Abstract
Background
Systemic inflammation is observed in horses with heaves and could also be present in horses with a lesser degree of pulmonary inflammation.Hypothesis/objectives
It was hypothesized that racehorses with inflammatory airway disease (IAD) have increased concentration of circulating acute phase proteins. The objective of this study was to compare serum acute phase proteins of racehorses with and without lower airway inflammation.Animals
Serum from 21 client-owned Standardbred racehorses with exercise intolerance and lower airway inflammation and serum from 10 client-owned Standardbred racehorses with exercise intolerance without lower airway inflammation.Methods
In a case-control study, serum samples from previously characterized horses presented for exercise intolerance with or without lower airway inflammation based on bronchoalveolar lavage fluid cytology were analyzed for serum amyloid A protein (SAA), C-reactive protein (CRP), and haptoglobin using commercial ELISAs.Results
There was no significant differences between groups for SAA (non-IAD versus IAD, median (range): 3.47 (0.06-34.94) versus 6.33 (0.06-80) μg/mL, P = .49), CRP (10.87 (2.05-29.03) versus 4.63 (0.02-31.81) μg/mL, P = .23) or haptoglobin (900.36 (607.99-2018.84) versus 749.54 (530.81-1076.95) μg/mL, P = .09).Conclusions and clinical importance
In this population of poorly performing racehorses in training, serum SAA, CRP, and haptoglobin were not helpful in distinguishing between horses with IAD from horses with exercise intolerance from other causes.Free full text

Acute Phase Proteins in Racehorses with Inflammatory Airway Disease
Abstract
Background
Systemic inflammation is observed in horses with heaves and could also be present in horses with a lesser degree of pulmonary inflammation.
Hypothesis/Objectives
It was hypothesized that racehorses with inflammatory airway disease (IAD) have increased concentration of circulating acute phase proteins. The objective of this study was to compare serum acute phase proteins of racehorses with and without lower airway inflammation.
Animals
Serum from 21 client‐owned Standardbred racehorses with exercise intolerance and lower airway inflammation and serum from 10 client‐owned Standardbred racehorses with exercise intolerance without lower airway inflammation.
Methods
In a case–control study, serum samples from previously characterized horses presented for exercise intolerance with or without lower airway inflammation based on bronchoalveolar lavage fluid cytology were analyzed for serum amyloid A protein (SAA), C‐reactive protein (CRP), and haptoglobin using commercial ELISAs.
Results
There was no significant differences between groups for SAA (non‐IAD versus IAD, median (range): 3.47 (0.06–34.94) versus 6.33 (0.06–80) μg/mL, P = .49), CRP (10.87 (2.05–29.03) versus 4.63 (0.02–31.81) μg/mL, P = .23) or haptoglobin (900.36 (607.99–2018.84) versus 749.54 (530.81–1076.95) μg/mL, P = .09).
Conclusions and Clinical Importance
In this population of poorly performing racehorses in training, serum SAA, CRP, and haptoglobin were not helpful in distinguishing between horses with IAD from horses with exercise intolerance from other causes.
Abbreviations
- AST
- aspartate aminotransferase
- CK
- creatine kinase
- CI
- confidence interval
- CRP
- c‐reactive protein
- ELISA
- enzyme‐linked immunosorbent assay
- IAD
- inflammatory airway disease
- SAA
- serum amyloid A protein
Acute phase proteins are involved in the host response to inflammatory stimuli by modulating leukocyte function and migration, activating the complement cascade, preventing the loss of iron by the formation of stable complexes with free hemoglobin, and by having anti‐inflammatory properties in some cases.2, 3 They are produced mainly by hepatocytes following stimulation by inflammatory mediators including interleukin 1β, interleukin 6, and tumor necrosis factor.2 Their rise is most dramatic following infectious processes or severe trauma but they are also present in noninfectious inflammatory diseases such as arthritis, allergy, and surgery.4 While the main source is the liver, some acute phase proteins, including serum amyloid A protein (SAA), C‐reactive protein (CRP), and haptoglobin, are also expressed in the lungs.5, 6, 7 In horses, SAA, CRP, haptoglobin, α1‐acid glycoprotein, and fibrinogen are the main acute phase proteins that have been studied. Among those, only SAA is considered a “major” acute phase protein, with a very rapid (24–48 hours) and pronounced (10 fold or more) increase with inflammation, while haptoglobin and CRP tend to rise and decrease more slowly and to a lesser magnitude.3 SAA and haptoglobin were also found to be elevated in horses with heaves, a condition characterized by severe airway inflammation and airflow limitation.8 In contrast, horses with inflammatory airway disease (IAD) have only mild to moderate pulmonary function impairment (ie, they do not display respiratory difficulty at rest) expressed clinically by poor performance, exercise intolerance, or cough.9
The aim of the present study was to measure three acute phase proteins in serum of poorly performing racehorses previously characterized as having IAD or not, to identify blood markers that could help discriminate horses with and without lower airway inflammation. The hypothesis was that horses with IAD have higher serum concentrations of SAA, CRP, and haptoglobin compared to horses without evidence of pulmonary inflammation.
