Abstract
Purpose
Transarterial chemoembolization is accepted therapy for hepatocellular carcinoma (HCC). No randomized trial has demonstrated superiority of chemoembolization compared with embolization, and the role of chemotherapy remains unclear. This randomized trial compares the outcome of embolization using microspheres alone with chemoembolization using doxorubicin-eluting microspheres.Materials and methods
At a single tertiary referral center, patients with HCC were randomly assigned to embolization with microspheres alone (Bead Block [BB]) or loaded with doxorubicin 150 mg (LC Bead [LCB]). Random assignment was stratified by number of embolizations to complete treatment, and assignments were generated by permuted blocks in the institutional database. The primary end point was response according to RECIST 1.0 (Response Evaluation Criteria in Solid Tumors) using multiphase computed tomography 2 to 3 weeks post-treatment and then at quarterly intervals, with the reviewer blinded to treatment allocation. Secondary objectives included safety and tolerability, time to progression, progression-free survival, and overall survival. This trial is currently closed to accrual.Results
Between December 2007 and April 2012, 101 patients were randomly assigned: 51 to BB and 50 to LCB. Demographics were comparable: median age, 67 years; 77% male; and 22% Barcelona Clinic Liver Cancer stage A and 78% stage B or C. Adverse events occurred with similar frequency in both groups: BB, 19 of 51 patients (38%); LCB, 20 of 50 patients (40%; P = .48), with no difference in RECIST response: BB, 5.9% versus LCB, 6.0% (difference, -0.1%; 95% CI, -9% to 9%). Median PFS was 6.2 versus 2.8 months (hazard ratio, 1.36; 95% CI, 0.91 to 2.05; P = .11), and overall survival, 19.6 versus 20.8 months (hazard ratio, 1.11; 95% CI, 0.71 to 1.76; P = .64) for BB and LCB, respectively.Conclusion
There was no apparent difference between the treatment arms. These results challenge the use of doxorubicin-eluting beads for chemoembolization of HCC.Free full text

Randomized Trial of Hepatic Artery Embolization for Hepatocellular Carcinoma Using Doxorubicin-Eluting Microspheres Compared With Embolization With Microspheres Alone
Associated Data
Abstract
Purpose
Transarterial chemoembolization is accepted therapy for hepatocellular carcinoma (HCC). No randomized trial has demonstrated superiority of chemoembolization compared with embolization, and the role of chemotherapy remains unclear. This randomized trial compares the outcome of embolization using microspheres alone with chemoembolization using doxorubicin-eluting microspheres.
Materials and Methods
At a single tertiary referral center, patients with HCC were randomly assigned to embolization with microspheres alone (Bead Block [BB]) or loaded with doxorubicin 150 mg (LC Bead [LCB]). Random assignment was stratified by number of embolizations to complete treatment, and assignments were generated by permuted blocks in the institutional database. The primary end point was response according to RECIST 1.0 (Response Evaluation Criteria in Solid Tumors) using multiphase computed tomography 2 to 3 weeks post-treatment and then at quarterly intervals, with the reviewer blinded to treatment allocation. Secondary objectives included safety and tolerability, time to progression, progression-free survival, and overall survival. This trial is currently closed to accrual.
Results
Between December 2007 and April 2012, 101 patients were randomly assigned: 51 to BB and 50 to LCB. Demographics were comparable: median age, 67 years; 77% male; and 22% Barcelona Clinic Liver Cancer stage A and 78% stage B or C. Adverse events occurred with similar frequency in both groups: BB, 19 of 51 patients (38%); LCB, 20 of 50 patients (40%; P = .48), with no difference in RECIST response: BB, 5.9% versus LCB, 6.0% (difference, −0.1%; 95% CI, −9% to 9%). Median PFS was 6.2 versus 2.8 months (hazard ratio, 1.36; 95% CI, 0.91 to 2.05; P = .11), and overall survival, 19.6 versus 20.8 months (hazard ratio, 1.11; 95% CI, 0.71 to 1.76; P = .64) for BB and LCB, respectively.
Conclusion
There was no apparent difference between the treatment arms. These results challenge the use of doxorubicin-eluting beads for chemoembolization of HCC.
INTRODUCTION
In 2002, two groups reported a survival benefit for patients with locally advanced hepatocellular carcinoma (HCC) treated with transarterial chemoembolization (TACE) compared with best supportive care (BSC).1,2 The study by Lo et al2 compared cisplatin-based TACE to BSC whereas the study by Llovet et al1 used doxorubicin-based TACE and included a bland hepatic artery embolization (HAE) arm. The study by Llovet et al is thought by many to have demonstrated the superiority of TACE compared with both HAE and BSC; however, the sequential design allowed the trial to be stopped before any valid conclusion could be drawn regarding the effectiveness of bland HAE compared with BSC.1 No prospective trial has demonstrated the superiority of conventional TACE compared with bland embolization, embolization with drug-eluting microspheres, or radioembolization.3 The randomized phase II trial by Meyer et al4 used cisplatin-based TACE compared with HAE using polyvinyl alcohol particles alone and found no difference in survival. The purpose of this study was to determine what effect the addition of doxorubicin has on response and outcome after embolization with microspheres.
