Abstract
Free full text

Recent Advances in Subunit Vaccine Carriers
Abstract
The lower immunogenicity of synthetic subunit antigens, compared to live attenuated vaccines, is being addressed with improved vaccine carriers. Recent reports indicate that the physio-chemical properties of these carriers can be altered to achieve optimal antigen presentation, endosomal escape, particle bio-distribution, and cellular trafficking. The carriers can be modified with various antigens and ligands for dendritic cells targeting. They can also be modified with adjuvants, either covalently or entrapped in the matrix, to improve cellular and humoral immune responses against the antigen. As a result, these multi-functional carrier systems are being explored for use in active immunotherapy against cancer and infectious diseases. Advancing technology, improved analytical methods, and use of computational methodology have also contributed to the development of subunit vaccine carriers. This review details recent breakthroughs in the design of nano-particulate vaccine carriers, including liposomes, polymeric nanoparticles, and inorganic nanoparticles.
1. Introduction
The introduction of vaccines to prevent infectious disease has had a transformational effect on human health. For example, the declaration of the global eradication of wild poliovirus type 2 was one of the highlights of the Global Commission for the Certification of Poliomyelitis Eradication (GCC) meeting in September 2015. Further, vaccination against measles, a highly contagious disease caused by morbillivirus in the paramyxovirus family has resulted in a 79% decrease in measles deaths globally from 2000 to 2014. Another example was the introduction of a meningitis A conjugate vaccine, which expedited the near elimination of the deadly disease in the African “meningitis belt”. These stunning accomplishments emphasize the importance and need for vaccines that profoundly contribute to well-being of society.
A vaccine can be generally defined as a biological preparation that contributes to active immunity against a particular disease. The pioneering work by Edward Jenner for the smallpox vaccination, followed by Louis Pasteur for an anthrax vaccine, lead to development of vaccines against many other infectious diseases. The World Health Organization (WHO) has reported vaccines for the prevention and control of 27 infections, with many other vaccines in the discovery pipeline. A candidate vaccine against malaria (RTS,S) recently completed phase III clinical trials involving approximately 15,000 infants and young children in seven sub-Saharan African countries. Two Ebola candidate vaccines are also in the final stages of clinical trials. The research community is also making sustained efforts toward the development of vaccines against diseases which are difficult to treat, such as cancer [1,2], hepatitis C, and tuberculosis [3,4,5].
2. Types of Vaccines
Vaccination involves exposure of an antigen, derived from a disease-causing agent, to the immune system with the aim to develop active immunity against the antigen. When the vaccinated individual comes in contact with the causative micro-organism, a strong protective immune response occurs. The optimal properties of any vaccine include long-lasting immunity, lack of autoimmunity or hypersensitivity, ease of administration, and storage. Additionally, vaccine components should be safe, and, specifically, the vaccine itself should not result in the disease state.
The different types of vaccines (Table 1) include live attenuated vaccines, which consist of live, weakened, or modified disease causing micro-organisms, which results in a limited infection that is sufficient to invoke immune response, but not long-lived enough to cause the actual disease state [6,7]. The attenuation is achieved by repeated culturing of disease causing agent in a foreign host. The less virulent mutant adapted to the foreign host can be used for vaccination. Killed/Inactivated vaccines work with help of different chemical methods, radiation, or heat. The pathogen is inactivated so it cannot replicate in the host and is used as the vaccinating agent [6,7]. Bacterial vaccines have generally used dead micro-organisms, while viral vaccines are composed of inactivated agents. Recombinant/DNA are experimental vaccines composed of genes encoding antigen inserted into a vector (recombinant vaccine) [6,7]. The DNA can be injected or attached to a carrier, such as metallic nanoparticles (DNA vaccine). In situ generation of antigenic protein can lead to strong immune response. Subunit vaccines contain a purified antigen instead of using whole microorganisms. Purified antigens could be toxoid, subcellular fragment, or surface molecules, which are transported by different carriers [6,7]. Immune response to subunit vaccine differs based on the antigen used. Protein antigens usually give rise to T-cell dependent adaptive immune response while polysaccharide antigens generate T-cell independent response. Conjugated vaccines can be defined as subclass of subunit vaccines as protein carriers are used to carry polysaccharide based antigen.
