Abstract
Free full text

Impact of Tobacco Smoke and Nicotine Exposure on Lung Development
Abstract
Tobacco smoke and nicotine exposure during prenatal and postnatal life can impair lung development, alter the immune response to viral infections, and increase the prevalence of wheezing during childhood. The following review examines recent discoveries in the fields of lung development and tobacco and nicotine exposure, emphasizing studies published within the last 5 years. In utero tobacco and nicotine exposure remains common, occurring in approximately 10% of pregnancies within the United States. Exposed neonates are at increased risk for diminished lung function, altered central and peripheral respiratory chemoreception, and increased asthma symptoms throughout childhood. Recently, genomic and epigenetic risk factors, such as alterations in DNA methylation, have been identified that may influence the risk for long-term disease. This review examines the impact of prenatal tobacco and nicotine exposure on lung development with a particular focus on nicotinic acetylcholine receptors. In addition, this review examines the role of prenatal and postnatal tobacco smoke and nicotine exposure and its association with augmenting infection risk, skewing the immune response toward a T-helper type 2 bias and increasing risk for developing an allergic phenotype and asthmalike symptoms during childhood. Finally, this review outlines the respiratory morbidities associated with childhood secondhand smoke and nicotine exposure and examines genetic and epigenetic modifiers that may influence respiratory health in infants and children exposed to in utero or postnatal tobacco smoke.
Lung development begins during early embryogenesis and extends into late childhood.1, 2 Although the majority of postnatal lung growth occurs within the first 2 years of life, modest alveolar growth continues through the first 15 years of life.3 Exposure to tobacco smoke and nicotine during in utero and postnatal life has been shown to disturb lung development, increase susceptibility to lower respiratory tract infections, increase prevalence of wheezing, and exacerbate respiratory symptoms in children with chronic lung diseases.4, 5, 6, 7, 8 The goals of this review are to examine the effects of tobacco smoke and nicotine exposure on prenatal and postnatal lung development, assess the relationship between tobacco smoke and nicotine exposure and respiratory morbidities in children, and evaluate the role of genetics and epigenetics on the respiratory phenotypes of children exposed to tobacco smoke and nicotine.
Tobacco smoke contains more than 4,000 chemicals, many of which have been associated with deleterious health consequences.9 In particular, exposures to nicotine; carbon monoxide; acrolein; and carcinogens, including polycyclic aromatic hydrocarbons, aromatic amines, and tobacco-specific N-nitrosamines, can be detrimental, especially during fetal life and periods of rapid postnatal organ growth, including the lungs and brain.4, 10 Nicotine, the primary addictive substance in tobacco smoke, has been shown to cross the placenta in utero as evidenced by high levels of cotinine, a nicotine metabolite, in the amniotic fluid of pregnant smokers and in blood spots from neonates exposed in utero.11, 12 Nicotine has also been shown to be concentrated in the breast milk of mothers who smoke.13, 14 In addition to indirect maternal exposure, children can also be exposed to secondhand tobacco smoke or thirdhand tobacco smoke during postnatal life. Secondhand smoke (SHS) is a mixture of mainstream smoke exhaled from the smoker and sidestream smoke that enters the environment from the burning cigarette. More recently, thirdhand smoke exposure has been recognized as a source of tobacco smoke and nicotine exposure, particularly in indoor environments. Thirdhand smoke exposure occurs when constituents of tobacco smoke deposit on surfaces. These chemicals may attach to dust, be re-emitted into the air, or react with other chemicals in the environment.15 Because of oxidation and breakdown processes, thirdhand smoke may have enhanced toxicity compared with mainstream smoke. Furthermore, infants and young children may be at highest risk for toxicity from secondhand and thirdhand smoke exposure because they tend to spend more time indoors, have faster respiratory rates, have a greater relative body surface area, and have more hand or oral behaviors than do older children.