MATERIALS AND METHODS
Study Design
This study is a prospective single-center, single-blind, randomized phase II trial conducted at a tertiary cancer referral center in the United States and approved by the institutional review board at the center. No important changes were made to initial trial design or inclusion criteria.
Participants
Patients who were older than 18 years with a diagnosis of HCC, either biopsy proven or meeting European Association for the Study of the Liver (EASL) imaging criteria,5 and with Eastern Cooperative Oncology Group performance scores of 0 to 1 and Okuda stage I and II were eligible. Adequate organ function, including creatinine less than 2× the upper limit of normal and bilirubin less than 3.0 mg/dL, was required. Portal vein invasion at any level was allowed as long as liver function was preserved. Patients could have limited extrahepatic disease, such as a few small lung nodules or enlarged regional lymph nodes, at enrollment. Patients were staged by Okuda, Child-Pugh, TMN, and Barcelona Clinic Liver Cancer scores.6 Patients provided written informed consent both for trial participation and at the time of each procedure.
Random Assignment and Masking
Patients were randomly assigned to embolization using either Bead Block (BB; Biocompatibles UK, Farnham, Surrey, United Kingdom) or LC Bead (LCB; Biocompatibles UK), loaded with doxorubicin. Random assignment was stratified by the anticipated number of embolizations for complete treatment (one versus two) as left and right liver are never embolized concomitantly. Enrollment was by the interventional radiologist in the clinic, with random assignments generated by permuted blocks in the institutional database. Because LCB is red/purple when loaded, the operators were not blinded to treatment at the time of embolization; however, the patients and those interpreting the scans were blinded. The study was closed when target enrollment was achieved.
Hepatic Embolization Procedure
The morning of the procedure intravenous (IV) access was established and hydration begun with normal saline. An antiemetic (palonosetron hydrochloride 0.25 mg IV) and antibiotic (cephazolin 1 g IV) were administered. Procedures were performed using conscious sedation or general anesthesia. In the LCB group, doxorubicin 150 mg was loaded onto either 4 or 6 mL of LCB microspheres (37.5 or 50 mg/mL), depending on assessment of a combination of tumor volume and vascularity. The 150-mg dose was chosen as it was commonly used when the study protocol was developed.7-10 The embolization procedure for BB and for LCB is outlined in Figure 1.
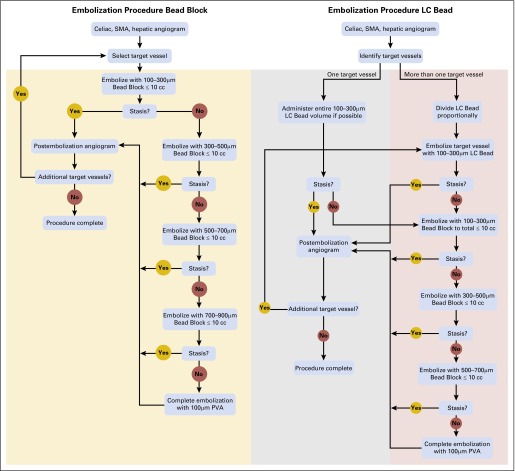
Flow diagram of embolization procedure for Bead Block and LC Bead. PVA, polyvinyl alcohol; SMA, superior mesenteric artery.
Embolizations were performed as selectively as possible, treating individual segmental arteries when feasible. When more than one target branch was present, LCB was divided proportionately, depending on the size and number of vessels to be treated, and administered sequentially into the target vessels, which were then recatheterized and embolized with BB as necessary to achieve stasis identical to that achieved in the BB group. Stasis was defined as the absence of antegrade flow within a vessel such that contrast filling the target vessel persisted, without washout, five cardiac beats after the injection of contrast.11
Complete treatment was defined as the treatment of all tumor that was identified on preprocedure imaging; in the case of multifocal bilobar disease, either the right or left liver was treated initially, embolizing the remaining liver at a second session to complete treatment. Decisions regarding retreatment were made on the basis of multiphase computed tomography (CT) obtained at 3-month intervals.
