Abstract
Free full text

Matrix control of pancreatic cancer: new insights into fibronectin signaling
Abstract
Pancreatic ductal adenocarcinoma (PDA) is a highly metastatic disease that resists most current therapies. A defining characteristic of PDA is an intense fibrotic response that promotes tumor cell invasion and chemoresistance. Efforts to understand the complex relationship between the tumor and its extracellular network and to therapeutically perturb tumor-stroma interactions are ongoing. Fibronectin (FN), a provisional matrix protein abundant in PDA stroma but not normal tissues, supports metastatic spread and chemoresistance of this deadly disease. FN also supports angiogenesis, which is required for even hypovascular tumors such as PDA to develop and progress. Targeting components of the tumor stroma, such as FN, can effectively reduce tumor growth and spread while also enhancing delivery of chemotherapy. Here, we review the molecular mechanisms by which FN drives angiogenesis, metastasis and chemoresistance in PDA. In light of these new findings, we also discuss therapeutic strategies to inhibit FN signaling.
Introduction
Pancreatic ductal adenocarcinoma (PDA) is a lethal cancer with a 5-year survival rate of 7% and a median survival of ~6 months, making it the 4th leading cause of cancer-related deaths in the United States [1] .However, a recent and alarming report has projected that PDA will become the 2nd leading cause of cancer-related deaths in the United States by 2030 [2]. The poor prognosis of PDA can be attributed to challenges in early detection, resistance to chemotherapy and frequency of metastasis.
A prominent characteristic of PDA that contributes to these deadly features is a dense stroma that can occupy more than half of the tumor mass. The stroma of PDA is composed of various extracellular matrix (ECM) proteins such as collagen and fibronectin (FN), which provide structural integrity and initiate signaling cascades that promote tumor cell survival, proliferation, and migration. A major cellular source of ECM components in PDA is a type of fibroblast, the pancreatic stellate cell (PSC). PSCs are scarce in the normal pancreas where they are quiescent until activated by injury, inflammation or cancer [3]. In an active state, PSCs exhibit enhanced ECM production and can be identified by expression of α-smooth muscle actin (SMA). Using SMA as a marker, studies have shown that increased PSC activation was associated with shorter overall survival in PDA patients [4–6], supporting the concept that the ECM is an active and vital component of the tumor microenvironment.
FN is a major ECM constituent that binds to adhesion receptors on multiple cell types. FN supports cell-ECM interactions and is essential for wound healing, development, and maintaining tissue homeostasis [7–11]. The expression of FN is elevated in many solid tumors, particularly PDA [12, 13]. Various cell types including cancer cells and endothelial cells can produce FN, although it is primarily secreted by fibroblasts [9]. The surge of infiltrating PSCs in PDA and subsequent release of FN promotes tumor progression, and thus FN has garnered substantial attention as a potential therapeutic target. Here, we review the effects of FN signaling in PDA on metastasis and chemoresistance, and the molecular mechanisms that regulate FN signaling. We also discuss the function of FN in tumor angiogenesis and strategies to target FN-integrin interaction.
FN signaling in PDA
Cell surface integrins are the most well characterized receptors for FN. Integrins are heterodimers composed of α and β subunits that mediate cell-ECM interaction. Activation of integrins initiates many important and dynamic cell processes such as migration, adhesion, proliferation, and survival [14]. For example, α5β1 and αvβ3 integrins are critical during angiogenesis as they bind FN, which provides proliferative and migratory cues to endothelial cells [15–17]. Furthermore, α1β1 and α2β1 integrins (VLA-1 and VLA-2, respectively), receptors for collagen I, participate in inflammation by mediating leukocyte attachment and extravasation [18, 19]. While FN can bind to various β1 and β3 integrins, α5β1 integrin is the primary receptor for FN [20]. Through integrin binding, FN regulates cell survival, proliferation, adhesion, migration, and differentiation in a variety of cell types [14].
Integrins participate in ‘inside-out’ and ‘outside-in’ signaling, events that are ligand-directed. This unique bi-directional signaling can stimulate changes in integrin conformation and activity to initiate events on the opposite membrane face. This is a spatially and temporally regulated process that involves an array of multi-protein complexes and has been reviewed extensively elsewhere [21, 22]. For instance, talin promotes integrin activation and cytosolic signaling. Talin binds to the cytoplasmic tail of β integrins and provides a mechanical link between integrins and the actin cytoskeleton. This link strengthens ECM-integrin binding by inducing an open high affinity conformation on the extracellular side of the receptor [23]. The ECM provides many integrin-binding sites and thus can propagate integrin clustering into large macromolecule structures known as focal adhesions [21]. Focal adhesions provide mechanical force and transmit signals between the inside and outside of the cell. Integrin tails lack enzymatic activity and thus signaling to downstream kinases is induced by the formation of focal adhesions.