Tobacco smoke exposure during prenatal and postnatal lung development is associated with an increase in respiratory morbidities during childhood. Tobacco smoke exposure can adversely affect lung development, cause small airway dysfunction and wheeze, and increase the frequency of upper and lower respiratory tract illnesses.16, 17, 18 It is unclear whether links exist between tobacco smoke exposure during childhood and increased risk of COPD during adult life. However, recently Chan and colleagues19 found an association between childhood pneumonia and diminished adult lung function. This finding suggests that that the risk of COPD in adult life may be influenced by SHS during childhood because children exposed to SHS are more likely to have a history of lower respiratory tract infections.
The detrimental effects of childhood tobacco smoke exposure on respiratory health may be increased by other confounding factors, including psychosocial stress, lower socioeconomic status, underlying chronic lung disease, genetic polymorphisms, and exposure to environmental pollutants.20 SHS exposure may also impair the effectiveness of corticosteroids used for the treatment of small airway disease.21 In addition, adverse health effects caused by SHS may be augmented in children with a genetic susceptibility to asthma, and exposure to tobacco smoke during in utero life may cause epigenetic changes in fetal lung.22
Tobacco Smoke Exposure in Children
Prenatal exposure of children to tobacco smoke remains high in the United States and worldwide, and caregiver questionnaires may underrepresent the actual number of children exposed to SHS.6 In the United States, an estimated 400,000 newborns are exposed every year to tobacco smoke during pregnancy, with 10.7% of pregnant mothers smoking during the last 3 months of pregnancy.23 The highest prevalence of smoking has been reported in women between 20 and 24 years of age (17.6%).23 Although multiple tobacco toxins likely affect the fetus, nicotine is the best studied component of tobacco smoke. Nicotine readily crosses the placenta and can cause significant in utero exposure to the fetus. Indeed, cotinine levels in neonates exposed to in utero tobacco smoke have been reported to be similar to that of active smoking adults.24, 25 Analysis from the Pregnancy Risk Assessment Monitoring System for 2001 and 2002 revealed that maternal smoking was associated with a longer length of stay in the neonatal intensive care unit, with an estimated smoking-attributable cost of $122 million annually in the United States.26
Postnatal exposure of children to SHS continues to be widespread. Oberg and colleagues,27 using data from 192 countries in 2004 reported that 40% of children worldwide were exposed to SHS. They also estimated that 5,939,000 lower respiratory tract infections in children younger than 5 years were because of SHS exposure and that 28% of the approximately 379,000 deaths annually because of SHS occur in children. Despite these and numerous other studies that have shown links between tobacco smoke exposure and increased morbidity and mortality in children, prenatal and postnatal exposure of children to tobacco and nicotine products remains unfortunately common.
Normal Lung Development
Human lung development spans both prenatal and postnatal life1 and can be adversely affected by exposure to tobacco smoke and nicotine during both of these periods. During normal embryogenesis (0-6 weeks’ gestation), lung buds develop from the ventral foregut by week 4 of gestation, with lobar and subsegmental branching with concomitant vascular growth taking place by week 6. During the pseudoglandular stage (7-16 weeks’ gestation), the conducting airways and preacinar pulmonary vessels form completely. Airway cartilage, smooth muscle, and mucous glands appear, and epithelial cell differentiation progresses from proximal to distal sites. The canalicular stage of lung development (16-24 weeks’ gestation) signals the earliest development of terminal respiratory units. This stage of lung development is characterized by the formation of type I and type II epithelial cells, capillary proliferation, thinning of pulmonary mesenchyme, and closer contact of capillary vessels to alveolar epithelium. The saccular stage of lung development (weeks 24-40) is characterized by increasing surface area of the gas-exchanging units, formation of true alveoli, and maturation of the surfactant in the type II epithelial cells. The majority of postnatal alveolar growth occurs during the first 2 years of life, with slower growth into adolescence.3, 28