End Points
The primary end point of the study was the estimate of response to treatment (RTT) of the target lesion(s) using RECIST 1.012 criteria as assessed on multiphase liver CT 2 to 3 weeks after complete treatment. Although RECIST 1.012 are the only validated imaging response criteria, they are insensitive to response at 2 to 3 weeks. After EASL suggestions,5 estimated tumor necrosis was computed. EASL recommends that the reduction in viable tumor volume be used to assess local RTT but does not specify how this should be accomplished.5 We defined categories according to the following criteria assessing enhancement of target tumor post-treatment: complete response, 0%, partial response, less than 50%, and stable disease, greater than 50%. Progressive disease was defined as a greater than 20% increase in enhancing tumor. With the advent of modified RECIST (mRECIST),13,14 an unplanned analysis using mRECIST was also performed. Recent evidence has demonstrated that objective response by either EASL or mRECIST, despite requiring validation, is valuable with regard to predicting survival, in contrast to RECIST.15 Progression by RECIST 1.0 and/or evidence of ≤ 5% necrosis after treatment was considered treatment failure and patients were taken off study. Each multiphase CT was interpreted by an experienced hepatobiliary radiologist (L.H.S. or R.K.D.), who assessed the target lesions that were selected on the pretreatment CT by using RECIST 1.0, applying EASL as well, with retrospective mRECIST assessment performed by R.K.D. Patients were restaged after each imaging study, noting the development of local tumor progression, distant hepatic progression, or extrahepatic progression.
Secondary End Points
Secondary end points included safety, progression-free survival (PFS) and overall survival (OS) as well as an exploratory comparison of response rate. Hospital stay after treatment in each group was recorded. After the initial CT was obtained to assess response, imaging was performed every 3 months for the first year and then at 3- to 6-month intervals, depending on disease stability, as long as the patients remained on study. RTT was determined at 3, 6, 9, and 12 months by both RECIST 1.0 and mRECIST.
Serious adverse events (SAEs) were assessed and reported as defined by Common Terminology Criteria for Adverse Events 3.0.
Statistical Analysis
This was a randomized phase II trial with the primary objective of estimating response within each treatment arm. With 50 patients in each arm, this could be accomplished to within ± 14%. Secondary objectives were to estimate PFS and OS. In addition, an exploratory comparison of RTT, PFS, and OS was also planned.
The primary analysis was based on intent to treat. Patients who were not evaluable (dead or off study) for response were counted as nonresponders. Response rates were estimated with binomial proportions, reported with a 95% CI for the difference, and compared by using Fisher’s exact test. PFS and OS probabilities were estimated using the Kaplan-Meier method and compared using the log-rank test. The number of patients with SAEs were compared by using Fisher’s exact test. All statistical tests were two-tailed with type I error set at 5%.
RESULTS
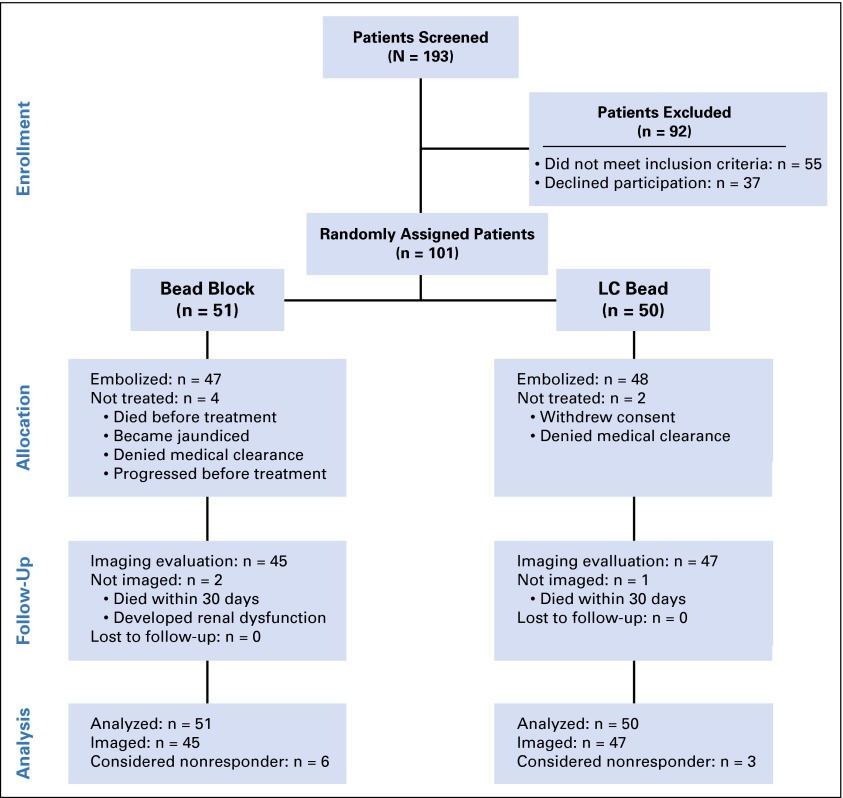
Of 101 patients enrolled between December 2007 and April 2012, 92 underwent 129 embolizations to complete their initial treatment, with a total of 209 embolizations during the entire study. Patients underwent a median of two embolizations, and the average hospital stay was 3.5 days per patient in each group. A mean dose of doxorubicin 132.7 mg was administered to patients in the LCB group in the first treatment cycle. The patient population and random assignment are shown in Figure 4. There were 19 patients with portal vein (PV) involvement; in 42% of these patients, the main PV was involved, in 21%, second-order branches (left or right), and in 36%, segmental branches. There were twice as many patients in the BB group who had PV involvement; however, two were not treated.