In addition to talin, proteins such as focal adhesion kinase (FAK), Src, vinculin, and paxillin are recruited to the cytoplasmic tail of β1 integrin and promote integrin signaling [24, 25]. FAK was one of the first phosphorylation targets discovered downstream of integrin activation [26]. FAK functions as a central signaling scaffold that is critical for the activation of downstream signaling pathways, namely the Rho-family GTPases, which support cytoskeletal changes and promote cell movement [27–30]. FAK interacts directly with paxillin, another important signaling scaffold that supports the binding of kinases such as Src [31–34]. FAK is also a key player in growth factor receptor crosstalk with integrins [35]. Furthermore, the duration and magnitude of integrin signaling is regulated by factors that bind to and inactivate the integrin such as filamin, ICAP1, DOK1, and SHARPIN [36]. The interaction of these proteins with the cytoplasmic tail of integrins prevents the binding of important integrin activators, such as talin [36]. Filamin, ICAP1, and DOK1 bind to β-integrin tails, with ICAP1 exclusively binding to and inhibiting β1 integrins [37]. SHARPIN engages α-integrin tails as well as β1 integrins and blocks integrin activity by perturbing talin interaction with β-integrin tails [38].
Two distinct sites on FN bind to its major receptor, integrin α5β1. Interaction at each site, the RGD domain and a synergy site located about 32 Å from the RGD site, is required for high affinity binding and optimal stimulation of the integrin [39]. FN signaling through α5β1 in PDA induces pro-tumorigenic effects in most studies, affecting PSCs, tumor cells, and endothelial cells. The remainder of this section will focus specifically on the various mechanisms by which FN signaling can promote cell survival, chemoresistance, and cell invasion and metastasis in PDA.
Cell survival and chemoresistance
The dense ECM characteristic of PDA contributes to the high level of resistance to current chemotherapies. The desmoplasia present in most PDA is thought to function as a physical barrier for drug delivery [40]. Tumors with a prominent ECM network are more resistant to the penetration of pharmacologic molecules [41]. Although many epithelial-derived cancers display increased desmoplasia, PDA exhibits a particularly strong stromal reaction. This is thought to be due to PSCs that actively secrete ECM components such as collagen and FN [42]. Targeting the stromal compartment is a promising strategy to increase chemosensitivty. For example, it has been shown that the hedgehog (Hh) signaling pathway contributes to desmoplasia via paracrine signaling from tumor cells to stromal cells [43]. Activation of the Hh pathway can induce FN and collagen expression [44]. Consistent with this, inhibition of the Hh pathway using the small molecular inhibitor IPI-926 decreased the overall stromal content in a genetically engineered mouse model (GEMM) of PDA and increased blood flow, which augmented delivery of gemcitabine by 60% [45]. Unfortunately, these tumors ultimately adapt to hedgehog inhibition, therefore alternative strategies to target the stromal response in PDA are critically needed. For example, inhibition of the hormone angiotensin by losartan was shown to decrease ECM production and consequently increase vascular perfusion [46]. Collagen and hyaluronan, another abundant ECM molecule and prognostic marker of poor outcome in many solid tumors [47], exert pressure on blood vessels resulting in vessel constriction and impaired perfusion [46]. Losartan reduced protein levels of collagen, hyaluronan, and SMA resulting in enhanced drug and oxygen delivery to the tumor [46]. Losartan also reduces TGF-β levels, and given that TGF-β can trigger secretion of ECM proteins, this may be an alternative mechanism by which ECM production is abated by losartan [48, 49]. Another group also showed that high interstitial fluid pressure, due to a dense stroma, causes vascular collapse resulting in poor perfusion of PDA [50]. Targeting the stroma in mouse PDA by degradation of hyaluronan resulted in a decrease in interstitial fluid pressure, widening of blood vessels, and ultimately increased delivery of chemotherapy to the tumor [50, 51]. This strategy has advanced to clinical testing in PDA patients using systemic administration of pegylated hyaluronidase (PEGPH20) [52].