Materials and Methods
This case–control study used serum stored at −80°C from horses recruited in a previous study on the relationship between bronchoalveolar cells and cytokine gene expression.1 Serum from thirty‐one client‐owned Standardbred racehorses were included in the study, 29 of them were part of Lavoie et al (2011) (some horses were excluded in one or the other study because serum or cells were not available). Briefly, and as described previously, horses presented for decreased performance and the clinical investigation included a physical examination, thoracic auscultation, lameness examination, complete blood count (available for 27 horses), plasma aspartate aminotransferase (AST) and creatine kinase (CK), upper airway endoscopy, and bronchoalveolar lavage. Trainers were requested to train at race speed the day before presentation and to refrain from administering medication 2 weeks prior to presentation. There were 13 mares, 12 geldings, and 6 males. Age ranged from 2 to 10 years in the IAD group (median: 4) and from 2 to 9 years (median: 3) in the non‐IAD group. Horses were considered as having IAD if, in addition to exercise intolerance, they had increased neutrophils (>5%), eosinophils (≥1%), or mast cells (≥2%), in their bronchoalveolar lavage (Exercise intolerant IAD group) while horses without lower airway inflammation formed the Exercise intolerant non‐IAD group. Horses with lameness or a suspected cardiac condition were excluded from the study but in both groups more than one potential cause of poor performance was identified (exercise‐induced pulmonary hemorrhage, suspected dorsal displacement of the soft palate, mildly elevated muscle enzymes). Experimental procedures were performed in accordance with the Canadian Council of Animal Care and approved by the Université de Montréal Animal Care Committee.
Quantification of Serum Acute Phase Proteins
Serum amyloid A, CRP, and haptoglobin were quantified using commercial enzyme‐linked immunosorbent assay (ELISA) kits according to the manufacturer's instructions and as previously described.8 Serum amyloid A was quantified using an assay1 previously validated in horses.10 C‐reactive protein and haptoglobin were quantified using CRP and haptoglobin ELISAs for equine biological samples.2, 3 Plates were washed using an automatic plate washer4 and absorbance was obtained using a microplate scanning spectrophotometer plate reader.5 Measuring ranges were 0.625–40 μg/mL for SAA, 2–320 μg/mL for CRP, and 100–3200 μg/mL for haptoglobin. Samples with saturated optical density values were attributed twice the highest standard concentration. An arbitrary value corresponding to one‐tenth of the lowest standard concentration was attributed to samples with undetectable acute phase proteins.
Statistical Analysis
Data for SAA, CRP, and haptoglobin were not normally distributed (as assessed by D'Agostino and Pearson omnibus test and Shapiro–Wilk normality test), nor were log transformed or square root transformed data. Differences between groups were therefore analyzed with Mann–Whitney and Kruskal–Wallis tests using a commercial software.6 Correlation between SAA, CRP, haptoglobin, bronchoalveolar lavage cell percentages, muscle enzymes, and age were explored with Spearman correlation test, with the same ranks attributed to data outside the quantification range. Fisher's exact test was used to compare proportions of horses with abnormally high acute phase proteins when possible. Differences were considered significant when P values were <.05.