At the time of embolization, the risk of particle embolization to the pulmonary circulation by using LCB 100 to 300 μm resulted in one patient assigned to LCB embolization being treated instead with a larger size BB rather than LCB because the study design did not allow for the use of larger LCB. This patient, although treated with BB, was evaluated in the LCB group as this was the initial intent. Patient demographics, tumor characteristics, and stages are presented in Table 1. Patients in both arms had similar clinical and pathologic features.
Table 1.
Demographics and Clinical and Lesion Characteristics
| Characteristic | Patients Receiving Bead Block (n = 51) | Patients Receiving LC Bead (n = 50) |
|---|---|---|
| Age, years (± SD) | 68.33 (± 9.72) | 65.52 (± 11.82) |
 > 65, No. (%) > 65, No. (%) | 22 (43) | 21 (42) |
| Male sex, No. (%) | 37 (73) | 41 (82) |
| Race, No. (%) | ||
 White White | 41 (80) | 38 (78) |
 Asian Asian | 7 (14) | 6 (12) |
 Black Black | 3 (6) | 5 (10) |
| Etiology, No. (%) | ||
 Hepatitis B Hepatitis B | 8 (17) | 7 (14) |
 Hepatitis C Hepatitis C | 15 (29) | 15 (30) |
 Alcohol Alcohol | 8 (17) | 10 (20) |
 Multiple Multiple | 6 (12) | 5 (10) |
| Prior therapy, No. (%)* | 13 (25) | 16 (32) |
| Staging, No. (%) | ||
 Okuda stage Okuda stage | ||
  Stage I (0) Stage I (0) | 39 (76) | 43 (86) |
  Stage II (1 or 2) Stage II (1 or 2) | 12 (24) | 7 (14) |
 Child’s Pugh score Child’s Pugh score | ||
  A (score 5-6) A (score 5-6) | 41 (80) | 45 (90) |
  B (score 7-11) B (score 7-11) | 10 (20) | 5 (10) |
 BCLC stage BCLC stage | ||
  Early stage (A) Early stage (A) | 10 (20) | 12 (24) |
  Intermediate stage (B) Intermediate stage (B) | 22 (43) | 23 (46) |
  Advanced stage (C) Advanced stage (C) | 19 (37) | 15 (30) |
| Lesion characteristic, No. (%) | ||
 Single Single | 12 (24) | 12 (24) |
 ≤ 3 ≤ 3 | 10 (20) | 7 (14) |
 Multifocal Multifocal | 29 (57) | 31 (62) |
 Mean diameter of lesion ± SD, cm Mean diameter of lesion ± SD, cm | 4.7 ± 3.7 | 4.3 ± 3.1 |
 Median, cm (range) Median, cm (range) | 3.4 (0.7-16.9) | 3.5 (0.8-16.9) |
 Mean sum of diameters ± SD, cm Mean sum of diameters ± SD, cm | 8.7 ± 4.5 | 10.8 ± 6.1 |
 Median sum of diameters, cm (range) Median sum of diameters, cm (range) | 7.7 (1.1-21.2) | 8.7 (0.8-27.3) |
| ECOG PS, No. (%) | ||
 0 0 | 44 (86) | 43 (86) |
 1 1 | 7 (14) | 7 (14) |
| Portal vein involvement, No. (%) | 13 (25) | 6 (12) |
| Extrahepatic disease, No. (%) | 20 (39) | 21 (42) |
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; ECOG, Eastern Cooperative Oncology Group; PS, performance status; SD, standard deviation.
Postembolization syndrome consisting of pain, fever, and/or nausea and vomiting was common, occurring after the first treatment cycle in 88% of patients who were embolized with BB and in 84% of patients treated with LCB. There was no significant difference in overall incidence or in any single element of postembolization syndrome. Six of 51 patients (11.7%) in the BB group and six of 50 patients (12%) in the LCB group experienced major complications, according to Society of Interventional Radiology reporting standards,16 within 30 days (Table 2). There was one death in each group within 30 days; one patient with a large infiltrative tumor who was treated with LCB developed liver failure and died 25 days postembolization, and one patient who was treated with BB died unexpectedly at home 2 weeks after first embolization, before treatment was completed.
Table 2.