In animal models, tobacco smoke and nicotine exposure during in utero or early postnatal life has been shown to alter expression of genes involved in cell proliferation, T-helper (Th) type 1 (Th1) and type 2 (Th2) immune balance, fibrosis, and viral immune responses (Table 1).29, 30, 31, 32, 33
Table 1
Effects of Tobacco Smoke and Nicotine Exposure on Gene Expression During Development
| Changes in Gene or Receptor Expression | Effects of Tobacco Smoke and Nicotine Exposure | References |
|---|---|---|
| ↓ N-myc expression | Nicotine caused fibroblast differentiation in nonhuman embryonic stem cells | 29 |
| ↑ α7 nAChRs | Prenatal nicotine exposure caused ↑ α7 nAChRs in airway, cartilage, and vessels in rhesus monkeys | 30 |
| ↓ Interferon-γ ↓Tbet ↑ Th2 signaling pathway (GATA3/Lck/Erk/STAT6) | Prenatal smoke exposure caused Th1 and Th2 changes in mice after Aspergillus sensitization | 31 |
| ↑ Connective tissue growth factor | ↑ lung fibrosis in rats exposed to prenatal or postnatal nicotine and hyperoxia | 32 |
| ↓ Interferon-inducible GTPases and genes activated by viral double-stranded RNA | ↓ expression of innate immunity response genes in neonatal mouse lung exposed to postnatal tobacco smoke | 33 |
nAChRs = nicotinic acetylcholine receptors; Tbet = Th1-specific transcription factor; Th1 = T-helper type 1; Th2 = T-helper type 2.
Nicotine Exposure and Lung Development
Nicotinic acetylcholine receptors (nAChRs) are ligand-gated ion channels that enable flow of cations through the channel when bound by acetylcholine and other nAChR agonists, including nicotine. These receptors consist of five transmembrane subunits that form a central ion pore.34 Heteromeric nAChRs possess α and β subunits, and α7 nicotinic receptors are homopentamer subunits. Human bronchial and endothelial cells have been reported to express α3/β4 and α7 nAChRs.35 nAChRs have been shown to be expressed in the brain and lung during early fetal life, thus representing a potential mechanism for the effects of nicotine on development.
In utero nicotine exposure has been reported to disrupt normal developmental processes in the brain and lungs.36, 37 The effects of nicotine may occur very early in lung development. For example, Ben-Yehudah and colleagues29 have shown that nonhuman primate embryonic stem cells express nAChRs. In their study, exposure of nicotine caused downregulation of n-Myc expression and differentiation of nonhuman primate embryonic stem cells into fibroblasts, demonstrating that exposure to nicotine during embryogenesis can affect embryonic stem cell differentiation. The effects of nicotine may also occur later in fetal development as is seen in animal studies. Sekhon and colleagues30 implanted nicotine pumps in pregnant rhesus monkeys and found reduced total body weight and alveolar hypoplasia in the offspring. They also found that nicotine increased expression of α7 receptors in airway cartilage and vessels of fetal lung and that other nicotinic receptor subtypes, although expressed in fetal lung, were not upregulated by nicotine.30 Prenatal nicotine exposure in mice has been shown to lead to diminished forced expiratory flows in adulthood, but interestingly not in α7 nAChR knockout mice.38 Other rodent studies have had similar findings with impaired lung growth and alveolar development.39, 40 A recent study of lung tissue from fetal and infant sudden death cases found a higher incidence of α7 nAChR immunostaining in lung associated with maternal smoking, and maternal smoking was linked to lung hypoplasia and hypoplasia of specific brainstem regions.41 In addition to nicotine, other components of tobacco smoke may be detrimental to the developing lung. Further research is needed to delineate the relative contributions of tobacco constituents to adverse lung development.
Vitamin C has been tested as a therapy to mitigate the effects of in utero smoke exposure. Proskocil and colleagues42 reported that vitamin C, when given to pregnant rhesus monkeys, improved lung function in nicotine-exposed offspring. A recent randomized placebo-controlled study found that the offspring of smoking mothers who received vitamin C during in utero life had better airflow and less wheezing through the first year of life compared with those of untreated mothers who smoked during pregnancy.43 The mechanism underlying the potential protective effect of vitamin C against in utero smoke exposure is not clear.
