patterns of metastatic progression. Bone lesions should be recorded separately (see Bone section on this page).
Prostate or prostate bed.
Specific imaging of the prostate or prostate bed is not required for every patient. If there is a question of locally persistent or recurrent disease, a directed MRI of the prostate or prostate bed and/or biopsy of the site is recommended.
Nodes or viscera.
PCWG3 advises that nodal disease be measured in the short axis and recorded by location: pelvic disease should be classified as locoregional, and extrapelvic disease (retroperitoneal, mediastinal, thoracic, or other) as metastatic. Nodes ≥ 1.5 cm in the short axis are considered pathologic and measurable. As per RECIST 1.1, lymph nodes that are ≥ 1.0 cm but less than 1.5 cm in the short axis may be pathologic and can be considered nonmeasurable/nontarget lesions.37 Visceral disease in metastatic patients should be designated separately as lung, liver, adrenal, or CNS and is considered measurable if an individual lesion is ≥ 1 cm in its longest dimension. Given that lung metastases are relatively frequent in mCRPC trials (7% prevalence), chest CT imaging is recommended.38
PCWG2 did not formally address trials in the nmCRPC population, where the aim is to delay/prevent the development of radiographically evident metastatic disease and death. To establish nonmetastatic status, note that nodes less than 1.0 cm in the short axis are nonpathologic; nodes 1.0 to less than 1.5 cm may be considered pathologic, but with clinical discretion; and nodes ≥ 1.5 cm are both pathologic and measurable. PCWG3 advises that trial entry be based on PSADT, and that the same standard imaging modalities (eg, bone scan, CT, and/or MRI) used to determine eligibility be used for monitoring the patient receiving treatment.
Bone.
The use of 99mTc-methylene diphosphonate radionuclide bone scintigraphy as the standard for bone imaging is retained in PCWG3, with the presence or absence of metastasis recorded first. A quantitative measure of disease burden, such as lesional number,39 the bone scan index,40,41 or lesion area,42 is also suggested, recognizing that these measures require further analytical and prospective clinical validation. Changes in lesions considered metastatic on bone scintigraphy should be followed and assessed serially using a bone scan assessment form (Appendix Fig A3, online only). Areas/lesions on bone scintigraphy that are suggestive can be assessed further with CT or MRI and followed separately, but such supplemental imaging should not be used to establish indicator lesions for the purposes of a trial.
Different modalities for imaging bone metastases can provide different information for the same patient (Appendix Fig A2). However, because of the lack of standards for reporting disease presence or changes after treatment, positron emission tomography imaging with sodium fluoride, fluorodeoxyglucose, choline, or prostate-specific membrane antigen, bone marrow MRI (body MRI), and other modalities that are in use to image bone, should be approached as new biomarkers subject to independent validation.
Neurologic.
PCWG3 upholds the PCWG2 recommendation to perform an MRI or CT of the brain for patients with small-cell/neuroendocrine tumors and to maintain a low threshold for performing an MRI of the base of the skull or spine to diagnose and/or detect impending neurologic compromise. Routine imaging of the brain for adenocarcinoma is not recommended.
Type of progression at entry into a trial.
PCWG3 advises recording whether progression was manifested by PSA alone, bone ± nodes by location, nodes by location only, or viscera (± other sites), and the proportion of patients who progress in each of these categories, because this is prognostic.5,6,10,11 PCWG3 also advises reporting whether progression by imaging at study entry involved the growth or enlargement of pre-existing lesions, the development of new lesions, or both. The criteria for progression can be found in Table 3.
Table 3.
Criteria for Progression at Trial Entry by Disease Manifestation
| Variable | PCWG2 (2008) | PCWG3 (2015) |
|---|---|---|
| Blood-based | ||
 PSA PSA | Obtain sequence of rising values at a minimum of 1-week intervals | Retained |
| 2.0 ng/mL minimal starting value | 1.0 ng/mL is the minimal starting value if confirmed rise is only indication of progression unless pure small-cell carcinoma | |
| Estimate pretherapy PSADT if at least three values available ≥ 4 weeks apart | Retained | |
| Imaging | ||
 Nodes Nodes | Nodal progression sufficient for trial entry independent of PSA | Retained |
| Measurable lesions not required for entry | Retained | |
| Use RECIST to record nodal lesions as target or nontarget | Modified RECIST 1.1 criteria, separate pelvic and extrapelvic disease, up to five nodal lesions total recorded | |
| Only lymph nodes ≥ 2 cm in diameter (long axis) were actionable as progressive disease | Previously normal (< 1.0-cm) lymph nodes must have grown by ≥ 5 mm in the short axis from baseline or nadir and be ≥ 1.0 cm in the short axis to be considered to have progressed | |
| If the node progresses to ≥ 1.5 cm in the short axis, it is measurable; nodes that have progressed to 1.0 to less than 1.5 cm are pathologic, subject to clinical discretion, and nonmeasurable | ||
| For existing pathologic adenopathy, progression is defined per RECIST 1.1 | ||
| Record presence of nodal and/or visceral disease separately | Retained with modification | |
| Nodal sites: | ||
 Locoregional: pelvic only Locoregional: pelvic only | ||
 Extrapelvic: retroperitoneal, mediastinal, thoracic, or other Extrapelvic: retroperitoneal, mediastinal, thoracic, or other | ||
 Viscera Viscera | Visceral progression sufficient for trial entry independent of PSA | Retained but recorded separately by site of spread (lung, liver, adrenal, CNS); up to five lesions per site of spread |
| Measurable lesions not required for entry | Retained | |
| Use RECIST to record visceral lesions as target or nontarget | Retained | |
| Record presence of nodal and/or visceral disease separately | Retained with modification | |
| Visceral sites: lung, liver, adrenal, CNS | ||
 Prostate/prostate bed (primary site) Prostate/prostate bed (primary site) | Record prior treatment of primary tumor | Retained |
| Perform directed pelvic imaging (CT, MRI, PET/CT, endorectal MRI, transrectal ultrasound) to document presence or absence of disease | Retained | |
 Bone Bone | Two new lesions | Retained |
| Confirm ambiguous results by other imaging modalities (eg, CT or MRI) | Retained, but only positivity on the bone scan defines metastatic disease to bone | |
 Other sites of disease Other sites of disease | Patients with treated epidural lesions and no other epidural progression are eligible | Retained |
| Type of progression at trial entry | Not addressed | Report separately: |
 PSA only PSA only | ||
 Bone only ± nodal disease Bone only ± nodal disease | ||
 Nodal disease only (no bone disease present) Nodal disease only (no bone disease present) | ||
 Visceral (lung, liver, adrenal, CNS) disease (± other sites) Visceral (lung, liver, adrenal, CNS) disease (± other sites) | ||
| Record new lesions and site of new lesions v growth of pre-existing lesions, or both | ||
| Other markers | ||
 Patient-reported outcomes Patient-reported outcomes | Not addressed | For pain palliation analyses, presence of clinically meaningful pain at baseline (eg, ≥ 4 on a 10-point pain intensity scale) is a prerequisite; for pain progression analyses, patients may have any level of pain at baseline, including no pain |
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging; PCWG2, Prostate Cancer Clinical Trials Working Group 2; PCWG3, Prostate Cancer Clinical Trials Working Group 3; PET, positron emission tomography; PSA, prostate-specific antigen; PSADT, PSA doubling time; RECIST, Response Evaluation Criteria in Solid Tumors.