Table 1
Advantages and disadvantages of different vaccine types.
| Vaccine Type | Advantages | Disadvantages |
|---|---|---|
| Live attenuated | A single dose of this type of vaccine is more potent as infectious agent can replicate in host. | May cause disease itself. |
| Multiple doses may not be required. | Since vaccine is composed of live organism, storage is very critical. | |
| Since micro-organism itself is used, immune response against all antigens is generated. | Cannot be given to immunosuppressed individuals. | |
| Killed/Inactivated | Safe to use in immunosuppressed patients. | Less immunogenic than live attenuated vaccines. |
| Can’t cause disease state. | May require more booster doses to achieve desired immunity. | |
| Storage conditions are not critical compare to live attenuated vaccines. | ||
| Recombinant/DNA | Better stability compare to traditional vaccines. | High production cost compare to other vaccine types. |
| Storage conditions not critical. | Mutation in host DNA is possible in case of DNA vaccines. | |
| Better control on vaccine design as desired gene can be added or deleted. | ||
| Subunit | Safe to use in immunosuppressed patients. | Less immunogenic than live attenuated vaccines. |
| Cannot cause disease state. | Particular antigen or antigens should be identified causing the disease. | |
| Because of the purified antigenic component, less chances of side-effects. | ||
| Conjugated | Safe to use in immunosuppressed patients. | Conjugation chemistry is difficult to control which could cause batch-wise variation. |
| Cannot cause disease state. | Choice of carrier protein is crucial as they could be immunogenic causing suppression of antigenic immune response. | |
| Because of the purified antigenic component, less chances of side-effects. |
3. Immunology of Vaccines
Immunology is a science that studies the structure and function of the immune system, which itself is sub-classified into innate and adaptive immune systems. The innate immune system is non-specific and quickly forms the body’s first line of defense. Phagocytes, soluble peptides, and proteins are complement molecules, while natural killer (NK) cells are key elements of innate immune system. On the other hand, the adaptive immune system gives a pathogen or antigen a specific response along with immunological memory. The adaptive response takes days to weeks to develop. T lymphocytes and B lymphocytes are essential for the adaptive immune response.
Dendritic cells (DCs) play important roles in both innate as well as adaptive immunity. These antigen presenting cells (APCs) are the messengers that call for help from the adaptive immune response when an infection outruns innate immunity [8]. The immature dendritic cells function as phagocytes in innate immunity, participating in capture, uptake, and processing of antigen. While moving to secondary lymphoid tissue, these cells gain capacity to activate naïve T cells (CD4+ and CD8+). These APCs stop phagocytosis and present antigen on their surfaces with a high density during this migration. Such cells in lymph nodes are called mature or activated dendritic cells.
Activation of naïve T cells requires binding antigen to specific T cell receptors (TCR), along with a co-stimulatory signal. The receptor responsible for the co-stimulatory signal is called CD-28 and presents on T cells that bind to the B7 ligand on dendritic cells. Proliferation and differentiation of naïve T cells into effector cells is initiated by these intracellular signals. CD8+ T cells or CD4+ T cells form based on antigen presentation by either MHC-class I or MHC-class II molecules, respectively, on dendritic cells. In most cases, extracellular antigens are presented by MHC-class II on APCs, which in turns activate CD4+ T cells, also known as T helper cells. On the other hand, intracellular antigens presented by MHC-class I molecules on APCs lead to the activation of CD8+ T cells, also known as cytotoxic T cells.
Although the above-mentioned distinction is not absolute, the phenomenon of presentation of antigen located in the MHC-class II pathway to MHC-class I pathway is called “cross presentation”. Such presentation helps to develop both CD4+, as well as CD8+ response, against antigens. The understanding of cross-presentation is important to the success of producing an effective immune response to a vaccine [9]. There is a major focus on increasing cross-presentation of antigen in DCs targeting vaccines.
In the case of subunit vaccine preparation, polysaccharide antigens are poorly immunogenic. Therefore, immuno-adjuvants are added along with antigen in the vaccine carrier to augment the immune response. Another approach is to conjugate bacterial proteins to these antigens, as mentioned earlier. Immuno-adjuvants, especially Toll-like receptor (TLR) ligands are increasingly used in vaccine design [10,11]. Signals from TLRs on dendritic cells improve the processing and presentation by APCs. They also play a role in maturation of dendritic cells.
4. Minimal Subunit Vaccine Development
The classical approach of conjugating carbohydrate antigens to a protein, such as bovine serum albumin (BSA) and keyhole limpet hemocyanin (KLH), is reviewed elsewhere [12,13]. The problems associated with these conjugated vaccines include: Variation in antigen loading, immunogenic linkers, and antigenic carrier proteins lead to the development of minimal subunit vaccines. Research on these vaccines is happening in three areas: Minimal-peptide antigens, improved immune-adjuvant and the use of novel vaccine carriers.
Single molecule two-component vaccines with monomeric, trimeric tumor-associated carbohydrate antigens (TACAs), along with TLR ligands were developed [14,15,16,17,18]. To facilitate antibody class switching from IgM to IgG, a helper T-cell epitope was incorporated into a two-component vaccine to obtain a fully synthetic multi-component vaccine, including phospholipid-based liposomes [19,20], which serves as the carrier. Several polysaccharide antigens specific to cancer [21,22,23,24,25], HIV [26], and other infections [25,27], have been identified and incorporated into this type of vaccine. Investigating new immuno-adjuvants with good safety profiles is another way to potentially advance this platform. Adjuvants can enhancing antigen presentation through dendritic cell (DC) maturation, produce a depot effect, or induce APCs to release cytokines [28,29,30]. Different types of adjuvants, such as bacterial, gel-type, emulsifier, and synthetic, are known [31].