Maternal Smoking and Sudden Infant Death Syndrome
Tobacco smoke exposure has been linked to an increased risk of sudden infant death syndrome (SIDS). A 2012 study found that maternal smoking was reported in 38.6% of infants dying of SIDS.44 Mitchell and Milerad45 reported that the pooled relative risk of SIDS associated with maternal smoking was 2.86; furthermore, if a causal association was assumed, one-third of SIDS deaths may have been avoided if the infant had not been exposed to smoke in utero.
The mechanisms underlying this link between prenatal tobacco smoke exposure and SIDS have not been clearly elucidated. Rodent studies have indicated that nicotine exposure during prenatal life may alter central and peripheral respiratory chemoreception.46, 47 A study in neonatal rats found that pups exposed to prenatal cigarette smoke had prolonged gasping following a hyperthermic or hypoxic exposure.48 Another study reported that neonatal mice exposed to nicotine in utero had decreased ventilation and a reduced response to hypercarbia when exposed to 100% nitrogen for 20 seconds and 10% CO2 for 20 minutes.49
SHS and Lung Function in Children
Both in utero and postnatal SHS exposure have been associated with increased respiratory symptoms or reduced lung function in exposed children. It has been difficult, however, to parse out the effects of in utero exposure versus postnatal exposure, particularly because many children are exposed to both. In 3-year-old children within the Generation R cohort, there was an increased risk of wheezing with any prenatal or postnatal tobacco smoke exposure, but at 4 years of age, those with postnatal exposure alone did not have increased wheezing.5, 50 Hayatbakhsh and colleagues51 also found that men with prior in utero smoke exposure had significant reductions in FEV1 and midexpiratory flow. Another study reported that Chinese school-aged children exposed to SHS had increased cough and reduced lung function compared with findings in unexposed children.52 A Finnish study that examined young children with a history of multitrigger wheezing found that children of mothers who smoked, validated by cotinine levels, were more likely to have increased airway resistance and higher fraction of exhaled nitric oxide levels than were nonexposed children.17 Aberrant growth and inflammation of the small airways from tobacco exposure during in utero and early postnatal life may, in part, account for the observed symptoms and outcomes in these studies. Wongtrakool and colleagues53 found an increase in α7 nAChRs and stimulated lung branching in nicotine-treated embryonic murine lung explants, suggesting that in utero nicotine exposure may lead to dysanaptic airway growth and reduced lung function in exposed offspring.
SHS Exposure and Childhood Respiratory Infections
Respiratory tract infections represent a major source of morbidity in infancy and early childhood.54 These infections have developmental consequences, and they are increasingly recognized as a source of subsequent respiratory disease sequelae and diminished adult lung function.19, 55 Since the 1980s, studies have demonstrated increased risk for lower respiratory tract infections in infants exposed to maternal smoke.56, 57 The risk for subsequent infection, however, is not uniform between prenatal and postnatal exposure. Prenatal maternal smoking may carry a higher risk of subsequent infection, suggesting a developmental mechanism for the increased vulnerability,58, 59 but the underlying mechanisms that account for this increased infection risk are not fully understood. Limited animal and human data suggest that tobacco products alter both the innate and adaptive immune response.
SHS and Pulmonary Immune Responses
Coordinated activation of the innate and adaptive immune systems is critical to effective clearance of pathogens and resolution of infections. The immune system in fetal life faces the added challenge of preventing potentially lethal inflammatory responses to maternal alloantigens. Consequently, fetal T-helper cells exhibit a predominantly antiinflammatory Th2 response.60 After birth, the immune response normally shifts toward a Th1 immune response. In animal models, exposure to in utero and early postnatal tobacco smoke has been associated with a Th1 and Th2 imbalance and increased susceptibility to lower respiratory tract infections.31 Human studies have found altered cytokine profiles in tobacco-exposed neonates. Prenatal exposure to tobacco smoke can cause decreased levels of cord blood interferon-γ, and continued environmental tobacco smoke exposure has been shown to suppress interferon-γ levels through 11 years of age.59, 61 Alteration in interferon-γ production may be particularly relevant for infection risk; Sumino and colleagues62 correlated decreased monocyte interferon production at birth with increased risk of early life infection. In addition to decreasing interferon-γ levels, in utero smoke increases the levels of cytokines associated with Th2 immune profiles. Noakes and colleagues63 found that cord blood from neonates exposed to smoke in utero had increased levels of IL-13 after antigen challenge. However, there are sparse human data on immune cell profiles and function to support the cytokine studies. Indeed, in a 2013 study comparing neonates born to smoking and nonsmoking mothers, Almanzar and colleagues64 found no difference in lymphocyte subsets between the two groups.