Complications and Serious Adverse Events
| Adverse Event | Bead Block | LC Bead | Total |
|---|---|---|---|
| Cholecystitis | 2 | 0 | 2 |
| Liver abscess | 1 | 0 | 1 |
| Liver failure (transient) | 1 | 0 | 1 |
| Pancreatitis | 0 | 1 | 1 |
| Puncture site | 1 | 0 | 1 |
| DVT | 0 | 1 | 1 |
| VTE | 0 | 0 | 0 |
| PE | 1 | 4 | 5 |
| Total | 6 | 6 | 12 |
| Number of SAEs (P = .93) | |||
 Grade 3 Grade 3 | 41 | 49 | 90 |
 Grade 4 Grade 4 | 19 | 21 | 40 |
 Grade 5 Grade 5 | 1 | 1 | 2 |
 Total Total | 61 | 71 | 132 |
Abbreviations: DVT, deep vein thrombosis; PE, pulmonary embolus; SAE, serious adverse event; VTE, venous thrombosis embolic.
Overall, 33 patients in each group did not experience an SAE. In the remaining 35 patients, a total of 61 SAEs were reported in the BB group and 71 in the LCB group, most commonly elevation in ALT, AST, or bilirubin immediately postembolization. No difference in the number or grade of SAE (P = .93, Table 2), number of SAEs per patient (P = .75), highest grade of SAE (P = .57), or relationship to treatment (P = .24) was found between the two groups.
The median follow-up time for survivors was 34 months; 73 patients had died at the time of data analysis. There were three RECIST responders in each group, yielding response rates of 5.9% and 6% in the BB and LCB groups, respectively (95% CI for the difference, −9% to 9%; P = 1.0). There was no difference in response rate between the two groups, either within 2 to 3 weeks (the primary end point) by EASL, RECIST 1.0, and mRECIST, or at quarterly follow-up by RECIST 1.0 or mRECIST (Fig 2).
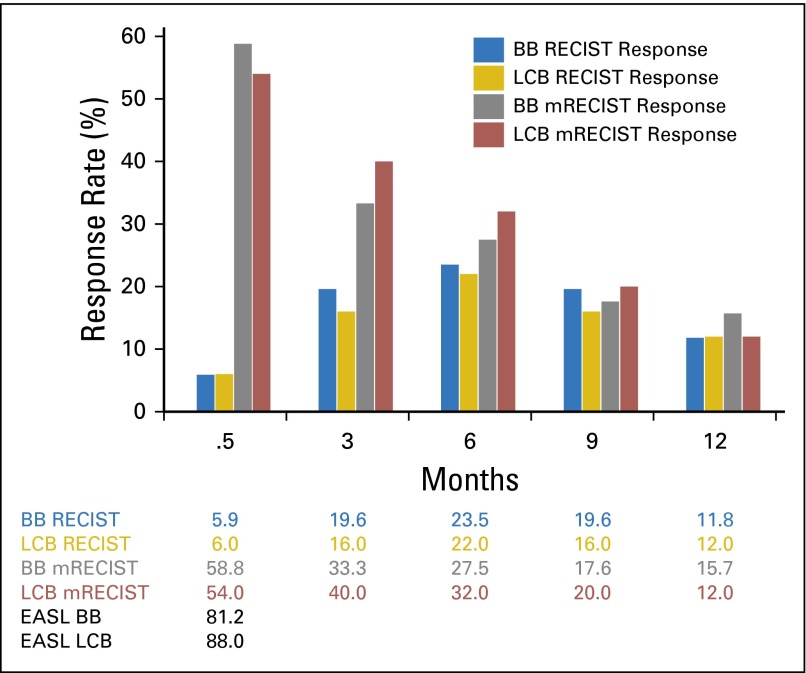
Response rate bar graph. There was no difference between Bead Block (BB) and LC Bead (LCB) at any time point. RECIST 2 to 3 weeks: P = 1.0 (95% CI, −9.3% to 9.1%]; 3 months: P = .79 (95% CI, −11.3% to 18.5%); 6 months: P = .89 (95% CI, −14.8% to 17.9%); 9 months: P = 1.0 (95% CI, −11.3%–18.5%); and 12 months: P = 1.02 (95% CI, −12.9% to 12.4%). Modified RECIST (mRECIST) 2 to 3 weeks: P = .69 (95% CI, −14.5% to 24.1%); 3 months: P = .54 (95% CI, −25.4% to 12.1%); 6 months: P = .67 (95% CI, −22.4% to 13.3%); 9 months: P = .8 (95% CI, −17.6% to 12.9%); and 12 months: P = .77 (95% CI, −9.8% to 17.1%). European Association for the Study of the Liver (EASL) at 2 to 3 weeks: P = .26 (95% CI, −21.7% to 6.5%).
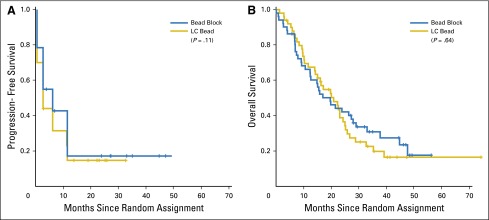
Median PFS by RECIST was 6.2 months for BB and 2.8 months for LCB (P = .11; hazard ratio [HR], 1.36; 95% CI, 0.91 to 2.05; Fig 3A). Median OS was 19.6 months for BB and 20.8 months for LCB (P = .64; HR, 1.11; 95% CI, 0.71 to 1.76; Fig 3B). There was also no difference in OS for the 92 treated and imaged patients, with a median survival of 21.4 months in the BB group and 20.8 months for LCB (P = .64; HR, 1.31; 95% CI, 0.81 to 2.12). Comparing only patients who were treated, there was no difference between BB and LCB in any other measure, including PFS or response rate, at any time point.