In recent years, advancement of vaccines has focused on one major area; i.e., vaccine carriers [32,33,34,35]. Liposomes, archeosomes, virosomes, virus-like particles (VLPs), polymeric, and inorganic micro-, nano-particles have all been developed. Some of these particulate carriers show adjuvant properties as well. Herein, we present recent case studies of advancements in these vaccine carriers with their physio-chemical attributes.
5. Liposomes
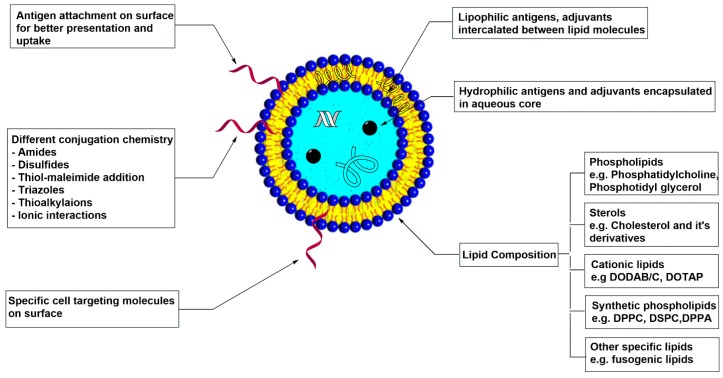
Liposomes are biocompatible, completely biodegradable, self-assembled vesicular structures composed of lipid bilayers. Liposomes have been extensively used as drug delivery vehicles for years, but, in last decade, their use as a vaccine carrier has increased (Figure 1). Considering their wide applications, liposomes have been extensively reviewed by many groups [36,37,38,39,40]. These reviews cover methods of preparation, physio-chemical properties, such as particle size, charge, lamellarity, and their effects on drug or vaccine delivery and different conjugation chemistry.
Liposomes are advantageous over other vaccine carriers due to their tolerability by the human body and lack of toxicity, along with their chemical and structural flexibility. Chemical flexibility refers to the ability of the liposome to encapsulate either a hydrophilic antigen or adjuvant or a lipophilic component, which can intercalate between the lipid molecules [41]. Surface conjugation of hydrophilic antigens is also possible, which facilitates a better uptake by phagocytes because of better accessibility of antigen [42,43,44,45,46]. Structural flexibility refers to the tuning of liposomal properties by modifying lipid composition. For example, the use of cationic lipids has increased, as cationic liposomes are known to improve cytosolic release of antigens by affecting endosomal membrane integrity [47,48,49,50].
There are different methods of preparation of liposomes, such as physical dispersion, solvent dispersion, and detergent solubilization, which are reviewed elsewhere [51,52,53,54]. The general procedure for liposomal formulation involves dissolution of lipid content in an organic solvent. The organic solvent is then evaporated to obtain a thin film of lipid. After drying, the film is hydrated with an aqueous system containing hydrophilic antigen and adjuvants. The resulting vesicular structures are then subjected to freeze-thaw cycles, sonication, or membrane extrusion to ensure entrapment efficiency, size, and lamellarity according to application. In the case of hydrophobic components, they are dissolved in organic solvents along with lipids.
5.1. Physiochemical Properties
Recent advances in physiochemical properties, namely particle size, fusogenicity, and lipid compositions of liposomal vaccine carriers, are discussed briefly.
Particle size: This important property of a vaccine vehicle governs cellular trafficking to secondary lymph nodes, antigen uptake, and cellular responses. Monolova and co-workers studied the kinetics of trafficking of small versus large Virus Like Particles (VLPs) [55]. They observed free drainage of small particles (20–200 nm) towards lymph nodes (LN), while large particles (500, 1000 nm) are dependent on dendritic cells (DCs) for transport to LN. Several experiments have been performed to evaluate the effect of liposomal size on TH1 and TH2 responses [56].
Fusogenicity: Ability to fuse with the plasma membrane or endosomal membrane, i.e., fusogenicity has been widely exploited. Greater cytoplasmic delivery of extracellular antigen elicits a higher cellular immune response. Miyabe and co-workers recently discovered a new class of adjuvant, cyclic di-GMP, which induces the production of type I interferons that can enhance immuno-stimulatory activity [57]. They also reported the use of the pH-sensitive and highly fusogenic synthetic lipid, YSK05. During the comparative study of cationic liposomes, commercially-available transfection reagent and their optimized lipid composition with YSK05, they observed higher INF-β (pg/ml) production with the YSK05 fusogenic lipid. Increased expression of CD80 and CD86, as well as higher CTL activity, suggests the importance of fusogenicity.