Clinical subtypes of CRPC that are based on pattern of spread.
The five clinical subtypes defined in PCWG2 on the basis of the pattern of spread in individual patients are retained with modifications: (1) locally recurrent CRPC after radical prostatectomy or persistent disease in the prostate or prostate bed after radiation therapy on ADT, with no evidence of metastases on imaging; (2) nonmetastatic (nmCRPC): a rising PSA with no detectable disease in the primary site, in lymph nodes beyond the true pelvis by CT/MRI (lymph nodes ≤ 1.5 cm in the short axis in the pelvis are eligible), in bone by radionuclide bone scan or CT, or in visceral organs; (3) nodal spread within the pelvis (lymph nodes > 1.0 cm) and/or beyond the pelvis (specify) and no evidence of bone or visceral disease43; (4) bone disease with or without nodal disease and no evidence of visceral spread; (5) visceral disease with or without spread to other sites; includes spread to lung, liver, or adrenal and CNS sites, each reported separately. It is recommended that research studies report the proportion of patients in each subtype. Stratification by pattern of metastatic spread is also encouraged.
MOLECULAR AND BIOLOGIC SUBTYPES
With the report of the CRPC genotypic landscape,44 PCWG3 strongly endorses incorporating detailed molecular assessments of tumors into clinical trial strategies to better understand the disease biology and to identify predictors of sensitivity to a specific therapy. This requires the molecular characterization of an individual patient’s tumor at the time treatment is considered. A profile of the primary tumor obtained at diagnosis may not be sufficiently informative for a castration-resistant metastatic site, because the biologic drivers of growth and cell-surface targets can change as the disease progresses through various treatments. Molecular biomarkers in metastatic lesions can be assessed through a directed biopsy45,46 or by using blood-based assays of CTCs or cell-free nucleic acids (RNA or DNA or proteins), recognizing that the biologic profiles of different lesions and blood-based assays in the same patient may not be the same. The number of tumor cells within a single metastatic site that harbor a specific alteration may also vary and, as such, simply detecting its presence at a low frequency may not predict sensitivity.
ASSESSING PROGNOSIS
Prognosis indicates the likelihood that a patient or patient group will develop a disease manifestation if left untreated. Predicted survival time is one measure estimated commonly by using nomograms. Validated prognostic tools such as nomograms include patient demographics and clinical measures to estimate outcomes at a trial-based level as risk-stratification tools.16,33,47-51 Adding a measure of metastatic disease burden and type of radiographic progression (new lesions versus growth of existing lesions) on the basis of physician assessment or computer-based assessments of bone or other disease sites may permit more reliable prognostication.42,52 The incorporation of prognostic molecular biomarkers is an active area of research.
INTERVENTION
In PCWG3, the term intervention includes both the therapy itself and the post-treatment assessments to determine dose, schedule, and antitumor effects. PCWG3 emphasizes the importance of developing trials that provide the following information tailored to the mechanism and postulated therapeutic target(s) of the agent: population-specific pharmacokinetics in the castrate state, the use of pharmacodynamic measures specific to the postulated effect of a particular agent on the malignant process to confirm or refute the hypothesized mechanism of action, and the optimal timing to assess the pharmacodynamic measures and to inform the determination of the optimal dose and schedule to study in future trials. The optimal time to assess for antitumor effects, which can be delayed for many types of treatment including those that affect immune function, should also be determined by assessing disease status at fixed intervals.
MEASURING OUTCOMES AND REPORTING
PCWG3 reaffirms the recommendation to use control/relieve/eliminate end points to assess antitumor effects of therapies that are anticipated to kill tumor cells, particularly in early clinical development. For therapies not expected to kill tumor cells, delay/prevent end points should be used. PCWG3 discriminates between activity-estimating end points in early-phase trials (such as declines in PSA, changes in CTCs, and time to progression) where the objective is to demonstrate sufficient antitumor activity to justify further study, versus registration trials for regulatory approval where clinical benefit is the objective. For instance, in early-phase trials, time to progression, which censors deaths, may be used, whereas in registration trials, progression-free survival, which includes deaths as events, is preferred. The suitability of efficacy end points to demonstrate clinical benefit is context dependent, and consultation with regulatory authorities is strongly recommended when selecting and defining end points for a trial intended to support drug approval.53 Although demonstrating overall survival may be challenging as a primary end point, it is assumed that all trials will continue to follow patients for survival and analyze and report survival results, particularly in randomized clinical trials. PCWG3 also recommends that all therapies administered subsequent to the intervention, including start and stop dates when available, be recorded, until death. This is essential because the availability and use of life-prolonging treatments post protocol may reduce the ability to demonstrate the survival benefit of an effective treatment.
On-treatment evaluations should include physical examinations, symptom assessments, and laboratory studies to assess safety, with appropriate attribution to the disease or therapy. Imaging should include cross-sectional imaging of the chest, abdomen, and pelvis, as well as bone scintigraphy, regardless of whether patients have involvement of those sites at baseline. Imaging strategies restricted to known sites of disease risk missing disease progression at new sites.
PCWG3 advises that disease assessments be performed at fixed intervals to better understand when antitumor effects occur (Table 4). To minimize patient exposure to ineffective treatment, and to better assess the timing of the antitumor effects of an agent for which the optimal timing is not known, an 8- to 9-week assessment interval for the first 6 months and every 12 weeks thereafter is advised. This recommendation is based on the findings of several trials.5,10,54 The shorter interval also helps clarify bone scan flare (the development of new lesions on a first follow-up scan that may actually represent a favorable response to treatment). Using the shorter interval will also inform the optimal assessment interval in subsequent trials, particularly important for biologic therapies whose antitumor effects may be delayed. Notable is that the applicability of the immune-modified RECIST criteria to prostate cancer has not been established, in particular, whether an early increase in the size of a nodal or visceral lesion represents the recruitment of immune effector cells or tumor growth.55 It is also noteworthy that neither the RECIST criteria nor the immune-modified RECIST criteria address changes in osseous disease.
Table 4.