DISCUSSION
The design of this study attempted to circumvent problems that are common to trials of embolization for HCC. We isolated the effect that could be ascribed to doxorubicin by assuring that the sole difference in treatment between the two treatment arms was whether the microsphere was loaded with doxorubicin or not. In addition, we selected RTT as the primary end point to avoid favoring one of the treatment arms. Because ischemia results in virtually immediate tumor cell death after HAE, and findings consistent with tumor necrosis can be seen on imaging within 24 hours, we chose the scan that was obtained 2 to 3 weeks after treatment to assess the primary end point. LCB elutes doxorubicin over a period of hours to days, and tissue concentration of doxorubicin can remain elevated for weeks17,18; thus, response may be enhanced beyond the immediate post-treatment time period. For that reason, we also evaluated RTT at 3-month intervals without finding any difference between BB and LCB.
Conventional TACE has garnered widespread popularity for treating unresectable HCC and is the only method of transarterial treatment that has been demonstrated to provide a survival advantage in randomized trials.1,2 Alternate methods include embolization with particles alone, with drug eluting beads, or with 90Y loaded microspheres—to date, the superiority of any one method has not been demonstrated.19 Although ischemia clearly plays a role in the treatment effect seen after embolization, the benefit of added chemotherapy in the embolization mixture has yet to be defined. Both a meta-analysis from 200220 and a systematic review from 200721 concluded that there was no evidence that chemoembolization was more effective than embolization alone. Early reports of systemic doxorubicin failed to demonstrate efficacy for treatment of HCC.22,23 More recently, in a randomized phase II study comparing doxorubicin plus sorafenib with doxorubicin plus placebo,24 the doxorubicin plus sorafenib group, in an exploratory comparison, showed a survival improvement of 13.7 months versus 6.5 months for the doxorubicin plus placebo arm (P = .006). Although this may suggest synergy between doxorubicin and sorafenib compared with the historical control phase III trial that helped establish sorafenib as a standard of care,18 it also supports the lack of activity of doxorubicin in the treatment of HCC. Despite this lack of efficacy, doxorubicin is the most commonly used agent for TACE, alone or in combination with other chemotherapeutic agents.21 When drug-eluting microspheres were developed, they were loaded with doxorubicin to treat HCC.8,25
Llovet et al1 used doxorubicin in their TACE arm, whereas the bland HAE arm used gelfoam for embolization. Gelfoam is a temporary occlusive agent that results in more proximal vessel occlusion. Tumor cell death after HAE results from ischemia; consequently, smaller particles that will block the intratumoral vessels should be more effective and permanent agents are preferred.26 Despite the use of gelfoam alone in the HAE arm, the probability of survival at 1 and 2 years was 75% and 50%, respectively, for HAE, and 82% and 63%, respectively, for TACE, with a mean survival of 25.3 months for HAE and 28.7 months for TACE. This similarity suggests that the therapeutic effect of TACE may primarily be an embolic or ischemic effect and not related to doxorubicin.
Using cisplatin, Lo et al2 also demonstrated a survival benefit for TACE compared with BSC. Sahara et al27 found that TACE with cisplatin, mitomycin C, and epirubicin was more toxic than TACE with epirubicin alone, and resulted in more postembolization hepatic artery injury, but no improvement in radiographic response, PFS, or survival at 1 or 2 years. The one agent common to all of these studies was an embolic. Of interest, the results of TACE seem to be independent of the type or number of chemotherapeutic drugs used, a point emphasized in the systematic review by Marelli et al21 that included 40 TACE studies. If indeed the chemotherapeutic was the active agent, this would be quite unusual, unless it is to be hypothesized that all of the drugs are active against HCC. A similar finding in a systemic chemotherapy trial or meta-analysis would be viewed with skepticism.