Lipid composition: The effect of selection of lipids on liposomal properties, such as particle size, stability, and ability of inducing maturation of DCs, is a prerequisite in order to achieve an optimized vaccine carrier. The effect of lipid composition on membrane fluidity has been studied using gel-liquid transition temperature and membrane phase behavior experiments [58,59,60,61,62]. The selection of a lipid determines membrane fluidity, which is linked to cellular trafficking and antigen presentation to APCs. In addition, the depot effect of an antigen at the site of injection is based on the choice of lipid. This effect has been studied by Christensen, et al. [63]. The effect of degree of lipid saturation on TH1-directed immune response was observed with rigid, saturated dimethyl dioctadecyl ammonium (DDA) lipid and fluid, unsaturated dimethyl dioleoyl ammonium (DODA) lipid. More than 100 times the priming ability and an elevated level of co-stimulatory molecules was observed with rigid lipids, which was correlated to higher retention capacity of lipids. A DoE (design of experiments) approach was used by Soema, et al. to study effect of different lipids such as Egg phosphatidylcholine (EPC), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), 1, 2-dioleoyl-3-trimethylammonium propane (DOTAP) and 3beta-[N-(N′,N′-dimethylaminoethane) carbamoyl] cholesterol (DC-Chol) on DC maturation [64]. They observed that the liposomes containing DOTAP lipids were able to induce DC maturation and had a higher zeta potential value, which dictates the colloidal stability of the formulation.
5.2. Liposome-Polymer Hybrid Vaccine Carrier
Recently, the use of cationic liposomes has increased owing to their higher internalization by macrophages and dendritic cells compared to neutral, as well as anionic liposomes. Electrostatic interaction with the negatively-charged plasma membrane and the ability to disrupt endosomal membrane are beneficial for higher immune-stimulatory response [65].
The major challenge of cytotoxicity of these cationic liposomes at high concentration was addressed by Fan, et al. [66]. Cationic DOTAP liposomes were surface-modified using thiolated hyaluronic acid (HA) biodegradable polymer with ionic complexation. Liposome-HA particles were coated with PEG (polyethylene glycol) using dithiol conjugation in the presence of an oxidizing agent, chloroamine T, for better stability and steady antigen release. The optimal concentration of HA was decided based on fluorescence resonance energy transfer (FRET) analysis and effect on particle size of hybrid particle after ionic interaction. Enhanced biocompatibility as well as reduced cytotoxicity of hybrid particles compare to DOTAP liposomes was observed using bone marrow derived dendritic cells (BMDCs) with various concentrations. In all, liposomal polymeric nanoparticle based intranasal vaccine with F1-V (fusion protein of fraction 1 pilus and LcrV antigen from Yersinia pestis, causative agent of pneumonic plague) and MPLA as an adjuvant was discovered.
5.3. Dendritic Cells (DCs) Targeting Liposomal Vaccine Carrier
DCs are the leading antigen presenting cells as a result of their ability to cross-represent extra cellular antigen via MHC class I molecule and potency to promote T-cell proliferation. As a result, targeting DCs for enhanced immune response has been exploited with the help of different receptors expressed by DCs.
In recent studies, Karmakar, et al. observed improved antigen uptake and cellular response by targeting Fcγ receptors on DCs [67,68,69]. The natural abundance of specific natural anti-Rhamnose antibodies in the human population was exploited using L-rhamnose as a targeting ligand [70]. Indirect targeting was achieved via the presence of anti-Rhamnose antibodies, generated in mice, bound to the rhamnose ligand on the liposomal vaccine. Higher CD8+ T-cell specific INF-γ production was observed with targeting ligands on liposomes.
C-type lectin receptors (CLRs) on DCs were exploited by Jiang, et al. [71]. CLRs are known to bind to galactose, Lewis X mono or oligosaccharides, and N-acetylgalactosamine. Higher levels of pro-inflammatory cytokines with galactosylated liposomal vaccine were observed, verifying the significance of DC targeting to immunological response.
Due to the tailoring properties and versatility that can be achieved with liposomes, these carrier systems are under investigation for further developments in vaccine formulation. Recent advancements with liposomal vaccine delivery systems for different diseases are summarized in Table 2. About 39 clinical studies involving liposomes as vaccine carriers are listed on Clinical Trials.gov. Some of those are listed with their respective statuses in Table 3.
Table 2
Recent advancements in liposomal vaccine carrier system.