Animal models, however, support immune cell phenotypic changes after in utero smoke and nicotine exposure (Fig 1). Neonatal mice exposed to nicotine in utero had enhanced M2 phenotype in their alveolar macrophages.65 Similarly, in a primate model, Wang and colleagues66 demonstrated decreased Th1 cytokine production after in utero and postnatal smoke exposure, which correlated with decreased recruitment of Th1 immune cells to the lung parenchyma. In addition, decreased expression of interferon-inducible GTPases and type 1 interferon response pathway genes induced by viral double-stranded RNA were found in the lungs of neonatal mice exposed to SHS.33
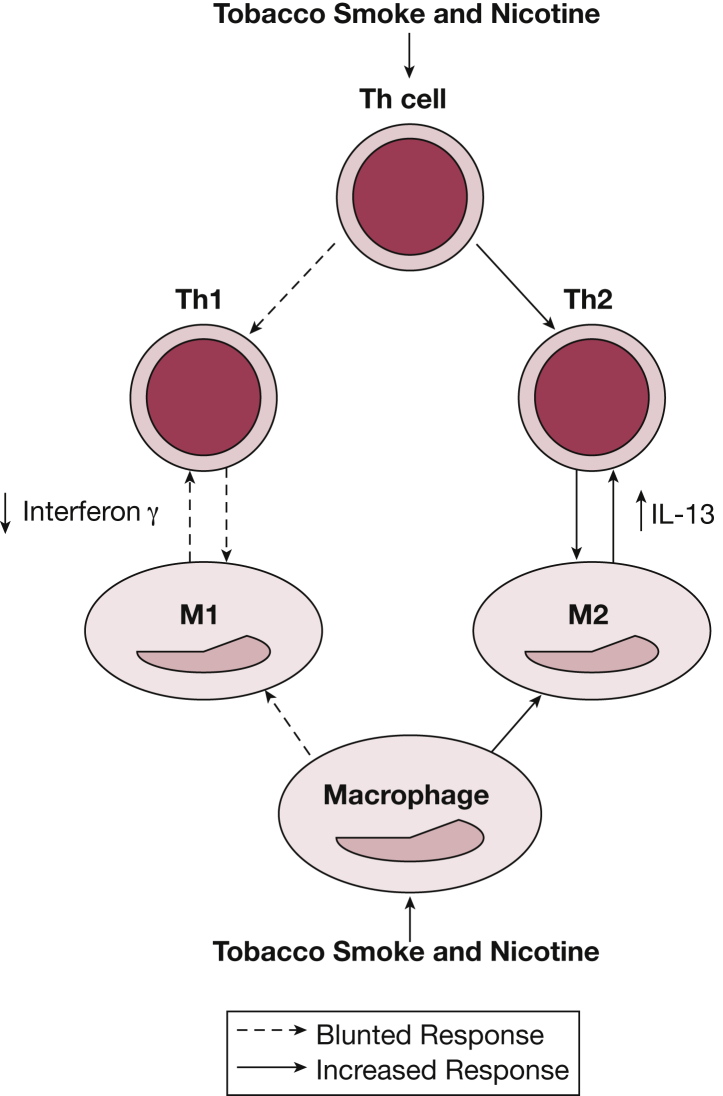
Schematic of phenotypic immune cell changes caused by tobacco smoke or nicotine exposure. Macrophages (M) exposed to tobacco smoke and nicotine are skewed toward an M2 phenotype, and Th cells exposed to tobacco smoke and nicotine are skewed toward a Th type 2 (Th2) phenotype. This preferential skewing caused by tobacco smoke and nicotine exposure results in increased levels of IL-13 and decreased levels of interferon-γ. Th = T-helper.