Results
There were no significant differences between groups (Exercise intolerant IAD versus Exercise intolerant non‐IAD), sex or age for any of the 3 proteins analyzed (Figs 1A, A,2A,2A, A,3A3A and and4),4), nor was there an association between the type of inflammation found in bronchoalveolar lavage and acute phase proteins (Figs 1B and and3B),3B), except for CRP concentrations in horses with and without increased neutrophils in their bronchoalveolar lavage fluid (P = .047) (Fig 2B). More specifically, median (range) for Exercise intolerant non‐IAD versus Exercise intolerant IAD were 3.47 (0.06–34.94) versus 6.33 (0.06–80) μg/mL (P = .49) for SAA, 10.87 (2.05–29.03) versus 4.63 (0.02–31.81) μg/mL (P = .23) for CRP and 900.36 (607.99–2018.84) versus 749.54 (530.81–1076.95) μg/mL (P = .09) for haptoglobin. Mean ± SD were 6.52 ± 10.18 versus 15.66 ± 23.50 μg/mL with a difference between the means of 9.14 (95% confidence interval (CI): −3.24 to 21.52) for SAA, 12.50 ± 9.63 versus 9.23 ± 10.99 μg/mL with a difference between the means of 3.27 (95% CI: −4.66 to 11.19) for CRP, and 968.49 ± 394.08 versus 766.32 ± 152.34 μg/mL with a difference between the means of 202.17 (95% CI: −61.59 to 465.93). Neutrophils and other cells percentages in the IAD and the non‐IAD groups are summarized in Table 1. Seven horses had SAA levels considered above normal values (> 20 μg/mL3), 6 of which were in the IAD group (no significant association, P > .2, Fisher exact test). Two horses with IAD had concentration above the detection limit and 5 horses had undetectable levels of SAA, 4 of them in the IAD group. Four horses had undetectable level of CRP, all of them in the IAD group. There was no correlation between concentrations of SAA, CRP, and haptoglobin, or between these parameters and peripheral leukocytes, neutrophils, fibrinogen, or bronchoalveolar lavage cytology (P values >.05). Muscle enzymes CK and AST were not correlated with acute phase proteins. There was a weak positive correlation between SAA and age (r = .36; P = .047).
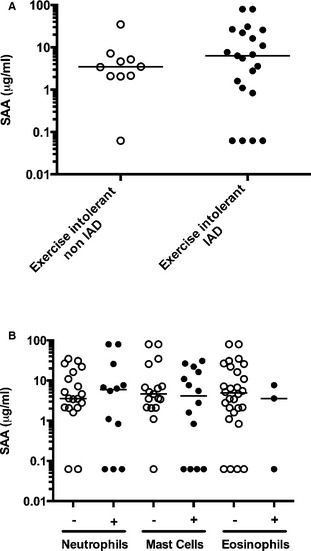
(A) Serum amyloid A protein (SAA) in horses with inflammatory airway disease (IAD, closed circles, n = 21) and exercise intolerance from other cause (open circles, n = 10). (B) SAA in bronchoalveolar cytology with (closed circles) or without (open circles) elevated neutrophils, mast cells, and eosinophils (all horses). One bronchoalveolar lavage fluid can be classified in more than one category. Horizontal bar represents the median.
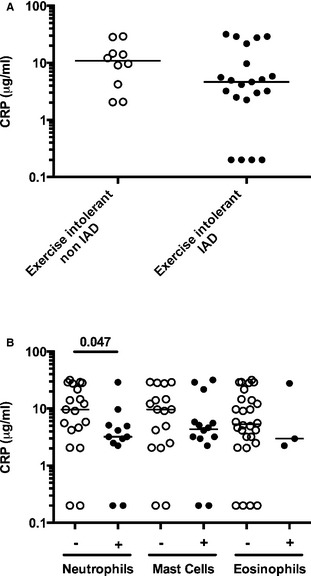
(A) C‐reactive protein (CRP) in horses with inflammatory airway disease (IAD, closed circles, n = 21) and exercise intolerance from other cause (open circles, n = 10). (B) CRP in bronchoalveolar cytology with (closed circles) or without (open circles) elevated neutrophils, mast cells, and eosinophils (all horses). One bronchoalveolar lavage fluid can be classified in more than one category. Horizontal bar represents the median.