Results of this trial are reported on the basis of intent-to-treat by using RECIST 1.0 to report overall RECIST 1.0 progression, not local tumor progression; thus, results will not be comparable to many other reports of embolization for HCC. Nine patients who were either not treated or not imaged, and thus not evaluable, were counted as progressed, which contributes to the low median PFS of 6.2 and 2.8 months in the BB and LCB groups, respectively. Sixty-one patients progressed within the first year, but only nine progressed locally and could rightly be considered to have experienced treatment failure. Tumors of two patients classified by RECIST 1.0 as progressive disease at 2 to 3 weeks on the basis of only larger tumor measurement were completely nonenhancing, or necrotic, by imaging, a well-recognized problem when using RECIST after local regional treatments, which prompted the development of mRECIST in 2010.13 Whereas the 2% mortality rate is higher than reported in some studies, it is within accepted Society of Interventional Radiology parameters and below the threshold level of 4%. Although a 1% mortality rate was reported for patients without extrahepatic disease or PV involvement in studies by Llovett et al1 and Lammer et al,8 rates of 6% to 7% have been reported for more advanced disease.28
Our trial has some of the shortcomings reported in other HCC trials: a small study population, with patients receiving other surgical and systemic therapies before and after enrollment. The results serve as a cautionary reminder of the potential pitfalls that may be encountered with the informal application of therapies available for HCC outside of the domain in which they were defined. The median OS is shorter than reported in previous studies1,2; however, comparisons across trials are fraught with selection bias. This trial included a number of patients with larger tumors, and allowed PV tumor thrombus and oligo-metastatic disease. It is important to note that this is a single-institution study defined by a certain technical expertise, particular methodology, and clinical practice; however, these factors were identical in both arms and, yet, there was no difference in any outcome measure. Our results should be interpreted within the context of a randomized phase II study. It is doubtful that any further efforts will be spent on a randomized phase III trial of this nature because a large trial would be required to detect any small difference that might exist—a difference unlikely to be clinically meaningful. Despite the initial disappointment in evaluating this approach with the addition of sorafenib to TACE,29 future efforts might be better focused on synergistic or adjuvant effects of other therapies added to local treatments. Another approach might be the addition of transarterial or other local therapies to systemic treatment in the metastatic setting.30 Whereas our study was not powered to detect small to moderate differences in outcome, none of the end points approached statistical or clinical significance, calling into question the role of doxorubicin for transarterial therapies.
In conclusion, we report no difference in response, PFS, or OS after treatment with BB versus LCB. Given the comparable safety profile, progression rate, and survival, HAE should continue to be considered a reasonable therapeutic option and an alternative to embolization with doxorubicin-loaded microspheres.
Acknowledgment
LC Bead provided by Biocompatibles UK, Farnham, Surrey, United Kingdom.
GLOSSARY TERMS
| Hepatocellular carcinoma (HCC): | A type of adenocarcinoma, the most common form of liver cancer. |
Footnotes
Supported by Grant No. 1 R21 CA128391-01 from the National Institutes of Health and by Grant No. P30 CA008748 (Cancer Center Support Grant) National Institutes of Health National Cancer Institute.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Presented in part at the 10th Annual American Society of Clinical Oncology Gastrointestinal Cancers Symposium, San Francisco, CA, January 24-26, 2013, and at the 39th Annual Meeting of the Society of Interventional Radiology, San Diego, CA, March 22-27, 2014.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT00539643.
AUTHOR CONTRIBUTIONS
Conception and design: Karen T. Brown, Mithat Gonen, Anne M. Covey, Lynn A. Brody, Gerald P. O’Neill, Lawrence H. Schwartz, Ronald DeMatteo, Ghassan K. Abou-Alfa
Provision of study materials or patients: William R. Jarnagin, Michael I. D'Angelica, Peter J. Allen, Ronald DeMatteo
Collection and assembly of data: Karen T. Brown, Richard K. Do, Anne M. Covey, George I. Getrajdman, Constantinos T. Sofocleous, Kristian N. Johnson, Alessandra R. Garcia, Christopher Beattie, Binsheng Zhao, Lawrence H. Schwartz
Data analysis and interpretation: Karen T. Brown, Richard K. Do, Mithat Gonen, Anne M. Covey, Joseph P. Erinjeri, Kristian N. Johnson, Christopher Beattie, Stephen B. Solomon, Lawrence H. Schwartz, Ronald DeMatteo, Ghassan K. Abou-Alfa
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized Trial of Hepatic Artery Embolization for Hepatocellular Carcinoma Using Doxorubicin-Eluting Microspheres Compared With Embolization With Microspheres Alone