Suggested Frequency of Assessment for Commonly Used Measures in Metastatic Prostate Cancer Clinical Trials
| Measure* | PCWG2 Frequency (2008) | PCWG3 Frequency (2015)† |
|---|---|---|
| Clinical | ||
 Symptoms/ performance status Symptoms/ performance status | Every cycle | Retained |
| Blood-based markers | ||
 PSA PSA | By cycle (every 3 or 4 weeks) | Retained |
 ALK, LDH ALK, LDH | By cycle (every 3 or 4 weeks) | Retained |
 Serum chemistry, CBC Serum chemistry, CBC | Not addressed | By cycle (every 3 to 4 weeks) |
 Circulating tumor cells Circulating tumor cells | Not addressed | By cycle (every 3 to 4 weeks) if available |
| Imaging | ||
 Bone scans Bone scans | Every 12 weeks | Every 8 to 9 weeks for first 24 weeks, then every 12 weeks† |
 CT/MRI CT/MRI | Every 12 weeks | Every 8 to 9 weeks for first 24 weeks, then every 12 weeks† |
| Patient-reported outcomes | Not addressed | By cycle (every 3 to 4 weeks) |
 Analgesic consumption (opioids/no opioids) Analgesic consumption (opioids/no opioids) | Not addressed | By cycle (every 3 to 4 weeks) |
Abbreviations: ALK, alkaline phosphatase; CT, computed tomography; LDH, lactate dehydrogenase; MRI, magnetic resonance imaging; PCWG2, Prostate Cancer Clinical Trials Working Group 2; PCWG3, Prostate Cancer Clinical Trials Working Group 3; PSA, prostate-specific antigen.
APPLYING THE CONTROL/RELIEVE/ELIMINATE VERSUS DELAY/PREVENT FRAMEWORK
PCWG3 retains the recommendation to report outcomes by manifestation (eg, host or tumor biomarkers, symptoms, site of spread) and not to report grouped categorizations of response such as complete response, partial response, or progression on the basis of multiple manifestations. In patients who show evidence of benefit, PCWG3 advises continuing therapy in the case of an isolated PSA rise after an initial decline until radiographic or clinical progression is manifest. Table 5 describes the updated PCWG3 recommendations for reporting outcomes by disease manifestation. Of particular note is the use of the 2+2 rule to distinguish flare from true progression in patients with osseous disease at baseline (Fig 2).4,6 Also recommended are reporting the proportion of patients who have not progressed at fixed time intervals (eg, 6 or 12 months) and reporting separately the time receiving treatment.
RADIOGRAPHIC PROGRESSION-FREE SURVIVAL
Radiographic progression-free survival (rPFS) is the time interval from random assignment to the date when the first site of disease is found to progress (using a manifestation-specific definition of progression), or death, whichever occurs first. To better understand the effect of therapy on an individual site of disease, PCWG3 advises the date of progression in all specific sites be reported independently whether it is bone, nodes (pelvic or extrapelvic), visceral (lung, liver, adrenal, or CNS), or other. Reporting the proportion of patients who remain radiographic progression free at fixed time points (eg, 6 and 12 months) is also advised.
PCWG3 also emphasizes the importance of analytically and clinically validating the definitions of progression for each site of disease, as was performed for the bone scan progression end point proposed in PCWG2 that became part of the rPFS definition in the phase III registration trials of abiraterone (NCT00887198), enzalutamide (NCT01212991), and orteronel (NCT01193244). Noteworthy is that in all three trials, the local radiology interpretations were highly concordant with the centralized interpretation. The association between rPFS and overall survival is still being investigated, but an association was demonstrated in at least one of these trials.56
SKELETAL-RELATED EVENTS AND SYMPTOMATIC SKELETAL EVENTS
Delay/Prevent
PCWG2 did not consider skeletal-related events (SREs), which include asymptomatic nonclinical fractures ascertained by serial imaging, clinical pathologic fractures, spinal cord compression, and surgery or radiation therapy to bone. Reduction in the frequency of SREs is an approvable end point in its own right, used in several trials to support the approval of zoledronic acid and denosumab.57,58 The registration study of radium-223 used a composite bone end point, symptomatic skeletal events (SSEs), defined as symptomatic fracture, radiation or surgery to bone, or spinal cord compression.9,59
PCWG3 advises reporting SSEs that include only symptomatic events of clear clinical significance, in contrast to SREs, which also include asymptomatic fractures. The prospective evaluation of composite end points such as SSEs that incorporate rPFS and clinical progression is warranted, to determine which end point may be most associated with clinical benefit and/or survival after treatment with a specific agent class.
PROs
Control/Relieve/Eliminate
PCWG3 supports standardizing an approach for reliable and quantitative assessment of the patient experience in prostate cancer trials. PCWG3 reaffirms that demonstrating pain palliation requires a patient population with clinically meaningful pain at baseline (eg, ≥ 4 on a 10-point pain intensity scale) and a response defined as a clinically meaningful score improvement at a subsequent time point (eg, a 30% relative or 2-point absolute improvement from baseline at 12 weeks, confirmed at least 2 weeks later, without an overall increase in opiate use).34 Serial (eg, daily) assessments at each time point can improve the stability of values. These principles may be extended to any patient-reported disease-related symptom end point for which a clinically meaningful baseline score has been determined, together with a responder definition that is based on a sustained clinically meaningful score improvement.60 Physical functioning should be assessed serially using a validated instrument (eg, EORTC QLQ-C30 or PROMIS).
Delay/Prevent
For a time-to-pain-progression analysis, patients with any level of baseline pain are eligible. Those without pain are followed for development of pain, whereas those with baseline pain are followed for pain progression (eg, a 2-point increase without an overall decrease in opiate use). A pain assessment should be administered at treatment discontinuation and once again if feasible (eg, 2 to 4 weeks later). When product labels are correct, they should include time-to-pain-progression end points, such as the time to development of opiate-dependent pain.61 Time to deterioration of physical function should also be included, with a priori thresholds defining clinically meaningful deterioration score changes.
Description of Adverse Events
Collection of patient-reported symptoms related to adverse events from treatment should be considered using the National Cancer Institute’s PRO-CTCAE.36 Inclusion of PRO-CTCAE should be considered in both early- and late-phase clinical trials. Results from PRO-CTCAE analyses can help determine optimal dosing and tolerability and can inform the risk:benefit evaluation of treatments.
PROGRESSION FROM nmCRPC TO mCRPC
Lymph Nodes
PCWG3 recommends that lymph nodes that were previously normal in size (< 1.0 cm) or pathologic in size must have grown by at least 5 mm in the short axis from baseline or nadir and be ≥ 1.0 cm in the short axis to be considered to have progressed. If the node progresses to ≥ 1.5 cm in the short axis, it is pathologic and measurable. Nodes that have progressed to between 1.0 and less than 1.5 cm are pathologic subject to clinical discretion and are nonmeasurable. These guidelines apply to studies involving patients with both metastatic and nonmetastatic disease. For existing pathologic adenopathy, progression is defined per RECIST 1.1.