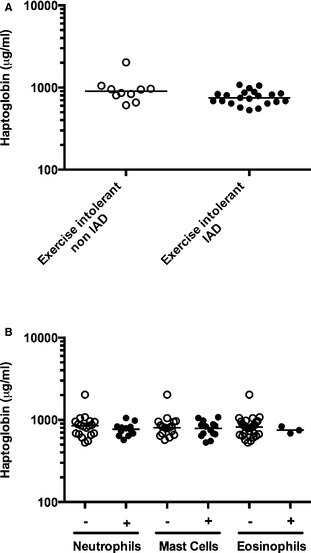
(A) Haptoglobin in horses with inflammatory airway disease (IAD, closed circles, n = 21) and exercise intolerance from other cause (open circles, n = 10). (B) Haptoglobin in bronchoalveolar cytology with (closed circles) or without (open circles) elevated neutrophils, mast cells, and eosinophils (all horses). One bronchoalveolar lavage fluid can be classified in more than one category. Horizontal bar represents the median.
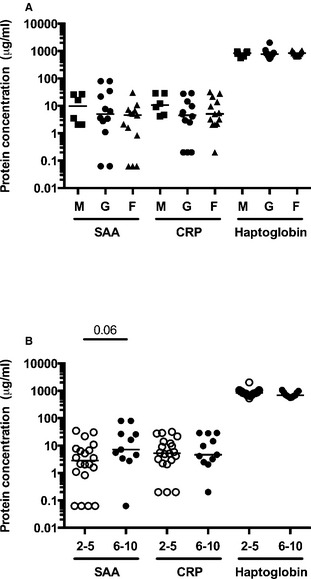
Protein concentrations of serum amyloid A protein (SAA), C‐reactive protein (CRP), and haptoglobin by sex (A) and age group (in years) (B) (all horses). M: male, G: gelding, F: mare. Horizontal bar represents the median.
Table 1
Bronchoalveolar lavages differential cell count.
| Group | Neutrophils % Median: Range (Horses >5%) | Mast Cells % Median: Range (Horses ≥2%) | Eosinophils % Median: Range (Horses ≥1%) |
|---|---|---|---|
| Non‐IAD | 3:1–5 (0) | 1: 0–1 (0) | 0:0–0 (0) |
| IAD | 7:1–19 (12) | 2: 0–3 (14) | 0:0–5 (3) |
Non‐IAD: Exercise intolerant horses without inflammatory airway disease (n = 10). IAD: Exercise intolerant horses with inflammatory airway disease (n = 21). Neutrophils %: percentage of neutrophils. Horses >5%: number of horses with percentage of neutrophils above 5%. Same for other cell types.
Only one horse with IAD (of 27 with complete blood count available) had peripheral leukocytes and neutrophils above the reference range (leukocytes: 13 × 109/L (5.5–12.5 × 109/L), neutrophils 9 × 109/L (2.7–6.7 × 109/L)). This horse had low concentrations of SAA, CRP, and haptoglobin. All horses had normal fibrinogen concentration (≤4 g/L, n = 27). There was no difference in age and proportion of mares between Exercise intolerant IAD and Exercise intolerant non‐IAD horses (Fig 4).
Discussion
The main finding of this study was that in this population, SAA, CRP, and haptoglobin concentrations were not detectably different between exercise intolerant horses with and without IAD, or between horses with different types of cell population in their bronchoalveolar lavage, except for slightly lower concentration of CRP in horses with airway neutrophilia. It is therefore unlikely that these 3 acute phase proteins would be useful as a diagnostic marker of lower airway inflammation in horses presented for exercise intolerance.