Along with modifying infection risk, the SHS-skewed immune response toward a Th2 bias may result in an allergic phenotype, small airway inflammation, and asthmalike symptoms. In animal models, exposure to in utero and early postnatal tobacco smoke has been associated with a Th1 and Th2 imbalance and an increased susceptibility to airway reactivity.31 Wang and colleagues66 examined the Th1 and Th2 pulmonary responses in lungs of infant monkeys exposed to in utero and early postnatal tobacco smoke and found decreased expression of upstream and downstream interferon-γ pathway genes that regulate interferon-γ.
Thus, smoke exposure may render neonates vulnerable to infection and promote development of allergic disease by changing the activation profile of immune cells. However, further research is needed to establish the changes in cellular immunity in humans and to define the molecular pathways underlying these changes.
Epigenetic Effects of Tobacco Smoke Exposure
Although the deleterious clinical consequences of early life tobacco exposure have been well established over the past several decades, the molecular mechanisms underpinning these changes remain incompletely understood. In recent years, epigenetic interactions with tobacco smoke have emerged as potentially intriguing mechanisms for postnatal lung disease. Epigenetic modification of gene expression and phenotype plays an important role in fetal development; furthermore, the epigenome that develops in utero and in early life persists throughout life and is heritable.67
Tobacco-related epigenetic changes, particularly DNA methylation, were first noted in the context of cancer development and progression.68, 69 Subsequently, epigenetic differences in global DNA methylation have been found between smokers and nonsmokers.70, 71 Because of difficulties in sampling, the majority of epigenetic studies of fetal methylation patterns in humans have been performed in cord blood and placenta. Cord blood studies have implicated smoking-induced methylation changes in multiple specific genes, including CYP1A1, GF1, FOXP3, and AHRR,72, 73, 74 which play a role in detoxification and immune regulation and, therefore, may contribute to childhood lung disease. Among the best understood and most consistently recovered changes in methylation patterns involves the AHRR gene, which encodes for the aryl hydrocarbon receptor repressor, a feedback inhibitor that has been suggested to attenuate effects of tobacco smoke toxins.75 Studies of human cord blood have shown decreased methylation in AHRR loci in neonates similar to immune cell methylation patterns in adult smokers. These neonatal changes correlated with maternal levels of cotinine.74 These epigenetic changes in AHRR methylation persist well into postnatal life. Novakovic and colleagues76 recently demonstrated persistent AHRR hypomethylation in blood monocytes at 1 year of age, and a separate longitudinal study detected persistent changes at 7 years of age.72, 77 Changes in AHRR methylation may contribute to altered detoxification function and subsequent disease development. Placental studies also have demonstrated changes in global and gene-specific methylation.78
Although these findings are highly suggestive, placental and cord blood studies have limitations. Significant variability in methylation patterns between cord blood and placental and saliva samples suggests tissue-specific epigenetic patterns,79 which may lead to differing functional consequences.80 Currently, knowledge of tissue-specific epigenetic effects of tobacco smoke exposure remains limited. One of the first articles looking at the effects of in utero smoke exposure on fetal lung-specific methylation found changes in methylation of DPP10, a gene associated with asthma.22 Ultimately, further work is needed to correlate cord blood and placental and tissue-specific epigenetics with functional measures of lung disease.