| Disease | Lipid composition | Antigen | Adjuvant | Ref. |
|---|---|---|---|---|
| Pneumonic Plague | DOTAP, DOPE | Ovalbumin (OVA) F1-V recombinant fusion protein of Y. pestis | MPLA | [66] |
| Hepatitis B | SPC, MPC, SA | HBsAg | MPLA | [72] |
| Tuberculosis | DDA | BCG | TDB | [73] |
| Yeast lipids | Alpha crystalline protein 1 (Acr 1) | - | [74] | |
| HIV | DMPC, DMPG, Chol, and MPLA (ALF liposomes) | CN54 gp140 protein | MPLA,QS21 (a triterpenoid glycoside saponin) | [75] |
| DOPC, DOPG, DSPE-PEG | MPER peptide | LACK-1 and HIV-30 (CD4+ epitope) | [76] | |
| Cancer | DOTAP, DOPE, PC, DSPE-PEG | OVA-peptide, TRP-2peptide | Alpha-galactoceramide (α-GC) | [77] |
| EYPC, DOPE, 3-Methyl glutarylated poly(glycidol) (MGluPG) and 3-methylglutarylated dextran | OVA | IFN-g-encoding plasmid DNA | [78] | |
| EPC, DSPE-PEG, Cholesterol, Sterylated R8 | - | Alpha-galactoceramide (α-GC) | [79] | |
| POPE, YSK05, Cholesterol, DMG-PEG | - | Cyclic diGMP | [57] | |
| PC, Cholesterol, (α and β) Galactosyl-DLPE | OVA | [71] | ||
| DPPC, Cholesterol, Rha-TEG-Cholesterol | Tn | Pam3Cys | [67] | |
| DNA vaccine | EPC, Cholesterol, DSPE-PEG | IVTT mix , plasmid DNA (β galactosidase) | IVTT | [80] |
| Alzheimer’s disease | POPG, DOPC, Cholesterol, S1P | Amyloid-beta peptide (A β) | CFA/IFA | [81] |
| Foot and Mouth Disease (FMD) | Lecithin, Cholesterol | Inactivated FMDV | polyinosinic–polycytidylic acid (poly I:C) and oligonucleotide CpGmotif (CpG) | [82] |
Table 3
Selected clinical studies involving liposomal carrier system.
| Condition | Sponsor/Collaborators | Status |
|---|---|---|
| Lung cancer | Eastern Cooperative Oncology Grp.; National Cancer Institute (NCI) | Phase II |
| Chronic Lymphocytic Leukemia (CLL) | XEME biopharma Inc.; National Cancer Institute (NCI) | Phase I |
| Tuberculosis | National Institute of Allergy and Infectious Disease (NIAID) | Phase I |
| Non-small cell lung cancer | EMD Serono; Merck KGaA | Phase III |
| Breast cancer | EMD Serono | Phase III |
| Influenza | NasVax Ltd. | Phase II |
| Tuberculosis | Statens Serum Institut | Phase I |
6. Polymeric Nanoparticles
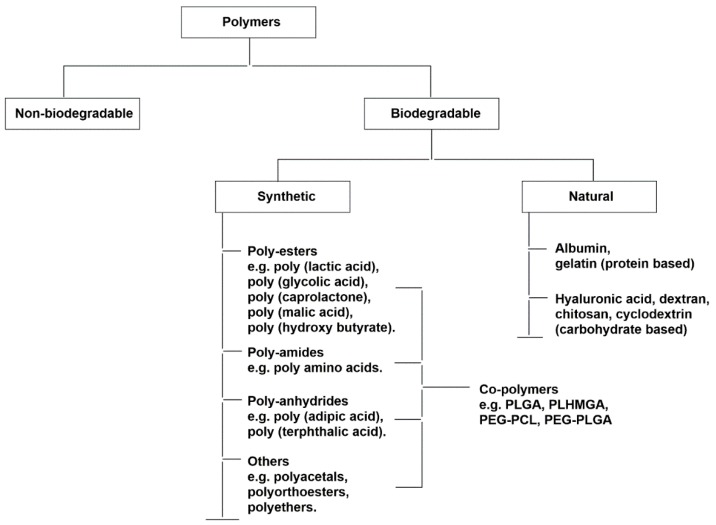
Polymeric nanoparticles are also under investigation for their potential use as vaccine carrier platforms [83,84,85,86,87,88]. Apart from similar advantages, such as biocompatibility, safety, and flexibility of liposomal carrier systems, a wide variety of natural, as well as synthetic, bio-degradable polymers and block co-polymers create opportunities to tailor and improve these materials (Figure 2). By varying nanoparticle size, shape, surface charge, type of polymer, and polymer concentration, researchers can optimize polymeric nano-particulate carriers. Recent advances in these areas are discussed further.
Physiochemical Properties
Particle size: Experimental data are available on particle size and its effects on drug distribution and respective immunological applications. Recently, Silva and co-workers evaluated micro- and nano-poly (lactic-co-glycolic acid) (PLGA) particles, their phagocytosis by DCs, and respective immune responses [89]. They observed superior antigen presentation with nano-PLGA particles than micro-particles with balanced TH1 and TH2 type antibody response compared to vaccination with incomplete Freund’s adjuvant (IFA). Another study, with hydrophilic polyester (poly(D,L lactic-co-hydroxy methyl glycolic acid) (pLHMGA)) nanoparticles carrying synthetic long protein derived from HPV 16 E7 onco-protein and TLR 3 ligand, showed comparable results with IFA formulation without any local adverse effects of IFA [90].