Visceral
The date of first metastasis is the date on which an unequivocal visceral lesion by RECIST 1.1 is determined. No confirmatory scan is required unless the protocol uses modified criteria for immunotherapy.62 Visceral disease should be reported as being with or without other patterns of spread (node, bone).
Bone
Documentation of radiographic evidence of metastatic disease should include the time of the unequivocal development of new sites on bone scintigraphy. Given the earlier disease setting, it is advised that the scanning interval be increased to 16 weeks rather than the 8 weeks recommended for trials in mCRPC (Table 4). With 8-week intervals, it is less clear whether the detection of new sites of disease on a first follow-up bone scan represents flare of a pre-existing subclinical metastatic lesion(s) or a true transition from a nonmetastatic to a metastatic state.
In such cases, PCWG3 advises that the event be recorded, and that both the treatment and serial radiographic assessments be continued until two additional new lesions are detected, assuming the treatment is being tolerated well and there is no clinical reason to discontinue it. Both the date of the initial detection of metastatic disease and the date of subsequent progression should be recorded and both intervals, metastasis-free survival (MFS) and rPFS, should be reported. Because the optimal method to confirm new metastatic bone disease is uncertain at this time, the definition of MFS should be discussed with regulatory authorities if the MFS end point is being considered for a trial intended to support drug approval.
Use of Confirmatory Scans With Other Modalities
For patients who develop equivocal bone lesions while on study, PCWG3 discourages the use of other scanning modalities, including MRI, positron emission tomography, or other investigational scans that were not used to determine eligibility for the study. Supplemental scanning using imaging modalities with varied sensitivity that were selected by personal preference or regional practice may introduce bias.
MIXED RESPONSES
Individual metastatic foci in a particular site of disease or in the patient as a whole may be biologically distinct. Such biologic heterogeneity can result in a mixed response to treatment, wherein one site responds favorably and another unfavorably. This phenomenon has been considered historically as disease progression that requires a change in therapy. PCWG3 advises focusing on the entirety of disease rather than on a subset of index lesions. A review of the disease as a whole using each modality used at baseline is recommended. Biopsies of these growing, nonresponding, and new lesions may provide insights into mechanisms associated with resistance.
Control/Relieve/Eliminate
Whether favorable changes are occurring in all lesions in an organ or disease site, or in a subset of lesions, should be reported; whether they are occurring in one organ site or across sites of involvement should be reported separately.
Delay/Prevent
Progression may not occur in all sites. PCWG3 encourages reporting whether worsening represents the growth of one to two existing foci in an organ or disease site, or the development of new lesions in an organ or disease site that was previously uninvolved.
NLCB: PROGRESSION VERSUS THE DECISION TO DISCONTINUE THERAPY
PCWG2 encouraged the continuation of treatment if a rising PSA or worsening of an isolated disease site that was not clinically significant was the sole indicator of disease progression and the patient was otherwise tolerating therapy. Now, recognizing the biologic heterogeneity of individual metastatic lesions, PCWG3 draws the distinction between documenting progression for consistency of reporting (eg, recording the date of documented progression in a site of disease such as a lymph node that is unlikely to adversely affect prognosis) versus the decision to stop therapy.
To address this, PCWG3 introduces the no longer clinically benefiting (NLCB) reporting metric defined as the date and the specific reason(s) a therapy was ultimately discontinued. This end point permits individualized provider-patient decisions to continue or discontinue a treatment based on the primary therapeutic objective for which it is being administered and assessed, be it quality of life, PROs, or survival. As an example, in cases in which multiple sites of disease continue to respond but one to two sites grow, focal therapy such as radiation or surgery could be administered to the resistant site(s) and systemic therapy continued. Similarly, therapy may be continued if progression by PSA or imaging is slow and the disease-related symptoms that were present at baseline remain controlled. Important here is to record in detail the specific reasons why a therapy was ultimately discontinued, which may include clinical deterioration (clarifying whether it is disease or therapy related) or need for a change in systemic therapy. PCWG3 cannot define the risks/benefits at the individual level for continuation of therapy beyond progression, but sets the goal of prospectively defining the circumstances in which scenarios are identified where continuing a therapy is justified. Clinical trials evaluating whether therapy A should be stopped and a new one started, continued alone, or continued with a new one added, are ongoing.
To illustrate the concept of NLCB, we recommend use of a swimmers plot (Fig 3), in which each lane documents the individual patient experience on a trial over time. This plot includes significant adverse events encountered, treatment discontinuations, and the points at which the criteria for progression by a specific disease manifestation were met while treatment was continued up through the time treatment was ultimately discontinued. The information in each swim lane can also be used to record the dates a focal therapy (eg, external beam radiation) was used to treat nonresponding sites in cases in which multiple other sites of disease are still responding. In both cases, the patient may still be benefiting overall from a treatment, justifying its continuation. These plots are suggested for phase I and II studies and may be particularly useful in trials designed to assess the patient experience across treatment groups.
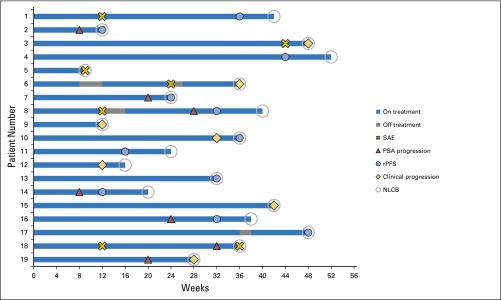
Swim lanes illustrating the patient experience on a trial. For early-phase trials, the swimmers plot is used to track individual patients on a trial, including time on and off therapy, adverse events, progression events, and the point at and the reason for which therapy is stopped. Progression in individual disease manifestations and other significant events are recorded, including those that did not lead to trial discontinuation and those that did. Pragmatically, each line shows the duration on therapy. Note that this particular example depicts a trial in progress. NLCB, no longer clinically benefiting; PSA, prostate-specific antigen; rPFS, radiographic progression-free survival; SAE, serious adverse event.
SUMMARY
PCWG3 seeks to move the drug development process closer to common clinical scenarios encountered in routine practice. It also aims to allow a more complete characterization of the host and his disease, not only at the start of therapy, but also over time as it evolves under treatment, which may help establish the value and the benefit of continuing a therapy when there are signs that the disease is beginning to progress.