In addition to the physiological barrier that the ECM imposes, it also induces activation of intracellular signaling pathways important for growth and survival of cancer cells. Ligation of α5β1 by FN has been widely shown to promote survival and proliferation in a variety of cancer cell types. For example, earlier studies done in the human PDA cell line Mia Paca-2 showed that cells plated onto FN-coated dishes displayed an RGD-dependent increase in interleukin-8 (IL-8A), which is a chemokine important for PDA progression and metastasis [53]. In addition to Mia Paca-2, the human pancreatic cancer cell lines Panc-1 and Capan-1 showed increased resistance to cytotoxic agents including gemcitabine, cisplatin and doxorubicin when seeded on FN-coated dishes [54]. The pro-survival effect that FN has on cancer cells is primarily mediated by FAK dependent activation of the PI3K/AKT/mTOR pathway [55, 56]. Activation of this pathway results in upregulation of Bcl-2 through inhibition of Bad, ultimately blocking cytochrome c release from mitochondria, which results in reduced apoptosis [57, 58]. Furthermore, FN-mediated activation of FAK also triggers cell proliferation through the recruitment of SH2-binding proteins such as Src and Grb2, which directly activate the Ras pathway [59].
FN (and to a lesser extent collagen and laminin) also promotes pancreatic cancer cell survival through a modest increase in reactive oxygen species (ROS). ROS are important signaling molecules that, when present in moderate amounts, can stimulate proliferation and promote survival through the Ras-Raf-MEK-ERK and NF-κB pathways respectively [60]. The authors of this study found that Mia PaCa-2, Panc-1, and Capan-1 cells cultured on FN stimulated NADPH-oxidase and 5-lipoxygenase-dependent ROS production [61]. Consequently, these cells showed increased survival, which was reversed when ROS production was inhibited by treatment with antioxidants [61]. Therefore, FN-induced ROS production can be a critical pro-survival mechanism of pancreatic cancer cells.
Stromal cells found within the tumor microenvironment can promote PDA tumorigenicity and chemoresistance by enabling ECM-cancer cell interaction [62, 63]. For example, subcutaneous co-injection of pancreatic cancer cells and PSCs resulted in larger tumors compared to those mice that were injected with cancer cells only [64, 65]. These co-injected tumors showed an increased fibrotic response and increased cancer cell proliferation. Similarly, an orthotopic mouse model of PDA showed enhanced cancer cell proliferation, reduced apoptosis, and increased metastatic burden when PSCs were co-injected [66]. While the authors did not analyze FN expression in these tumors it is plausible that FN produced by PSCs promoted tumor progression. Further studies using PSC engineered to lack expression of FN and subsequently co-injected with tumor cells could be used to demonstrate the function of FN as a pro-tumorigenic element in this setting. Moreover, while these mouse studies highlight the importance of PSCs in tumor progression, they do not provide direct information on the effect of PSC presence on chemoresponse, thus it will be important to monitor the effect of anti-cancer agents in this context. However, PSCs contribute to chemoresistance in vitro, for example, a co-culture model consisting of PSCs and PDA cells revealed increased resistance to the anti-cancer drug, etoposide, compared to PDA cells cultured alone [67]. Conditioned media from PSCs also reduced sensitivity to etoposide in PDA cells. Taken together, these studies emphasize the importance of stromal cells and their subsequent ECM production in regulating tumor behavior and chemoresponse, and highlight FN as a promoter of cell survival and drug resistance in PDA.
Cell invasion and metastasis
FN promotes metastasis in many cancers, including PDA [68, 69]. Metastasis occurs when cancer cells have acquired invasive and migratory characteristics. FN is important in cell adhesion and spreading where it promotes FAK-dependent activation of Rho, leading to actin stress fiber formation and enhanced migration [70, 71]. Moreover, FN-integrin interaction has been shown to stimulate p21-activated kinase (Pak), a downstream target of Rac/Cdc42 and promoter of cell motility [72].