Particle shape and geometry: The importance of physical attributes, other than particle size, such as shape and elasticity, have not been comprehensively studied. However, recent studies suggest an impact of these other aspects on circulation time, phagocytosis, immune cell targeting, and cargo release. Kumar and co-workers synthesized polystyrene particles of different shapes and sizes to investigate their interactions with the immune system [91]. Spherical polystyrene particles (193 nm and 521 nm) were stretched by the film stretching method to obtain rod-shaped particles (376 nm and 1530 nm in length) containing a model antigen (ovalbumin). Smaller spherical nanoparticles (193 nm) produced higher antibody titers when compared to large spheres, but, interestingly, in the case of rod-shaped particles, the trend was reversed (high titers for 1530 nm than 376 nm). TH1 and TH2 biased immune response was observed to be based on shape, as spherical particles swayed the immune response to a strong TH1, while the rod-shaped particles shifted the response to TH2.
Cylindrical, 80 × 180 nm, hydroxy-poly (ethylene glycol) (PEG) was shown to elicit an improved immune response with sustained antigen presentation [92]. Hydrogel PEG particles were prepared using particle replication in nonwetting templates (PRINT) technology to achieve better control of NP size, charge, and surface functionality. Anionic cylindrical particles were observed to have better lymphatic drainage compare to other variable nanoparticles. These rod/cylindrical particles seem to be advantageous over traditional spherical polymeric particles owing to their higher cellular uptake, as well as antigen loading. A computational model for nanoparticle transport and distribution was studied by Tan and co-workers, which simulated the Brownian dynamics and fluid mechanics of nanoparticle in blood vessels [93].
Elasticity of the polymeric platform also plays a role in phagocytosis, which is crucial for antigen uptake and presentation. Anselmo and co-workers synthesized hydrogel nanoparticles composed of PEG diacrylate (PEGDA) via the nano-emulsion method [94]. The elastic modulus of the particle was controlled by volume fraction of PEGDA in the nano-emulsion. Hard nanoparticles were observed to be phagocytosed with a rate of 3.5-fold, or higher, compared to soft nanoparticles. Long circulation of both types of nanoparticles were attributed to the presence of PEG, which provides stability to particles by avoiding opsonization, reticuloendothelial system (RES) clearance, and decreased interactions with the extracellular matrix (ECM).
The significance of cellular and/or humoral response against any antigen is well established. The intracellular fate of the antigen decides the induction of an antigen-specific immune response. Antigens present in endosomes lead to specific humoral immune responses, while endosomal escape of antigens, followed by proteosomal processing, leads to the specific cellular immune response. The effect of hydrophobicity of polymers on endosomal escape and their membrane interactions have started to gain attention. Shima and co-workers modified the hydrophilic backbone of poly (γ-glutamic acid) (γ-PGA) with hydrophobic L-phenylalanine ethyl ester (L-Phe) with different degrees of grafting [95]. The membrane disruptive property of γ-PGA-Phe nanoparticles was observed to be dependent on surface hydrophobicity. It can be concluded that the balance between hydrophilicity and hydrophobicity is important for cytosolic delivery of antigens, which could be polymer specific. The role of hydrophobicity in manipulating the rate of polymer degradation is also well known. In the case of the PLGA polymer, a higher ratio of hydrophilic monomer component, i.e., glycolic acid, causes an increased rate of degradation.
PLGA nanoparticles: Among all natural, as well as synthetic, bio-degradable polymers, PLGA has been extensively used for vaccine delivery. PLGA is an Food and Drug Administration (FDA)-approved aliphatic co-polymer, composed of varying degrees of lactic acid and glycolic acid monomers. As mentioned earlier, PLGA nanoparticles with various antigens are capable of inducing a stronger CD8+ T-cell immune response compare to soluble antigen. There are several reports of the chemical modifications and degradation of antigens loaded in PLGA particles, exposing some of the drawbacks of the carrier. However, PLGA continues to be used as a vaccine carrier. Some recent reports of this particulate carrier with different modification are mentioned in Table 4.
Table 4
Modifications of PLGA nanoparticles for vaccine delivery.