PCWG3 emphasizes the distinction between adenocarcinomas and other histologies, moves away from the pre- and post-taxane categorization, and recommends eligibility on the basis of prior therapies received. This should result in a greater focus on developing specific protocols for the distinct clinical phenotypes. An important addition to PCWG3 is the recommendation to describe the percentage of patients with a specific disease pattern; in some cases, stratification for a specific pattern of disease may be indicated. Focusing PROs on core concepts of disease-related symptoms, physical functioning, and adverse events of therapy is advised, with the goal of improving the standardization of PRO in prostate cancer clinical trials.
Serial biologic profiling of the disease before treatment, while receiving treatment, and at progression is also advised. To establish clinical significance, PCWG3 advises evaluating the strength of the association between early changes in individual outcome measures, be they clinical, biochemical, PROs, imaging, CTCs, or other molecular determinants, and later events such as rPFS or clinical progression-free survival and/or overall survival. Standards for the interpretation of outcomes for therapies that affect the immune system are also needed. Although improvements in symptoms and/or functional status can be clinical benefits in their own right, determining the clinical significance of a statistically significant change in a PRO measure is another area of focus. Finally, PCWG3 underscores the distinction between the need to consistently report progression by individual disease manifestation in the order in which they occur (be it PSA, imaging, or other surrogate) from the need to terminate a treatment because the patient is NLCB from the therapy he is currently receiving.
AUTHOR CONTRIBUTIONS
Conception and design: Howard I. Scher, Michael J. Morris, Walter M. Stadler, Celestia Higano, Ethan Basch, Karim Fizazi, Emmanuel S. Antonarakis, Tomasz M. Beer, Michael A. Carducci, Kim N. Chi, Paul G. Corn, Johann S. de Bono, Robert Dreicer, Daniel J. George, Elisabeth I. Heath, Maha Hussain, Wm. Kevin Kelly, Glenn Liu, Christopher Logothetis, David Nanus, Mark N. Stein, Charles J. Ryan, Oliver Sartor, Eric J. Small, Matthew Raymond Smith, Cora N. Sternberg, Mary-Ellen Taplin, George Wilding, Peter S. Nelson, Lawrence H. Schwartz, Susan Halabi, Philip W. Kantoff, Andrew J. Armstrong
Provision of study materials or patients: Howard I. Scher, Michael J. Morris
Collection and assembly of data: Howard I. Scher, Michael J. Morris, Celestia Higano, Tomasz M. Beer, Lawrence H. Schwartz, Susan Halabi, Andrew J. Armstrong
Data analysis and interpretation: Howard I. Scher, Michael J. Morris, Walter M. Stadler, Ethan Basch, Emmanuel S. Antonarakis, Tomasz M. Beer, Michael A. Carducci, Robert Dreicer, Daniel J. George, Maha Hussain, Dana E. Rathkopf, Susan F. Slovin, Charles J. Ryan, Oliver Sartor, George Wilding, Lawrence H. Schwartz, Susan Halabi, Andrew J. Armstrong
Manuscript writing: All authors
Final approval of manuscript: All authors
Acknowledgment
Supported by National Cancer Institute (NCI) Grant No. P50-CA92629 SPORE in Prostate Cancer, NCI Cancer Center Support Grant No. P30 CA008748, the Department of Defense Prostate Cancer Research Program (PC071610), the Prostate Cancer Foundation, the Sidney Kimmel Center for Prostate and Urologic Cancers, and funds provided by David H. Koch through the Prostate Cancer Foundation. We thank the following institutions and individuals for their input into these recommendations: the US Food and Drug Administration, the Prostate Cancer Clinical Trials Consortium (which is supported by the US Department of Defense and the Prostate Cancer Foundation), and other international investigators in prostate cancer drug and biomarker development.
Appendix
The Dual-Objective Framework for Therapeutic Objectives
The Prostate Cancer Clinical Trials Working Group 3 (PCWG3) retains the dual-objective structure described by the Prostate Cancer Clinical Trials Working Group 2 (PCWG2), categorizing therapeutic objectives as control/relieve/eliminate versus delay/prevent. Delay/prevent objectives include manifestations that are clinically significant trial end points in their own right, such as symptomatic skeletal events or prolonged survival. This model aligns with the indications and uses of the currently approved drugs (see Appendix Table A1). Notable is that pain is the only control/relieve/eliminate end point for which therapies are approved or indicated.
The Biomarker Context of PCWG3
Drug development programs are designed to demonstrate clinical benefit with acceptable safety in well-controlled studies.63 Optimally, the clinical benefit should fulfill an unmet need. PCWG3 considers all aspects of the clinical trial process within a biomarker context, from discovery or definition, to analytical assay validation, to clinical validation.64,65 The biomarker may be a clinical measure (eg, functional status), patient-reported outcome, tumor or host biochemical measure, imaging parameter, or molecular determinant. PCWG3 encourages defining and evaluating biomarkers for specific contexts of use during the early phases of drug development to enable validation in later-phase trials. The demonstration of a biomarker’s clinical usefulness (which shows that the risk/benefit outcome for the patient is improved when the biomarker test result is used in making the therapeutic decision) is also emphasized.66
Calculating Prostate-Specific Antigen Doubling Time
Prostate-specific antigen (PSA) doubling time should be calculated using a linear regression model of the normal logarithm of PSA and time.67 The calculation should be based on (1) at least three consecutive PSA values with each value ≥ 0.2 ng/dL, (2) inclusion of the most recent PSA values during androgen deprivation therapy, and (3) interval between first and last PSA values of ≥ 8 weeks but ≤ 12 months.20,21,67
Table A1.