Epithelial cancer cells can often undergo epithelial-to-mesenchymal transition (EMT), which converts polarized epithelial cells into mobile and invasive cells, a requisite for some forms of metastasis to occur. It is well established that FN contributes to EMT, in fact, TGF-β (the major driver of EMT), stimulates FN expression and deposition into the ECM, leading to enhanced cell motility [73]. In turn, FN promotes assembly and deposition of latent TGF-β binding protein (LTBP), which is a protein that facilitates latent TGF-β secretion and activation [74]. Inhibition of the major transcription factors of the TGF-β pathway, such as Snail and Smad, results in a reduction of FN expression and subsequent decreased motility [75, 76]. Moreover, Smad-independent pathways of TGF-β induced FN expression have also been identified. For example, it has been shown that TGF-β-induced activation of the c-Jun terminal kinase (JNK) pathway can trigger FN expression in human fibrosarcoma cells [77].
It was recently shown in breast cancer cells that FN induces EMT in the absence of serum and TGF-β [78]. This study also showed that neutralizing TGF-βR1 while cells were plated on FN in the absence of serum still showed an increase in Smad2 activation compared to cells plated on matrigel. These findings suggest that stromal FN is capable of inducing an EMT response in settings with low or no TGF-β activity; however, the response is amplified in the presence of TGF-β. Therefore, TGF-β and FN may cooperate to induce EMT in breast cancer cells. Given that TGF-β signaling and FN expression are prominent in PDA [79], a similar cooperation may operate in regard to EMT induction in this tumor; however such findings have yet to be confirmed.
Recently, FN was discovered as a novel transcriptional target downstream of Pak1, which is upregulated in human PDA compared to normal pancreatic tissue [80]. In this study, FN mRNA and protein levels were significantly increased in various human pancreatic cancer cell lines expressing Pak1. Conversely, FN was downregulated in pancreatic cancer cells where Pak1 was stably knocked down. Further investigation showed that Pak1 induced FN expression via the NF-kB–p65 pathway. The consequence of this increased FN expression was enhanced invasion of human pancreatic cancer cells. Another recent and striking example of FN promoting invasion of pancreatic tumor cells was revealed using circulating tumor cells (CTCs) isolated from mouse PDA. CTCs showed enhanced anchorage-independent cell growth driven by non-canonical Wnt2 signaling. Overexpression of Wnt2 in these cells resulted in a significant induction of FN whereas knockdown of FN in these cells blocked the effects of Wnt2 on anchorage-independent growth [81]. These examples highlight that FN can promote invasion and support that FN signaling participates in metastasis.
Another recent study using mouse models of PDA to investigate liver metastasis accentuates the importance of FN during this process. Costa-Silva and colleagues [82] showed that mouse PDA-derived exosomes (cell-derived vesicles that carry molecular cargo) elicit a fibrotic response in the liver, referred to as the “pre-metastatic niche”, a site that supports engraftment of incoming metastatic cells. It is becoming increasingly apparent that exosomes mediate communication between tumor cells and their environment [83]. The authors of this study saw a significant upregulation of FN in the pre-metastatic niche but not other ECM proteins such as collagen type I or vitronectin. This exosome-mediated fibrotic response was followed by enhanced recruitment of macrophages to the pre-metastatic niche. The recruitment of macrophages and other immune cells is typically considered a pro-tumorigenic response in PDA [84]. To determine whether FN is required for macrophage infiltration in this context the authors used a conditional FN knockout mouse model (Rosa– CreER+;Fnfl/fl). This model revealed a reduction in macrophage recruitment and a reduced fibrotic response in the exosome-mediated liver pre-metastatic niche [82]. These results suggest that FN deposition in the pre-metastatic niche provides a suitable environment for liver metastases. Furthermore, it has been shown that macrophages and other immune cells secrete factors to surrounding PSCs to stimulate FN expression, thereby further enhancing the desmoplastic response and immune cell recruitment and ultimately the metastatic potential of the disease [85]. Altogether, the evidence points towards FN as being a critical factor for cell invasion and as a therapeutic target to prevent metastatic spread in PDA.
Angiogenesis
Angiogenesis, the formation of new blood vessels from pre-existing vessels, is a requisite for all solid tumors to persist [86]. In addition to important growth factors such as vascular endothelial growth factor (VEGF), adhesion to the ECM via integrins is essential for angiogenesis. The use of knockout mice revealed that integrins β1, αv, α4, and α5 are required for vasculogenesis and angiogenesis [87]. However, each of these specific integrins can influence angiogenesis in different ways. For example, deletion of integrin αv in mice is embryonic lethal; however, a considerable amount of development occurs in the absence of this integrin with some animals surviving to birth but ultimately dying due to intracerebral and intestinal hemorrhages [88]. Moreover, integrin α5 knockout mice die at embryonic day E10.5 due to severe angiogenic defects [89]. Similarly, deletion of FN results in angiogenic abnormalities and death at day E9.5 [8].