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Karen T. Brown
Consulting or Advisory Role: Surgical Specialties, Guerbet
Research Funding: GE Healthcare
Richard K. Do
No relationship to disclose
Mithat Gonen
No relationship to disclose
Anne M. Covey
No relationship to disclose
George I. Getrajdman
Consulting or Advisory Role: CareFusion
Constantinos T. Sofocleous
Stock or Other Ownership: Sirtex Medical
Honoraria: Surefire Medical, Perseon, NeuWave Medical, Siemens Healthcare Diagnostics
Consulting or Advisory Role: Perseon, Guerbet
Research Funding: HS Medical, NeuWave Medical, Perseon, MedWaves
William R. Jarnagin
No relationship to disclose
Michael I. D'Angelica
No relationship to disclose
Peter J. Allen
No relationship to disclose
Joseph P. Erinjeri
No relationship to disclose
Lynn A. Brody
Stock or Other Ownership: Sirtex Medical, General Electric (I)
Gerald P. O'Neill
No relationship to disclose
Kristian N. Johnson
No relationship to disclose
Alessandra R. Garcia
No relationship to disclose
Christopher Beattie
No relationship to disclose
Binsheng Zhao
No relationship to disclose
Stephen B. Solomon
Leadership: Devicor Medical Products
Stock or Other Ownership: Johnson & Johnson, Progenics, Aspire Bariatrics
Consulting or Advisory Role: GE Healthcare, AngioDynamics, Covidien
Research Funding: GE Healthcare (Inst)
Patents, Royalties, Other Intellectual Property: GE Healthcare patents pending (Inst), Aspire Bariatrics
Lawrence H. Schwartz
Consulting or Advisory Role: Novartis, Celgene, ICON Clinical Research, BioClinica
Ronald DeMatteo
No relationship to disclose
Ghassan K. Abou-Alfa
Consulting or Advisory Role: Astellas Pharma, Celsion, Eli Lilly/ImClone, Exelixis, IntegraGen, Jennerex, MedImmune, Novartis, Vicus Therapeutics, Aduro Biotech (I), AstraZeneca (I), Celgene, Pharmacyclics (I), Sanofi (I), Silenseed (I), Cipla, Array BioPharma (I), BioAlliance Pharma, Boston Scientific, Boston Therapeutics, CASI Pharmaceuticals, Merrimack, Onxeo, Sillajen, Bristol-Myers Squibb (I), EMD Sorono (I), Gilead Sciences (I), Medergy (I), Momenta Pharmaceuticals (I)
Research Funding: Abbott Laboratories (Inst), Amgen (Inst), Bayer AG (Inst), Eli Lilly/ImClone (Inst), Exelixis (Inst), Genentech (Inst), Novartis (Inst), Polaris (Inst), Roche (Inst), Vicus Therapeutics (Inst), CASI Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Polaris
REFERENCES
Articles from Journal of Clinical Oncology are provided here courtesy of American Society of Clinical Oncology
Full text links
Read article at publisher's site: https://doi.org/10.1200/jco.2015.64.0821
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4966514?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1200/jco.2015.64.0821
Article citations
Locoregional Therapies for Hepatocellular Carcinoma in Patients with Nonalcoholic Fatty Liver Disease.
Biomedicines, 12(10):2226, 30 Sep 2024
Cited by: 0 articles | PMID: 39457538 | PMCID: PMC11504147
Review Free full text in Europe PMC
Targeted redox-responsive peptide for arterial chemoembolization therapy of orthotropic hepatocellular carcinoma.
Abdom Radiol (NY), 49(11):3925-3934, 11 Jul 2024
Cited by: 0 articles | PMID: 38990300 | PMCID: PMC11519146
Examining the Efficacy and Safety of Combined Locoregional Therapy and Immunotherapy in Treating Hepatocellular Carcinoma.
Biomedicines, 12(7):1432, 27 Jun 2024
Cited by: 1 article | PMID: 39062006 | PMCID: PMC11274263
Review Free full text in Europe PMC
Embolization alone is as effective as TACE for unresectable HCC: systematic review and meta-analysis of randomized controlled trails.
BMC Gastroenterol, 24(1):195, 07 Jun 2024
Cited by: 0 articles | PMID: 38849765
Review
Transarterial embolization is an acceptable bridging therapy to hepatocellular carcinoma prior to liver transplantation.
World J Transplant, 14(2):90571, 01 Jun 2024
Cited by: 0 articles | PMID: 38947974
Go to all (185) article citations
Other citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT00539643
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Transcatheter arterial chemoembolization with doxorubicin-eluting superabsorbent polymer microspheres in the treatment of hepatocellular carcinoma: midterm follow-up.
J Vasc Interv Radiol, 25(2):248-55.e1, 02 Dec 2013
Cited by: 12 articles | PMID: 24295569
Degradable starch microspheres versus ethiodol and doxorubicin in transarterial chemoembolization of hepatocellular carcinoma.
J Vasc Interv Radiol, 25(2):240-247, 28 Nov 2013
Cited by: 17 articles | PMID: 24291001
Transarterial Chemoembolization Using 100-μm Drug-Eluting Microspheres in Patients with Hepatocellular Carcinoma: A Prospective Study and Midterm Follow-up.
J Vasc Interv Radiol, 31(11):1784-1791, 03 Oct 2020
Cited by: 2 articles | PMID: 33023805
Conventional transarterial chemoembolization vs microsphere embolization in hepatocellular carcinoma: a meta-analysis.
World J Gastroenterol, 20(45):17206-17217, 01 Dec 2014
Cited by: 26 articles | PMID: 25493037 | PMCID: PMC4258593
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NCI NIH HHS (2)
Grant ID: P30 CA008748
Grant ID: R21 CA128391