Acute phase proteins can increase in blood with noninfectious and infectious respiratory conditions. High SAA concentration has been documented in horses with influenza virus, Streptococcus zooepidemicus, and Rhodococcus equi infections,11, 12, 13 and CRP is elevated in horses with pneumonia.14 Among noninfectious conditions, both SAA and haptoglobin are increased in horses with heaves compared to controls kept in the same environment.8 The lack of differences between IAD and non‐IAD horses studied here could be because of the lesser degree of inflammation in IAD compared to heaves,9 or because of a relatively high baseline level of inflammation in this population of poor performers. The higher levels and frequency of detection of SAA in this study compared to levels and frequency previously reported in heaves supports the latter hypothesis (90 and 81% of non‐IAD and IAD horses had detectable SAA in this population, while only in 17% of controls and 67% of heaves‐affected horses had detectable SAA levels8). Nevertheless, the lack of difference in SAA, CRP, and haptoglobin between horses with and without lower airway inflammation in the present study is in agreement with, and further extends, the previous findings that SAA and fibrinogen concentrations are not different between poorly performing coughing and noncoughing thoroughbred racehorses in training.15 Training alone should not result in high concentrations of acute phase proteins but they might increase in a population of poor performers maintained in training. Specifically, SAA do not increase with acute strenuous exercise alone16 or following training of experienced horses.17 However, it increases following prolonged strenuous endurance exercise and in inexperienced endurance horses.17, 18 Similarly, haptoglobin and fibrinogen increased only at the end of a 2.5‐month training program, suggesting that fatigue or subclinical disease developed over time.19 This suggests that training in adverse conditions could lead to an increase in acute phase protein concentrations and could account for the apparent high baseline SAA in our population of horses. Interestingly, haptoglobin was found to decrease 2 and 7 days following acute strenuous exercise in Standardbred racehorses.16 Since trainers were asked to train horses at race pace before presentation, it could have created an unexpected confounding factor for haptoglobin in our population. Other potential confounding factors that could have modified acute phase protein blood levels are the administration of anti‐inflammatory drugs and the presence of exercise‐induced pulmonary hemorrhage. Even if trainers were requested to refrain from administering medication 2 weeks prior to presentation, horses were not tested for anti‐inflammatory drugs (or other medication) before entering the study. Evidence of exercise‐induced pulmonary hemorrhage was very common in this population of racehorses in training, independently of their group (8/10 and 19/21 horses in the non‐IAD and IAD group, respectively). The lack of difference between groups, whether it is because the pulmonary inflammation causes little systemic inflammation or because our non‐IAD horses were also exercise intolerant would be interesting to explore but nevertheless, in a clinical setting where one would try to differentiate IAD from non‐IAD in poorly performing horses, the acute phase proteins studied here are not promising. More specific markers of lung inflammation such as serum surfactant protein D might be more interesting in that context.20
There was a small positive correlation between age and SAA concentration in this population and a trend for a difference between horses of 2–5 years of age and 6–10 years of age. Variation of SAA with age in healthy human adults is not considered clinically relevant,21 but in one study, horses over 8 years of age had higher level of SAA than horses of other age groups (except for neonates).22 The lower CRP concentrations in horses with bronchoalveolar lavage neutrophilia (Fig 2B), when compared to other horses from the study was unexpected, especially since neutrophilic asthma is associated with systemic inflammation and increased CRP in humans.23 The difference observed here was small and of unknown biological significance. Finally, from the data available on record, it was not possible to identify the origin of increased acute phase protein in outlier individuals, not even in the one horse who had a 2.2–3.1 times increase compared to control's mean in all three acute phase proteins measured. Of note, all horses had normal fibrinogen concentrations and this could support the use of other acute phase proteins in a population of horses with undetected inflammatory conditions without overt signs of infections, and in which no other cause of counter performance can be identified.
One potential pitfall of this study is the small number of samples available, which could have prevented the identification of a significant difference between groups (type II error). A posteriori power calculation shows that with this sample size and the variation observed, a 50% change would have been unlikely to be detected for SAA and CRP (effective power: 8% and 31%) and likely to be detected for haptoglobin (effective power: 99%). Therefore, further studies with larger sample sizes could find differences of this magnitude between groups. While such potential findings could be helpful in understanding the pathophysiology of IAD, it remains that the large overlap observed between groups make these proteins unlikely diagnostic tools on an individual case level.