The requirement for α5β1 and FN in developmental angiogenesis is unequivocal; however, the contribution of these proteins in supporting tumor angiogenesis is still under active investigation. Early studies done by the Varner lab showed that antibody antagonists of the FN-α5β1 interaction blocked angiogenesis in the chick chorioallantoic membrane (CAM) assay [15]. Furthermore, α5β1 targeting antibodies, such as volociximab, inhibit tumor growth in various animal models of cancer [90–92]. Phase I clinical trials showed that volociximab was well tolerated by patients [93] and entered Phase II clinical trials for the treatment of metastatic renal cell carcinoma, melanoma, and PDA [94–97]. Volociximab has shown some preliminary efficacy, particularly when paired with standard chemotherapy, but overall has produced modest results. A study using volociximab in combination with chemotherapy for the treatment of non-small cell lung cancer observed a partial response in 8 out of 33 patients [98]. A more recently developed anti-α5β1 integrin monoclonal antibody, PF-04605412, showed promising anti-tumor effects in pre-clinical models [99]. In addition to neutralization of the integrin, PF-04605412 was engineered to exert its effects through antibody-dependent cellular cytotoxicity (ADCC) [99]. ADCC is a process by which immune effector cells target and lyse cells whose surface antigens have been recognized by specific antibodies [100]. PF-04605412 entered into a clinical trial for advanced solid tumors; however, due to dose related toxicities causing cytokine-mediated infusion reactions to occur, this drug has been discontinued [101].
Despite its preclinical promise, targeting the α5β1-FN interaction has not been as effective in patients, meriting further investigation to identify an explanation for its failure and improve efficacy. A new study by Murphy and colleagues [102] used genetic means to determine whether α5 and αv integrins and FN are required for pancreatic tumor angiogenesis in mice. First, the authors used a Cre-inducible system to simultaneously delete α5 and αv specifically in the endothelium of mice (Cdh5-CreERT2). These mice were crossed into the background of a RIP1-Tag2 model of pancreatic neuroendocrine cancer, which is induced by insulin-promoter-driven SV40 expression in beta cells produced by pancreatic islets [103]. Surprisingly, deletion of α5 and αv in the endothelium in RIP1-Tag2 mice did not reduce tumor angiogenesis. Moreover, to confirm that other integrins capable of binding to FN were not simply compensating for the loss of α5 and αv integrins, FN was deleted specifically in the endothelium and crossed into the RIP1-Tag2 model. Similarly, the loss of endothelial FN did not reduce tumor angiogenesis in this model as measured by the number of tumor angiogenic islets. Furthermore, post-natal global deletion of FN in the RIP1-Tag2 model modestly reduced the number of initial angiogenic islets; however, it did not reduce final tumor mass. Additionally, there was no difference in the CD31-stained tumor area between tumors with or without FN. The absence of FN did not suppress vascular basement membrane deposition or recruitment of fibrillins 1 and 2 [102].
This work is in direct contrast to the current idea that impeding FN-integrin interaction by inhibitors is the mechanism by which tumor angiogenesis is reduced in pre-clinical models. The authors provide a possible explanation, which is that other integrin-binding ECM proteins are able to compensate and thus support tumor angiogenesis given the aberrant expression of many ECM proteins in the tumor microenvironment compared to the developing embryo. This possibility remains to be investigated. Furthermore, one must consider the model used and whether this result is specific to the RIP1-Tag2 model or if a GEMM of PDA driven by the activation of pancreatic acinar cells would produce different results.