| Disease | Antigen | Immuno-Adjuvant | Modification | Ref. |
|---|---|---|---|---|
| Cancer (Melanoma) | Melan-A:26 , gp100:209 (peptides). OVA as model antigen. | Poly (I:C), CpG | Mannose functionalized delivery system (PLGA, PEG-PLGA and Mannose-PEG-PCL) was developed to target CD206/MR on DC. | [96] |
| MART-1 (peptide) | - | Biotinylated anti-human DEC-205 monoclonal antibodies were used to target DCs. | [97] | |
| Cancer | OVA as model protein antigen | Pam3Csk4, Poly (I:C) | Agonistic α-CD40-mAb were conjugated on the surface of PLGA nanoparticles for CD-40 targeted DC delivery. | [98] |
| Cancer cell membrane obtained from mouse-melanoma cells | - | PLGA nanoparticle were coated with cancer cell membrane to introduce multiple surface antigen which is challenging with traditional synthetic methods. | [99] | |
| OVA as model protein antigen along with SOCS1 siRNA | - | Silencing of immunosuppressive SOCS1 gene augmented pro-inflammatory cytokine response. | [100] | |
| Improved Hybrid polymer-lipid particle | BSA | - | Cholesterol coated PLGA particle showed improved stability with better cellular uptake and more controlled antigen release. | [101] |
| Malaria | Pfs25 (Plasmodium falciparum Transmission-Blocking Antigen) | - | - | [102] |
| VMP001 | MPLA | Lipid (DOPC, DOPG, mal-PE) coated PLGA particles were developed with surface presentation of antigen using maleimide-thiol conjugation. | [103] |
PLGA co-polymers, along with other co-polymers, are being investigated for different aspects of vaccinology to obtain an improved immune response. Higher surface conjugation, as well as physical adsorption of ovalbumin (OVA) on maleic anhydride (MA) grafted poly (lactic acid) (PLA) (PLA-g-MA) [104], compared to only PLA, emphasizes the untapped potential and scope of use for co-polymers in this area. To overcome plausible flaws in PLGA particles, pLHMGA particles were developed and shown to exhibit a cellular response with sustained release of antigen [105]. Kunda and co-workers developed a dry powder inhalation formulation for pneumonia with poly (glycerol adipate-co-ω-pentadecalactone) (PGA-co-PDL) nano-particles and L-leucine micro-particles to avoid exhalation of the nanoparticle because of low inertia [106].
7. Inorganic Nanoparticles
Recently, Zhang and co-workers presented promising CD8+ T cell results with polyelectrolyte multilayers (PEM) assembled on gold nanoparticles (AuNPs) [107]. PEMs are self-assembled structures constructed via layer-by-layer (LbL) deposition using electrostatic interactions between positively and negatively charged electrolytes. Positively charged antigen and negatively charged immuno-adjuvant on gold nanoparticles resulted in a new vaccine platform. Greater control over antigen loading is a key advantage in vaccine design. The concept of PEMs was further exploited by Chiu, et al. to develop vaccine capsules made up of PEMs without any vehicle [108]. Calcium carbonate was used as solid support for PEM deposition, which was later removed.
The ease of synthesis of these inorganic nanoparticles, with precise control over mono-dispersity, size and shape, higher cargo loading, and colloidal stability, outweigh some limitations, such as their non-biodegradability. Recently, AuNPs have been used in immunotherapy as they are inert and can be easily functionalized with desired molecules. Chiodo, et al. synthesized AuNPs with tetra- and pentamannosides in order to mimic clusters of HIV gp120 [109]. Similar efforts have been made to develop AuNP-based vaccines for cancer [110,111,112,113], influenza [114], malaria [115], FMD [116], and HIV [117], by conjugating respective antigens on the surface. Sungsuwan, et al. developed lipid-coated iron oxide nanaoparticles with mucin-1 (MUC-1) antigens for cancer therapy [118]. The antigen-modified lipid was used to coat iron oxide nanoparticles to bind antigens on the surface without covalent modification. Meningitis A capsular polysaccharide fragments carrying iron oxide particle have been recently developed by Ramella, et al. [119]. Carbon nanoparticles, carbon nanotubes, silica, as well as calcium phosphate nanoparticles, are also under investigation for vaccine development.
The optical and photothermal properties of inorganic particles have been exploited in drug delivery as a tumor imaging and targeting tool. Based on recent studies, these treatments can have applications in immune therapy [120,121,122]. Heat shock proteins and tumor antigens released from dying tumor cells can activate the immune system. Koboyashi, et al. observed anti-tumor immunity via magnetic-nanoparticle-induced hyperthermia [123]. Application of an external magnetic field to the targeted magnetic nanoparticles increases tumor cells temperature without causing any harm to normal cells. Along with expected tumor cell death, the treatment resulted in an unexpected tumor specific response. These observations could lead to treatment addressing disease states, as well as immunotherapy.
8. Conclusions and Future Directions
This review summarizes recent developments in particulate carries for subunit vaccines. Development of a wide variety of nanoparticles with target specific modifications has a profound impact on the efficacy of vaccines. Understanding the fluid dynamics of these carriers, based on their physio-chemical variations, is the key to a better bio-distribution and antigen presentation. With advances in technology, investigators can achieve better control over the synthetic parameters of these vehicles to ensure reduced toxicity, antigen stability, and enhanced immunogenicity.
With the availability of new multifunctional carriers, the possibilities for novel vaccines have been greatly expanded. Comparison of different adjuvant loadings may be more accessible with these newer material. The synergistic effects of multiple immuno adjuvants may be addressed with DoE and simple conjugation chemistry to these nano-carrier. Parallel immune response studies of different nano-carriers, having the same antigen loading and adjuvant, could be interesting and may help in further development of hybrid nano-particles. In conclusion, recent studies highlighted in this review represent a step forward in dealing with current challenges in vaccinology and present new directions for future vaccine design.
Acknowledgments
This work is supported in part by the University of Toledo and a grant from the National Institutes of Health (Grant No. GM094734).