Indications and Regulatory Labels for the Currently Approved Drugs for CRPC, Aligned With the PCWG3 Manifestation-Specific Outcome Framework
| Early manifestations (response): control, relieve, or eliminate |
 Approved to control pain or indicated for patients with symptoms Approved to control pain or indicated for patients with symptoms |
  Mitoxantrone plus prednisone for the treatment of patients with pain related to advanced hormone-refractory prostate cancer Mitoxantrone plus prednisone for the treatment of patients with pain related to advanced hormone-refractory prostate cancer |
  89Sr for the relief of bone pain in patients with painful skeletal metastases 89Sr for the relief of bone pain in patients with painful skeletal metastases |
  153Sm for relief of pain in patients with osteoblastic metastatic bone lesions that enhance on radionuclide bone scan 153Sm for relief of pain in patients with osteoblastic metastatic bone lesions that enhance on radionuclide bone scan |
  Radium-223* (symptoms from bone metastases with no involvement of other organs) Radium-223* (symptoms from bone metastases with no involvement of other organs) |
 No FDA drug approvals on the basis of: No FDA drug approvals on the basis of: |
  PSA declines PSA declines |
  Tumor shrinkage Tumor shrinkage |
  Bone scan improvement Bone scan improvement |
  Changes in circulating tumor cell number Changes in circulating tumor cell number |
| Later manifestations (progression): delay or prevent |
 Approved or shown to delay SREs or SSEs Approved or shown to delay SREs or SSEs |
  Zoledronic acid (SRE) Zoledronic acid (SRE) |
  Denosumab (SRE) Denosumab (SRE) |
  Abiraterone acetate (SRE)*,† Abiraterone acetate (SRE)*,† |
  Enzalutamide (SRE)*,† Enzalutamide (SRE)*,† |
  Radium-223 (SSE)*,‡ Radium-223 (SSE)*,‡ |
 Approved for delay of death (survival) Approved for delay of death (survival) |
  Docetaxel Docetaxel |
  Sipuleucel-T Sipuleucel-T |
  Cabazitaxel Cabazitaxel |
  Abiraterone acetate plus prednisone Abiraterone acetate plus prednisone |
  Enzalutamide Enzalutamide |
  Radium-223 Radium-223 |
 Delay of radiographic progression using PCWG2-based criteria, coprimary with survival Delay of radiographic progression using PCWG2-based criteria, coprimary with survival |
  Abiraterone acetate plus prednisone Abiraterone acetate plus prednisone |
  Enzalutamide Enzalutamide |
Abbreviations: CRPC, castration-resistant prostate cancer; FDA, United States Food & Drug Administration; PCWG2, Prostate Cancer Clinical Trials Working Group 2; PCWG3, Prostate Cancer Clinical Trials Working Group 3; PSA, prostate-specific antigen; SREs, skeletal-related events; SSEs, symptomatic skeletal events.
Fig A1.
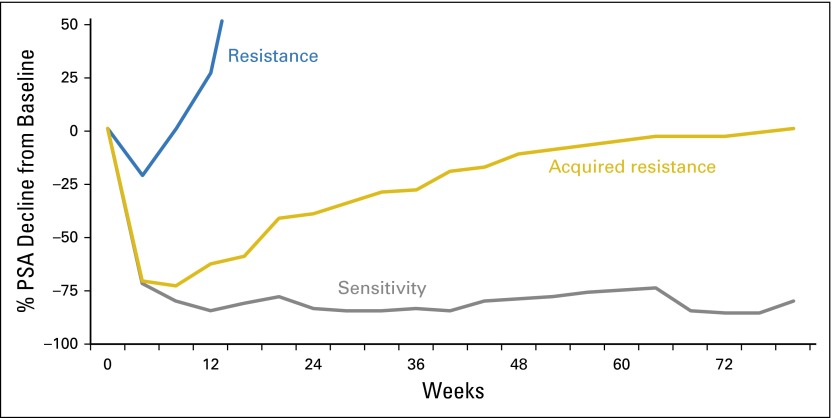
Three patterns of biomarker (eg, PSA) response to agents that effect cell kill. Shown are a substantial and durable decline consistent with marked sensitivity (gray line); a decline followed by a slow rise, indicative of partial sensitivity followed by acquired resistance (gold line); and a transient drop (or none at all) followed by an immediate rise, consistent with either de novo or intrinsic resistance (blue line). PSA, prostate-specific antigen.
Fig A2.
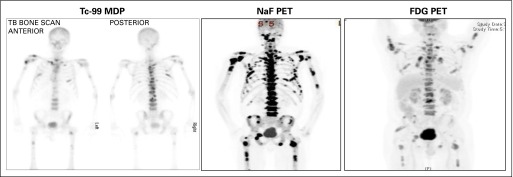
Radionuclide bone scan, sodium fluoride PET scan, and FDG PET scan of the same patient. Only FDG PET is tumor directed; Tc-99 MDP and NaF PET are bone directed. Note that neither the target of the tracer nor the clarity of the image necessarily implies a superior biomarker, and each modality must be validated analytically and clinically. 99mTc MDP, 99mTc methylene diphosphonate; NaF, sodium fluoride; FDG, fluorodeoxyglucose; PET, positron emission tomography.
Fig A3.

Bone scan assessment form.
Footnotes
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations From the Prostate Cancer Clinical Trials Working Group 3
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Howard I. Scher
Consulting or Advisory Role: Astellas Pharma, Bristol-Myers Squibb, Ferring Pharmaceuticals, OncologySTAT, Pfizer, Sanofi, Takeda Pharmaceuticals, WIRB-Copernicus Group, Medivation, BIND Therapeutics, Janssen, Roche/Genentech, Blue Earth, Celgene
Speakers' Bureau: WebMD
Research Funding: BIND Biosciences, Exelixis, Janssen Pharmaceuticals, Medivation, Janssen Diagnostics, Innocrin Pharmaceuticals
Travel, Accommodations, Expenses: Janssen Pharmaceuticals, Sanofi, AstraZeneca, Genentech, Pfizer, Takeda Pharmaceuticals, Ferring Pharmaceuticals, WIRB-Copernicus Group, Astellas Pharma, BIND Therapeutics, Roche, Medivation
Michael J. Morris
Consulting or Advisory Role: Astellas Pharma, Bayer, Millennium, Progenics, Tokai Pharmaceuticals
Research Funding: Bayer, Sanofi, Takeda Pharmaceuticals, Janssen Oncology, Agensys
Travel, Accommodations, Expenses: fujifilm
Walter M. Stadler
Honoraria: Bayer, Caremark, Medpacto, Johnson & Johnson, Merck, Sotio
Consulting or Advisory Role: Bayer, Caremark, Medpacto, Sotio, Johnson & Johnson, Merck
Research Funding: Active Biotech, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Dendreon, Exelixis, Novartis, Genentech/Roche, GlaxoSmithKline, Medivation, Pfizer, Merck, Millennium Pharmaceuticals
Other Relationship: UpToDate
Celestia Higano
Employment: CTI Biopharma
Leadership: CTI Biopharma
Stock or Other Ownership: CTI Biopharma
Consulting or Advisory Role: Dendreon, Bayer, Medivation, Ferring Pharmaceuticals, AbbVie, Genentech, Pfizer, BHR Pharma, Orion Corporation, Sanofi, Emergent BioSolutions, MorphoSys, Churchill Pharmaceuticals, Clovis Oncology