Others have also recently challenged the original dogma of anti-angiogenic therapy with data to support pro-angiogenic therapy as a means of enhancing therapeutic delivery in PDA [104]. The authors explored the dual effect of modestly stimulating angiogenesis while increasing overall blood flow on chemotherapeutic delivery. They used Cilengitide, an αvβ3/αvβ5 RGD-mimetic that at low levels can actually stimulate angiogenesis [105], in combination with Verapamil, a small molecule that can enhance vessel dilation and blood flow. Results showed that this therapeutic strategy increased tumor vascular density, blood flow, blood volume, and by doing so, enhanced chemotherapeutic delivery of gemcitabine in an orthotopic and GEMM of PDA, the KPC model (LSL-KrasG12D/+;Trp53R172H/+;Pdx1-Cre). Furthermore, their study also revealed an associated decrease in tumor hypoxia, desmoplasia, and metastasis. Increased hypoxia is seen in tumors as a result of anti-angiogenic therapy. Hypoxia has been linked to increased desmoplasia [106, 107] and is able to induce EMT, leading to increased metastatic spread [108]. Another potential feature of vascular promotion therapy is that it may lower the effective dose of certain chemotherapies thus lowering side effects. This study uncovers a new area of research that warrants further investigation into the efficacy of vascular promotion therapy in other tumor types and chemotherapies.
Taken together, these data highlight the need for a better understanding of the mechanism behind targeting angiogenesis in pre-clinical models and the possibility of a compensatory mechanism by other ECM proteins for supporting tumor angiogenesis in the absence of FN.
Regulation of FN signaling in PDA
Because FN is expressed abundantly in PDA, it is important to understand how FN signaling is regulated in PDA. This section will detail how FN binding to its receptor is regulated and how this affects downstream integrin signaling.
There are multiple extracellular molecules that can reduce FN signaling by directly binding to FN or through competition for its major integrin receptors. A class of proteins found within the ECM referred to as “matricellular proteins” mediate ECM-receptor interactions without serving a direct structural function within the ECM. These proteins are expressed highly during development and are often re-activated under pathological conditions such as cancer [109]. In 2007, Lomas et al. reported that a matricellular protein, fibulin-5, directly competes with FN for integrin binding in cell-based assays [110]. The authors showed that in the presence of higher or equal concentrations of recombinant fibulin-5 to FN, cells were more rounded and exhibited decreased migration and proliferation. Furthermore, evidence shows that integrin signaling can influence receptor tyrosine kinase activation and signaling [111]. To this extent, Lomas and colleagues found that cells plated on fibulin-5 failed to induce PDGFR and EGFR signaling compared to cells stimulated with FN. These results could be reversed by treatment with an activating β1 integrin antibody, showing that fibulin-5 competes with FN to negatively regulate β1 integrin function.
Using GEMMs of PDA, our lab has shown that expression of fibulin-5 is elevated in the stroma of PDA compared to normal pancreas [112]. Introducing a point mutation in the integrin-binding RGD sequence of fibulin-5 (RGD → RGE) blocks integrin binding while maintaining other functions of fibulin-5 [113, 114]. PDA GEMMs harboring this point mutation showed decreased tumor growth and increased survival. Further investigation revealed that RGE tumors had elevated levels of oxidative stress as a result of increased FN-induced integrin signaling. Elevated levels of oxidative stress had a negative effect on tumor angiogenesis in RGE tumors as evidenced by a decrease in microvessel density. Treatment with the antioxidant N-acetyl-cysteine (NAC) restored tumor growth and microvessel density in the RGE tumors, confirming that the initial decrease in tumor growth and microvessel density seen was a direct consequence of elevated ROS levels. Moreover, NAC treatment decreased survival in RGE tumor bearing mice compared to control untreated RGE mice [112]. Given the strong pro-tumorigenic effect of FN signaling in PDA as highlighted in the previous section, it seems counterintuitive that elevated FN signaling would result in smaller tumors. However, in the context of dysfunctional fibulin-5 in PDA, these tumors were subjected to chronic high levels of ROS resulting in decreased microvessel density and increased tumor cell apoptosis. These results highlight that an important function of fibulin-5 is to control integrin-induced ROS production by FN during angiogenesis. In summary, fibulin-5 is a negative regulator of FN signaling through passive ligation of FN-binding integrins. These results provide a more nuanced view that the effect of FN on tumorigenesis is context-dependent.
Expression of another matricellular protein, tenascin-C (TNC), is also elevated in the stroma of PDA. TNC binds to various ECM proteins and integrin receptors [115] and co-localizes with FN in tissue [116]. TNC reduces FN-integrin interaction, similar to fibulin-5. Early studies showed that fibroblasts and cancer cells display reduced attachment to fibronectin in the presence of TNC [116, 117]. In pancreatic tumor cells phosphorylation of downstream integrin targets, such as paxillin (early event) and Akt (later event), were increased in the presence of FN but decreased in the presence of TNC and FN. Similar findings were also seen in glioblastoma and breast cancer cells [116, 118]. This suggests that in the setting of abundant FN and TNC (as is in PDA), TNC functions as an endogenous inhibitor of FN signaling.