Intriguingly, the epigenetic effects of tobacco smoke may extend beyond direct effects on gene expression to transgenerational changes in lung development. In a well-established animal model of in utero nicotine exposure, Rehan and colleagues81 demonstrated increased airway resistance and contractility proteins in rats that were one generation (F2) removed from in utero nicotine exposure, which was supported by tissue-specific epigenetic differences. Human data evaluating the transgenerational effects of tobacco smoke on lung function have shown differing results, possibly because of unmeasured confounding variables such as socioeconomic status, distinct environmental exposures, or ethnicity. A grandmaternal effect on asthma risk was first suggested by the Children’s Health Study in 2005.82 In this nested case control study, grandmaternal smoking was associated with a 2.1 OR risk of developing childhood asthma; however, this effect was unable to be replicated using data from the Avon Longitudinal Study of Parents and Children.83 The Norwegian Mother and Child Cohort Study also found a strong association between grandmaternal smoking during pregnancy and childhood asthma.84
Ultimately, tobacco smoke-related modification of the epigenome appears to have longitudinal effects on DNA methylation patterns and may even alter transgenerational risk for the development of lung disease. There is a need for future research to define better the functional consequences of epigenetic modification, the heritable consequences of in utero smoke exposure, and the role for therapies that target smoke’s epigenetic modifications.
Gene-Environment Interactions and Tobacco Smoke Exposure
As with epigenetics, the interplay between genes and environmental exposures is becoming increasingly important to our understanding of the development of complex lung diseases. Both chromosomal linkage studies and analysis of specific polymorphisms have highlighted the role of genetics in determining susceptibility to in utero smoke exposure. Much of the research has focused on the association among genes, smoke exposure, and subsequent asthma; however, few studies have looked globally at the genome. The first genomewide interaction study of early life tobacco exposure and asthma was published in 2014 and identified novel polymorphisms on chromosomes 6 and 18.85 Linkage studies have also implicated chromosomal regions 1p, 1q, 5q, and 17p as modifying asthma risk after early life smoke exposure.
Specific candidate genes have emerged from the growing body of research on asthma genetics. Research on candidate genes has largely focused on xenobiotic detoxification and antioxidant pathways, including the GST family. Encoding for the glutathione S-transferase enzymes, GSTs play a role in metabolizing tobacco products and reactive oxygen species. Deletions of these genes are common, with 50% and 25% of whites being homozygous GSTM1 null and GSTT1 null, respectively. Polymorphisms have been implicated in asthma pathogenesis.86 The first study to associate in utero smoke and GST enzymes found that GSTM1 null children had increased risk of both diagnosed asthma and wheezing after in utero smoke exposure compared with findings in individuals with functional copies.87 Subsequent research has demonstrated physiologic changes, including increased airway resistance and decreased lung function after in utero smoke exposure in children with GSTTI and GSTP1 alleles.86, 88 Wu and colleagues89 reported in a population-based birth cohort that maternal smoking increased the risk of wheezing in children who had a GSTP1 AA genotype; however, the effect of maternal smoking on wheeze diminished with age. Additional candidate genes, CYP1A1, ADAM33, and TNF, have been suggested to modify the risk of subsequent asthma.85, 90, 91 Further genomewide association studies and clearer understanding of molecular pathways are needed to validate the role of identified candidate genes in the interaction between in utero smoke and lung development.
Electronic Cigarettes and Lung Development
With the increased popularity of electronic nicotine delivery systems among people of childbearing age, it is unknown what effect nicotine-containing e-cigarette aerosols will have on the lung function and respiratory health of children exposed during in utero and early postnatal life. One study reported that non-e-cigarette users exposed to e-cigarette aerosols in the environment had similar cotinine levels as those exposed to SHS from tobacco-containing cigarettes.92 Recent animal studies in mice have shown an adverse effect of e-cigarette aerosols on postnatal lung development and the innate immune system.93, 94 E-cigarette juices come in a variety flavors with and without nicotine. Lerner and colleagues95 reported increased levels of the proinflammatory cytokines, IL-6, and IL-8 in human epithelial cells and fibroblasts exposed to nicotine and nonnicotine flavored e-cigarette juices. Furthermore, they reported that the lungs of mice exposed to nicotine-containing e-cigarette aerosols had decreased total glutathione.
Taken together, these findings indicate that exposure to e-cigarette aerosols may increase oxidative stress and inflammation in the lung.95 Although data are limited regarding the pulmonary effects of e-cigarette aerosols on fetal development, the nicotine component of these aerosols may have developmental consequences similar to those of other forms of nicotine delivery. A recent study from the United Kingdom reported that offspring of mothers given nicotine replacement therapy during their first trimester or a month prior to conception had a significant increase in respiratory anomalies but not in any other organ-specific system.96 However, electronic nicotine delivery systems have the capacity to deliver a high level burst of nicotine. This in turn may cause different physiologic or pathologic changes in the developing lung compared with those seen with nicotine replacement therapy.