In conclusion, in this population of horses, there were no difference in the acute phase proteins measured between horses with inflammatory airway disease and other horses with exercise intolerance. Serum amyloid A, CRP, and haptoglobin are unlikely to be useful markers of IAD in that context.
Acknowledgments
The authors acknowledge Guy Beauchamp for statistical review.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Notes
The work presented here was performed at the Université de Montréal and supported by the Natural Sciences and Engineering Research Council of Canada (grant # 415448) and by an unrestricted grant from the Pfizer Clinical Research Fund.
The majority (29 of 31) of the samples analyzed in his study came from horses that were enrolled in a previous report on the relationship between bronchoalveolar lavage cytology and cytokine gene expression.1
Footnotes
1SAA Tridelta Phase™ ELISA, Tridelta Development Limited, Maynooth, Kildare, Ireland.
2Horse CRP ELISA, K‐Assay®, Kamiya Biomedical Company, Seattle, WA.
3Horse Haptoglobin ELISA, K‐Assay®, Kamiya Biomedical Company, Seattle, WA.
4ELX50 Autostrip washer, Thomas Scientific, Swedesboro, NJ.
5PowerWave™ X340, Biotek instruments, Inc, Winooski, VT.
6Prism 6.0, GraphPad Software, Inc, La Jolla, CA.
References
Articles from Journal of Veterinary Internal Medicine are provided here courtesy of Wiley
Full text links
Read article at publisher's site: https://doi.org/10.1111/jvim.12587
Read article for free, from open access legal sources, via Unpaywall:
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/jvim.12587
Citations & impact
Impact metrics
Citations of article over time
Article citations
Clinical findings and outcome predictors for multinodular pulmonary fibrosis in horses: 46 cases (2009-2019).
J Vet Intern Med, 38(3):1842-1857, 15 Apr 2024
Cited by: 1 article | PMID: 38619130 | PMCID: PMC11099712
Changes in Acute Phase Response Biomarkers in Racing Endurance Horses.
Animals (Basel), 12(21):2993, 31 Oct 2022
Cited by: 3 articles | PMID: 36359117 | PMCID: PMC9657625
Acute phase proteins levels in horses, after a single carbohydrate overload, associated with cecal alkalinization.
Front Vet Sci, 10:1043656, 02 Feb 2023
Cited by: 1 article | PMID: 36816195 | PMCID: PMC9932335
Neutrophil Extracellular Traps Are Found in Bronchoalveolar Lavage Fluids of Horses With Severe Asthma and Correlate With Asthma Severity.
Front Immunol, 13:921077, 13 Jul 2022
Cited by: 8 articles | PMID: 35911691 | PMCID: PMC9326094
Comparison of the proteomes in sera between healthy Thoroughbreds and Thoroughbreds with respiratory disease associated with transport using mass spectrometry-based proteomics.
J Equine Sci, 32(1):11-15, 16 Mar 2021
Cited by: 0 articles | PMID: 33776535 | PMCID: PMC7984915
Go to all (13) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Serum concentration of surfactant protein D in horses with lower airway inflammation.
Equine Vet J, 44(3):277-281, 23 Jun 2011
Cited by: 9 articles | PMID: 21696440
Serum Surfactant Protein D and Haptoglobin as Potential Biomarkers for Inflammatory Airway Disease in Horses.
J Vet Intern Med, 29(6):1707-1711, 19 Aug 2015
Cited by: 9 articles | PMID: 26289543 | PMCID: PMC4895656
Bronchoalveolar lavage fluid cytology and cytokine messenger ribonucleic Acid expression of racehorses with exercise intolerance and lower airway inflammation.
J Vet Intern Med, 25(2):322-329, 31 Jan 2011
Cited by: 31 articles | PMID: 21281348
Pulmonary Remodeling in Equine Asthma: What Do We Know about Mediators of Inflammation in the Horse?
Mediators Inflamm, 2016:5693205, 07 Dec 2016
Cited by: 8 articles | PMID: 28053371 | PMCID: PMC5174180
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Natural Sciences and Engineering Research Council of Canada (1)
Grant ID: 415448

 1
1