Lastly, a recent study identified that the cytokine endothelial monocyte activating polypeptide II (EMAP II) suppresses PDA progression by impeding FN-integrin interactions [119]. EMAP II binds directly to α5β1 integrin and significantly decreased proliferation, angiogenesis, and overall tumor growth of subcutaneous xenograft models of PDA. Interestingly, EMAP II also reduced host-derived as well as cancer cell-derived FN expression in these tumor models. Furthermore, in vitro assays revealed that FN-induced proliferation of human pancreatic cancer cell lines was decreased by addition of EMAP II. In summary, understanding the molecules that can regulate FN-integrin interactions opens new avenues to explore when considering innovative ways to block FN signaling.
Conclusions
This review provides evidence that the extracellular environment provides critical cues during PDA progression and maintenance. The effects of FN on PDA progression and the factors that regulate it are summarized in Figure 1. FN is an extracellular protein that is upregulated in pancreatic cancer and contributes significantly to chemoresistance and metastasis. FN induces distinct signals that promote survival and migration of PDA cells. Furthermore, mounting evidence supports the concept that FN binding to α5β1 is crucial in physiological angiogenesis as well as tumor angiogenesis. However, some pre-clinical models have put into question the absolute need of FN and its cognate receptors in supporting tumor angiogenesis, which may partially explain the disappointing clinical results of therapeutically targeting the FN-integrin interaction. Nonetheless, these findings should encourage deeper investigation into the mechanism underlying FN-targeting drugs.
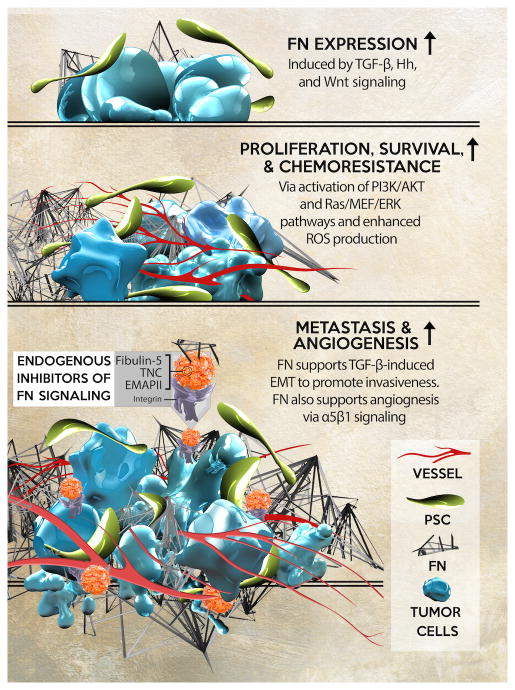
(Top) TGF-β, Hedgehog (Hh), Wnt, and NF-κB pathways increase FN expression and secretion from tumor and stromal cells. (Middle) FN activates pro- survival and proliferative pathways including AKT and the Ras-Ref-MEK-ERK pathway via activation of FAK. FN also induces ROS production via integrin activation leading to enhanced tumor cell proliferation. These factors contribute to desmoplasia and chemoresistance. (Bottom) FN supports TGF-β induced EMT and promotes migration and invasiveness and also supports angiogenesis via α5β1 signaling. Endogenous factors that block FN-integrin interaction include Fibulin-5, TNC, and EMAP II.
These studies have shed light on the biology of PDA and mechanisms of drug resistance in PDA; however, further efforts should be directed towards the clinical application of this knowledge. The current standard of care for patients with PDA provides only a slight improvement in mortality rates, with patients ultimately succumbing to the disease within a few months. This dismal prognosis has remained static for over a decade, underscoring the urgent need for novel targeted and combination therapies. FN is clearly a prominent factor in maintaining a rich ECM environment that is favorable for tumor and tumor-associated cells to migrate and thrive. These stroma-rich tumors increase fluid pressures leading to poor perfusion and ultimately low uptake of a given therapy. Therefore, targeting the stroma, be it through FN inhibition or an alternative mechanism has the potential to enhance drug delivery and accumulation into these characteristically dense tumors. Given that abrogating FN-integrin interactions have produced strikingly positive pre-clinical data, further investigative efforts should be pursued to refine this strategy for clinical translation.