Research Funding: Algeta/Bayer, Aragon Pharmaceuticals, AstraZeneca, Dendreon, Genentech, Medivation, Millennium, Sanofi, Teva, Exelixis, Oncogenex, Emergent BioSolutions
Travel, Accommodations, Expenses: Bayer, Medivation, Dendreon, Ferring Pharmaceuticals, Pfizer, Johnson & Johnson, AbbVie, Genentech, Amgen, Orion Pharma, Ockham, Teva, Sanofi, Astellas Pharma, Emergent BioSolutions, MorphoSys, Churchill Pharmaceuticals, Clovis Oncology
Ethan Basch
No relationship to disclose
Karim Fizazi
Consulting or Advisory Role: Astellas Pharma, Sanofi, Janssen, Amgen, Orion Pharma, Novartis, AstraZeneca, Bayer
Emmanuel S. Antonarakis
Honoraria: Sanofi, Dendreon, Medivation, Janssen Biotech, ESSA
Consulting or Advisory Role: Sanofi, Dendreon, Medivation, Janssen Biotech, ESSA
Research Funding: Janssen Biotech, Johnson & Johnson, Sanofi, Dendreon, Aragon Pharmaceuticals, Exelixis, Millennium Pharmaceuticals, Genentech, Novartis, Tokai Pharmaceuticals
Travel, Accommodations, Expenses: Sanofi, Dendreon, Medivation, Tokai Pharmaceuticals
Tomasz M. Beer
Stock or Other Ownership: Salarius Pharmaceuticals
Consulting or Advisory Role: Astellas Pharma, Bayer, Dendreon, Janssen Japan, Novartis, Sotio
Research Funding: Astellas Pharma, Bristol-Myers Squibb, Dendreon, Janssen Research & Development, Medivation, Oncogenex, Sotio
Michael A. Carducci
Consulting or Advisory Role: Medivation, Astellas Pharma, Blue Earth, AstraZeneca, Clovis Oncology
Kim N. Chi
Honoraria: Sanofi, Janssen Pharmaceuticals, Astellas Pharma
Consulting or Advisory Role: ESSA, Astellas Pharma, Janssen Pharmaceuticals, Sanofi, Lilly/ImClone, Amgen, Bayer, Takeda Pharmaceuticals
Research Funding: Janssen Pharmaceuticals, Astellas Pharma, Amgen, Bayer, Novartis, Sanofi, Exelixis, Tokai Pharmaceuticals, Oncogenex, Teva
Paul G. Corn
Honoraria: Sanofi
Consulting or Advisory Role: Sanofi
Travel, Accommodations, Expenses: Sanofi
Johann S. de Bono
Honoraria: AstraZeneca, Sanofi, Astellas Pharma, Pfizer, Genentech/Roche
Consulting or Advisory Role: AstraZeneca, Sanofi, Genentech/Roche, Astellas Pharma
Research Funding: AstraZeneca, Genentech, Sanofi
Patents, Royalties, Other Intellectual Property: Abiraterone Rewards to Inventors
Travel, Accommodations, Expenses: AstraZeneca, Astellas Pharma, GlaxoSmithKline, Orion Pharma, Sanofi, Genmab, Taiho Pharmaceutical, QIAGEN, Vertex
Robert Dreicer
Consulting or Advisory Role: Medivation, Roche, Merck, Asana Biosciences, Tokai Pharmaceuticals, Churchill Pharma
Research Funding: Progenics
Daniel J. George
Honoraria: Dendreon, Novartis, Sanofi
Consulting or Advisory Role: Bayer, Dendreon, Exelixis, Medivation, Novartis, Pfizer, BIND Biosciences, Sanofi, GlaxoSmithKline, Astellas Pharma, Progenics, Innocrin Pharma, Genentech, Clovis Oncology, Merck, Bristol-Myers Squibb
Speakers' Bureau: Dendreon, Novartis, BiPar Sciences/Sanofi
Research Funding: Exelixis, Genentech/Roche, Janssen Oncology, Novartis, Pfizer, Progenics, Astellas Pharma, Bristol-Myers Squibb, GlaxoSmithKline, Millennium Pharmaceuticals, Innocrin Pharma
Travel, Accommodations, Expenses: Astellas Pharma, Bayer, Dendreon, Pfizer, Sanofi, Progenics, Bristol-Myers Squibb, Merck, BIND, Medivation
Elisabeth I. Heath
Honoraria: Bayer Diagnostics, Dendreon, Sanofi
Research Funding: Tokai Pharmaceuticals, Seattle Genetics, Agensys, Dendreon, Genentech/Roche, Millennium Pharmaceuticals, Celldex, Inovio Biomedical, Celgene
Maha Hussain
Consulting or Advisory Role: Johnson & Johnson, Synthon
Research Funding: Astellas Pharma, Bayer, Genentech, Medivation, Millennium Pharmaceuticals, Pfizer
Patents, Royalties, Other Intellectual Property: Method of Treating Cancer, Serial No. 61/481/671
Wm. Kevin Kelly
Honoraria: Sanofi
Consulting or Advisory Role: Sanofi, Churchill Pharmaceuticals, Johnson & Johnson
Research Funding: Sanofi, Novartis, Bayer HealthCare Pharmaceuticals, Tesaro
Glenn Liu
Stock or Other Ownership: AIQ Services
Research Funding: Johnson & Johnson, Novartis, Millennium Pharmaceuticals, Cellectar, Pfizer
Patents, Royalties, Other Intellectual Property: Patent US 9161720B2 System and Method for Evaluation of Disease Burden
Other Relationship: AIQ Services
Christopher Logothetis
Honoraria: Bayer, AstraZeneca, Pfizer, Helsinn Therapeutics, Bristol-Myers Squibb, Janssen, Sanofi, Novartis, Medivation/Astellas Pharma
Consulting or Advisory Role: Astellas Pharma, Bristol-Myers Squibb, Johnson & Johnson, Pfizer, Novartis, Helsinn Therapeutics, Bayer, AstraZeneca, Sanofi, Exelixis, Churchill Pharmaceuticals, Clovis Oncology
Research Funding: Astellas Pharma, Bristol-Myers Squibb, Johnson & Johnson, Exelixis, Pfizer, Novartis, Karyopharm Therapeutics, Cougar Biotechnology, AstraZeneca, Medivation, Bayer, GlaxoSmithKline, Sanofi
Travel, Accommodations, Expenses: Bayer, AstraZeneca, Bristol-Myers Squibb, Pfizer, Janssen, Astellas Pharma, Novartis, Churchill Pharmaceuticals, Clovis Oncology, Sanofi
David Nanus
No relationship to disclose
Mark N. Stein
Research Funding: Janssen Oncology, Oncoceutics
Dana E. Rathkopf
Consulting or Advisory Role: Janssen Oncology
Research Funding: Janssen Oncology, Medivation, Celgene, Takeda Pharmaceuticals, Millennium Pharmaceuticals, Ferring Pharmaceuticals, Novartis
Susan F. Slovin
No relationship to disclose
Charles J. Ryan
Honoraria: Janssen Oncology, Astellas Pharma
Consulting or Advisory Role: Bayer, Millennium Pharmaceuticals, Astra Zeneca, Medivation
Research Funding: BIND Biosciences, Karyopharm Therapeutics, Novartis
Oliver Sartor