Gaps in Knowledge and Future Directions
Studies that continue to examine the effects of tobacco exposure during in utero life and throughout childhood are needed to understand fully the long-term effects of tobacco smoke exposures on adult lung function. In addition, studies that elucidate the role of epigenetic changes and genetic polymorphisms on respiratory disease phenotype in children exposed to SHS would help identify subgroups of individuals who may be at highest risk for respiratory morbidities and the development of COPD. Finally, studies are needed that examine the short- and long-term effects of e-cigarette aerosols on lung development and childhood respiratory health.
Acknowledgments
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
FUNDING/SUPPORT: This study was funded by a Center of Excellence Grant to the American Academy of Pediatrics from the Flight Attendant Medical Research Institute (S. A. M., J. M. C.); and the National Institutes of Health [Grant R01HL114800 (S. A. M.)].
References
Articles from Chest are provided here courtesy of American College of Chest Physicians
Full text links
Read article at publisher's site: https://doi.org/10.1378/chest.15-1858
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4944770?pdf=render
Citations & impact
Impact metrics
Article citations
The association of maternal smoking around birth with chronic respiratory diseases in adult offspring: A Mendelian randomization study.
Tob Induc Dis, 22, 27 Jun 2024
Cited by: 1 article | PMID: 38938749
Association of Fetal Lung Development Disorders with Adult Diseases: A Comprehensive Review.
J Pers Med, 14(4):368, 29 Mar 2024
Cited by: 0 articles | PMID: 38672994 | PMCID: PMC11051200
Review Free full text in Europe PMC
Status of home-based secondhand smoke exposure among children and its association with health risks in Japan.
Prev Med Rep, 38:102585, 02 Jan 2024
Cited by: 0 articles | PMID: 38283957 | PMCID: PMC10818243
Prevalence of pre-existing lung diseases and their association with income level among patients with lung cancer: a nationwide population-based case-control study in South Korea.
BMJ Open Respir Res, 10(1):e001772, 01 Nov 2023
Cited by: 0 articles | PMID: 37940354 | PMCID: PMC10632895
Effectiveness of behavior change interventions for smoking cessation among expectant and new fathers: findings from a systematic review.
BMC Public Health, 23(1):1812, 18 Sep 2023
Cited by: 0 articles | PMID: 37723506 | PMCID: PMC10506219
Review Free full text in Europe PMC
Go to all (90) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The impact of second-hand tobacco smoke exposure on pregnancy outcomes, infant health, and the threat of third-hand smoke exposure to our environment and to our children.
Przegl Lek, 69(10):717-720, 01 Jan 2012
Cited by: 12 articles | PMID: 23421018
Pulmonary Effects of Maternal Smoking on the Fetus and Child: Effects on Lung Development, Respiratory Morbidities, and Life Long Lung Health.
Paediatr Respir Rev, 21:27-33, 19 Aug 2016
Cited by: 118 articles | PMID: 27639458 | PMCID: PMC5303131
Review Free full text in Europe PMC
Gestational Exposure to Sidestream (Secondhand) Cigarette Smoke Promotes Transgenerational Epigenetic Transmission of Exacerbated Allergic Asthma and Bronchopulmonary Dysplasia.
J Immunol, 198(10):3815-3822, 05 Apr 2017
Cited by: 20 articles | PMID: 28381639 | PMCID: PMC5473031
In utero and childhood exposure to tobacco smoke and multi-layer molecular signatures in children.
BMC Med, 18(1):243, 19 Aug 2020
Cited by: 16 articles | PMID: 32811491 | PMCID: PMC7437049
Funding
Funders who supported this work.
NHLBI NIH HHS (2)
Grant ID: R01 HL114800
Grant ID: R01HL114800