Acknowledgments
This work was supported in part by grants from the American Cancer Society (ACS, RSG-10-244-01-CSM to R.A.B.), The Joe and Jessie Crump Medical Research Foundation (to R.A.B.), NIH (T32 GM008203 to M. T.), the Effie Marie Cain Scholarship in Angiogenesis Research (to R.A.B.). We gratefully acknowledge Drs. Michael Dellinger and Lance Terada for their helpful comments on the manuscript and Joel Hodge for preparation of the illustration (contact: moc.liamg@ngisedegdohleoj).
Abbreviations
| FN | Fibronectin |
| ECM | extracellular matrix |
| PSCs | pancreatic stellate cells |
| PDA | pancreatic ductal adenocarcinoma |
| SMA | α-smooth muscle actin |
| GEMM | genetically engineered mouse model |
| FAK | focal adhesion kinase |
| Hh | hedgehog |
| IL-8A | interleukin-8 |
| ROS | reactive oxygen species |
| EMT | epithelial-to-mesenchymal transition |
| LTBP | TGF-β binding protein |
| JNK | c-Jun terminal kinase |
| Pak | p21-activated kinase |
| CTCs | circulating tumor cells |
| NAC | N-acetyl-cysteine TNC, tenascin-C |
| EMAP II | endothelial monocyte activating polypeptide II |
| VEGF | vascular endothelial growth factor |
| CAM | chick chorioallantoic membrane |
| ADCC | antibody-dependent cellular cytotoxicity |
Footnotes
Disclosure of Potential Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.canlet.2015.12.027
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4927402?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
The Role of the Pancreatic Extracellular Matrix as a Tissue Engineering Support for the Bioartificial Pancreas.
Biomimetics (Basel), 9(10):598, 02 Oct 2024
Cited by: 0 articles | PMID: 39451804 | PMCID: PMC11505355
Review Free full text in Europe PMC
MR Molecular Image Guided Treatment of Pancreatic Cancer with Targeted ECO/miR-200c Nanoparticles in Immunocompetent Mouse Tumor Models.
Pharm Res, 41(9):1811-1825, 28 Aug 2024
Cited by: 0 articles | PMID: 39198318 | PMCID: PMC11436418
CTHRC1+ fibroblasts and SPP1+ macrophages synergistically contribute to pro-tumorigenic tumor microenvironment in pancreatic ductal adenocarcinoma.
Sci Rep, 14(1):17412, 29 Jul 2024
Cited by: 0 articles | PMID: 39075108 | PMCID: PMC11286765
Enhancing Neoadjuvant Virotherapy's Effectiveness by Targeting Stroma to Improve Resectability in Pancreatic Cancer.
Biomedicines, 12(7):1596, 18 Jul 2024
Cited by: 0 articles | PMID: 39062169 | PMCID: PMC11275208
Review Free full text in Europe PMC
PAK in Pancreatic Cancer-Associated Vasculature: Implications for Therapeutic Response.
Cells, 12(23):2692, 23 Nov 2023
Cited by: 0 articles | PMID: 38067120 | PMCID: PMC10705971
Review Free full text in Europe PMC
Go to all (67) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Cancer-associated fibroblasts in pancreatic adenocarcinoma.
Future Oncol, 11(18):2603-2610, 18 Aug 2015
Cited by: 28 articles | PMID: 26284509
Review
Reshaping the Tumor Stroma for Treatment of Pancreatic Cancer.
Gastroenterology, 154(4):820-838, 26 Dec 2017
Cited by: 112 articles | PMID: 29287624
Review
Hypoxia and Transforming Growth Factor β Cooperate to Induce Fibulin-5 Expression in Pancreatic Cancer.
J Biol Chem, 291(42):22244-22252, 16 Aug 2016
Cited by: 30 articles | PMID: 27531748 | PMCID: PMC5064003
Microenvironmental factors and extracellular matrix degradation in pancreatic cancer.
JOP, 15(4):280-285, 28 Jul 2014
Cited by: 6 articles | PMID: 25076320
Review
Funding
Funders who supported this work.
American Cancer Society (1)
Grant ID: RSG-10-244-01-CSM
Effie Marie Cain Scholarship in Angiogenesis Research
Joe and Jessie Crump Medical Research Foundation
NIGMS NIH HHS (1)
Grant ID: T32 GM008203
NIH (1)
Grant ID: T32 GM008203