Consulting or Advisory Role: Astellas Pharma, Bavarian Nordic, Bayer, Bellicum Pharmaceuticals, Biscayne Pharmaceuticals, Johnson & Johnson, Medivation, Oncogenex, Sanofi, Teva, Tokai Pharmaceuticals
Research Funding: Bayer, Johnson & Johnson, Progenics, Sanofi, Sotio, Endocyte
Eric J. Small
No relationship to disclose
Matthew Raymond Smith
Consulting or Advisory Role: Bayer, Janssen Oncology, Astellas Pharma, Medivation, Gilead Sciences
Cora N. Sternberg
Consulting or Advisory Role: Astellas Pharma, Bayer, Janssen Pharmaceuticals, Sanofi
Mary-Ellen Taplin
Honoraria: Medivation, Janssen-Ortho, Dendreon, Tokai Pharmaceuticals, Bayer
Consulting or Advisory Role: Medivation, Janssen-Ortho, Tokai Pharmaceuticals, Sanofi, Bayer, Guidepoint Global, Best Doctors, Gerson Lehrman Group, Dendreon, UpToDate
Research Funding: Janssen-Ortho, Medivation, Bayer
Travel, Accommodations, Expenses: Medivation, Janssen Oncology, Tokai Pharmaceuticals, Dendreon
George Wilding
No relationship to disclose
Peter S. Nelson
Honoraria: Janssen Research & Development
Consulting or Advisory Role: Janssen Research & Development, Medivation/Astellas Pharma, AstraZeneca
Lawrence H. Schwartz
Honoraria: Novartis, Celegene
Patents, Royalties, Other Intellectual Property: Varian Medical Systems
Other Relationship: ICON Clinical Research, BioClinica
Susan Halabi
Consulting or Advisory Role: Bayer, Genentech/Roche
Travel, Accommodations, Expenses: Bayer
Philip W. Kantoff
Consulting or Advisory Role: Aragon Pharmaceuticals, Amgen, Bayer, Bavarian Nordic, Celgene, Exelixis, Genomic Health, GTx, Janssen Pharmaceuticals, Millennium Pharmaceuticals, Pfizer, Teva, Astellas Pharma, Auven Therapeutics, Bellicum Pharmaceuticals, BIND Biosciences, Blend Therapeutics, Roche, Metamark Genetics, Medivation, Merck, MTG Therapeutics, OncoCellMDX, Oncogenex, Progenics, Sotio, Sanofi, Tokai Pharmaceuticals
Research Funding: Aragon Pharmaceuticals, Amgen, Astellas Pharma, Bayer, Bavarian Nordic, Dendreon, Exelixis, Janssen Oncology, Medivation, Sanofi, Oncogenex
Travel, Accommodations, Expenses: Sanofi, Janssen Pharmaceuticals, BIND Biosciences, Bavarian Nordic, Millennium Pharmaceuticals, Progenics
Andrew J. Armstrong
Honoraria: Dendreon, Sanofi
Consulting or Advisory Role: Bayer, Sanofi, Novartis, Dendreon, Medivation, Janssen Biotech
Speakers' Bureau: Dendreon, Sanofi
Research Funding: Dendreon, Sanofi, Bayer, Pfizer, Novartis, Bristol-Myers Squibb, KangLaiTe, Janssen Oncology, Medivation, Astellas Pharma, ImClone Systems
Patents, Royalties, Other Intellectual Property: Circulating tumor cell novel capture technology
Travel, Accommodations, Expenses: Dendreon, Medivation, Janssen Biotech
REFERENCES
Articles from Journal of Clinical Oncology are provided here courtesy of American Society of Clinical Oncology
Full text links
Read article at publisher's site: https://doi.org/10.1200/jco.2015.64.2702
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4872347?pdf=render
Citations & impact
Impact metrics
Article citations
A call for objectivity: Radiologists' proposed wishlist for response evaluation in solid tumors (RECIST 1.1).
Cancer Imaging, 24(1):154, 14 Nov 2024
Cited by: 0 articles | PMID: 39543673 | PMCID: PMC11566494
Review Free full text in Europe PMC
Matched-pair analysis of mCRPC patients receiving <sup>177</sup>Lu-labeled PSMA-targeted radioligand therapy in a 4-week versus 6-week treatment interval.
EJNMMI Res, 14(1):94, 14 Oct 2024
Cited by: 0 articles | PMID: 39402311 | PMCID: PMC11473467
Prognostic Role of PSMA-Targeted Imaging in Metastatic Castration-Resistant Prostate Cancer: An Overview.
Biomedicines, 12(10):2355, 16 Oct 2024
Cited by: 0 articles | PMID: 39457667 | PMCID: PMC11504290
Review Free full text in Europe PMC
Assessment of the therapeutic efficacy of [<sup>177</sup>Lu]Lu-PSMA-X compared to taxane chemotherapy in taxane-chemo-naïve patients with metastatic castration-resistant prostate cancer: A systematic review and meta-analysis.
Eur J Nucl Med Mol Imaging, 25 Oct 2024
Cited by: 0 articles | PMID: 39453485
Treatment response assessment in mCRPC: is PSMA-PET/CT going to take the lead?
Ther Adv Med Oncol, 16:17588359241258367, 07 Oct 2024
Cited by: 0 articles | PMID: 39386313 | PMCID: PMC11462558
Review Free full text in Europe PMC
Go to all (675) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials (3)
- (1 citation) ClinicalTrials.gov - NCT00887198
- (1 citation) ClinicalTrials.gov - NCT01193244
- (1 citation) ClinicalTrials.gov - NCT01212991
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Androgen Receptor Splice Variant 7 and Efficacy of Taxane Chemotherapy in Patients With Metastatic Castration-Resistant Prostate Cancer.
JAMA Oncol, 1(5):582-591, 01 Aug 2015
Cited by: 359 articles | PMID: 26181238 | PMCID: PMC4537351
Managing Nonmetastatic Castration-resistant Prostate Cancer.
Eur Urol, 75(2):285-293, 14 Aug 2018
Cited by: 60 articles | PMID: 30119985
Review
AR-V7 in Peripheral Whole Blood of Patients with Castration-resistant Prostate Cancer: Association with Treatment-specific Outcome Under Abiraterone and Enzalutamide.
Eur Urol, 72(5):828-834, 14 Aug 2017
Cited by: 52 articles | PMID: 28818355
The Evolving Biology of Castration-Resistant Prostate Cancer: Review of Recommendations From the Prostate Cancer Clinical Trials Working Group 3.
Oncology (Williston Park), 30(2):187-95, 199, 01 Feb 2016
Cited by: 6 articles | PMID: 26888794
Review
Funding
Funders who supported this work.
NCI NIH HHS (2)
Grant ID: P30 CA008748
Grant ID: P50 CA092629