Abbreviations
The following abbreviations are used in this manuscript:
| ALF | Army Liposome Formulation |
| CD 80 | Cluster of Differentiation 80 |
| CFA | Complete Freund’s adjuvant |
| DDA | Dimethyl dioctadecyl-ammonium bromide |
| DLPE | 1,2-dilauroyl-sn-glycero-3-phosphoethanolamine |
| DMG | 1,2-Dimyristoyl-sn-glycerol |
| DMPC | Dimyristoyl phosphatidylcholine |
| DMPG | Dimyristoyl phosphatidylglycerol |
| DOPC | 1,2-dioleoyl-sn-glycero-3-phosphocholine |
| DOPE | 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine |
| DOPG | 1,2-dioleoyl-sn-glycero-3-phoshpo-(1’-rac-gylcerol) |
| DOTAP | 1,2-dioleoyl-3-trimethylammonium propane |
| DPPC | Dipalmitoyl phosphatidylcholine |
| DSPE-PEG | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy (polyethyleneglycol)] |
| EPC | Egg phosphatidylcholine |
| HBsAg | Recombinant human hepatitis B virus surface antigen |
| IFA | Incomplete Freund’s adjuvant |
| INF-γ | Interferon γ |
| IVTT | in vitro transcription and translation |
| MART-1 | Melanoma antigen recognized by T-cells 1 |
| MHC | Major Histocompatibility Complex |
| MPC | Mannose-PEG1000-cholesterol |
| MPER | Membrane proximal external region |
| MPLA | Monophosphoryl lipid A |
| NK cells | Natural Killer cells |
| PC | Phosphatidylcholine |
| PEG | Polyethylene Glycol |
| POPE | 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine |
| POPG | 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylglycerol |
| SA | Stearyl amine |
| S1P | Sphingosine-1-phosphate |
| SPC | Soy phosphatidylcholine |
| TDB | d-(+)-trehalose 6,6-dibehenate |
| VMP | Vivax malaria protein |
Author Contributions
Abhishek Vartak and Prof.Steven Sucheck both wrote, revised and proofread this review article.
Conflicts of Interest
The authors declare no conflict of interest.
References
Articles from Vaccines are provided here courtesy of Multidisciplinary Digital Publishing Institute (MDPI)
Full text links
Read article at publisher's site: https://doi.org/10.3390/vaccines4020012
Read article for free, from open access legal sources, via Unpaywall:
https://www.mdpi.com/2076-393X/4/2/12/pdf?version=1461073332
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.3390/vaccines4020012
Article citations
An updated review of HSV-1 infection-associated diseases and treatment, vaccine development, and vector therapy application.
Virulence, 15(1):2425744, 13 Nov 2024
Cited by: 0 articles | PMID: 39508503 | PMCID: PMC11562918
Review Free full text in Europe PMC
Adenoviral fiber-knob based vaccination elicits efficient neutralizing antibodies and T cell responses against adenovirus infection.
Virol J, 21(1):246, 07 Oct 2024
Cited by: 0 articles | PMID: 39370512 | PMCID: PMC11457358
Reverse vaccinology approaches to design a potent multiepitope vaccine against the HIV whole genome: immunoinformatic, bioinformatics, and molecular dynamics approaches.
BMC Infect Dis, 24(1):873, 28 Aug 2024
Cited by: 1 article | PMID: 39198721 | PMCID: PMC11360854
Design of multivalent-epitope vaccine models directed toward the world's population against HIV-Gag polyprotein: Reverse vaccinology and immunoinformatics.
PLoS One, 19(9):e0306559, 27 Sep 2024
Cited by: 0 articles | PMID: 39331650 | PMCID: PMC11432917
Bovine Respiratory Syncytial Virus Nanovaccine Induces Long-Lasting Humoral Immunity in Mice.
ACS Pharmacol Transl Sci, 7(10):3205-3215, 17 Sep 2024
Cited by: 0 articles | PMID: 39421663
Go to all (134) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Nanoparticles and Vaccine Development.
Pharm Nanotechnol, 8(1):6-21, 01 Jan 2020
Cited by: 56 articles | PMID: 31647394
Review
Biomaterials for nanoparticle vaccine delivery systems.
Pharm Res, 31(10):2563-2582, 22 May 2014
Cited by: 122 articles | PMID: 24848341 | PMCID: PMC4198431
Review Free full text in Europe PMC
[Development of polymeric nanoparticles-based vaccine].
Nihon Rinsho, 64(2):279-285, 01 Feb 2006
Cited by: 1 article | PMID: 16454182
Review
Nanoparticulate Carriers Used as Vaccine Adjuvant Delivery Systems.
Crit Rev Ther Drug Carrier Syst, 36(5):449-484, 01 Jan 2019
Cited by: 5 articles | PMID: 32421952
Review
Funding
Funders who supported this work.
NIGMS NIH HHS (1)
Grant ID: R15 GM094734
National Institutes of Health (1)
Grant ID: GM094734