Abstract
Free full text

A randomized clinical trial aimed at preventing poor psychosocial and glycemic outcomes in teens with type 1 diabetes (T1D)
Abstract
Adolescents with type 1 diabetes have an increased risk for a variety of emotional and behavioral challenges as well as negative diabetes outcomes. This study was designed to compare the effectiveness of a depression-prevention, resilience promotion program with an advanced diabetes education program. Each program consisted of 9 group-based sessions. There were 264 adolescents enrolled in this multi-site randomized clinical trial. The primary outcomes were depressive symptoms and glycemic control; secondary outcomes included resilience skills, diabetes management and adherence, and diabetes-specific distress. The goal of the present paper is to describe the study design, the intervention, and the baseline characteristics of the sample. Preliminary data suggests that enrollment, randomization and retention were successful. Longitudinal follow-up and examination of mechanisms of action as they relate to psychosocial and glycemic outcomes will be explored in the future.
1. Introduction
Adolescents with type 1 diabetes (T1D) must balance a complex daily treatment regimen while also facing the emotional, social and academic demands of this developmental period. Not surprisingly, adolescents are at increased risk for anxiety and depressive symptoms,1–5 poor coping and problem-solving skills,6,7 poor regimen adherence,8,9 and negative diabetes-specific health outcomes.10–12 The mental and physical health risks of T1D add to its already staggering economic burden; the annual cost of diabetes in the United States for direct medical care exceeds $176 billion (a 41% increase from estimates in 2007)13 and individuals with diabetes have twice the healthcare costs of their peers without diabetes.13 The economic burden for individuals with T1D is greater than for those with T2D.14,15 The risk of depressive symptoms in individuals with diabetes is much higher than in the general population, with rates ranging between 13 and 33%,16–19 yet only 4% of youth with depressive symptoms receive mental health services.19 Moreover, individuals with both depression and diabetes incur 4.5 times the health care costs as those with diabetes alone.17,18,20
A handful of psychological interventions targeting adolescents with type 1 diabetes’ poor behavioral and emotional functioning demonstrate beneficial effects on disease management and psychosocial outcomes.21–24 These interventions also demonstrate prolonged benefit in preventing the development of later psychosocial difficulties. However, no prevention programs exist that equip adolescents with the behavioral skills and cognitive strategies shown to reduce depression and typical correlates of depressive and distress symptoms (e.g., suboptimal diabetes management and glycemic control). Most clinical interventions aimed at preventing depression have been developed for healthy youth,25 and none have been adapted for adolescents with T1D. The Penn Resilience Program (PRP)26–33 is a depression-prevention program that has been widely studied, both as a universal and as a selective intervention.27,28 PRP interventions have been led by both professionals and members of the lay community26,27 and it has been implemented in schools and in community mental health centers.26,27 This well-established prevention program promotes resilience and prevents depression. Our team adapted the intervention to include diabetes-specific content. The focus of the intervention is on preventing depression through a resilience promoting curriculum, which offers the potential to fundamentally change participants’ risk for depression and set adolescents on a trajectory toward improved adherence and health outcomes.34,35 Our research team adapted and pilot tested the PRP for adolescents with T1D (PRP T1D)36 and then integrated the feedback, rewrote the intervention, and pursued a randomized, controlled clinical trial. The goal of the Supporting Teen Problem Solving (STePS) randomized clinical trial was to test the efficacy of the intervention and to compare the effects of PRP T1D vs an advanced diabetes educational intervention (EI) on resilience characteristics, depressive symptoms, adherence behaviors, and glycemic outcomes. An advanced diabetes educational intervention was selected as the comparison group for two reasons: 1) to be consistent with a comparative effectiveness model for testing interventions and 2) to match for attention and time in intervention, versus standard care for diabetes. The present paper describes the study design, the intervention, and the baseline characteristics of the sample.
2. Primary research goals
The primary hypotheses being tested in the STePS study are: 1. Youth who receive the PRP T1D intervention will demonstrate fewer depressive symptoms and lower hemoglobin A1c levels compared to youth receiving the EI. 2. Youth who receive the PRP T1D intervention will demonstrate improved resilience skills, improved adherence, and reduced diabetes-specific emotional distress compared to youth receiving the EI. We further hypothesize that resilience and adherence function as mechanisms of change for both preventing depression and improving metabolic control. Our longitudinal, randomized, controlled design allows for documenting these hypothesized mechanisms of change that impact critical psychosocial and health outcomes in youth with T1D. Further, we will be able to test when effects occur as we hypothesize that some effects will be more immediate (e.g., improved resilience skills) versus those that take longer to appear (e.g., changes in glycemic control).
3. Study design
3.1 Conceptual Framework
Programs targeting the prevention of depression are limited to youth without chronic medical conditions or diseases.25,26,37,38 Among the programs most widely studied and with the greatest efficacy39 is the PENN Resiliency Program (PRP). PRP is unique in that it has been used as both a universal and selective prevention program, has been led by mental health professionals and members of the lay community, and has been evaluated in school, primary care, and juvenile detention settings. Over 15 studies have been published on PRP with over 2,000 children and adolescents.29 The average effect size on depression was 0.09 post-intervention (calculated as pooled effect size in meta-analysis), consistent with findings in other prevention studies.30,40,41 Importantly, and in contrast to other programs, PRP’s effects increase over time to 0.32 at six months, showing that the treatment effects of PRP continue to grow.31 In contrast to the increasingly robust PRP effect, a meta-analysis of 30 non-PRP prevention studies25 reported a weighted overall mean effect size of 0.16 at post-treatment and 0.11 at 6 month follow up, indicating relatively stable effects. Depression prevention studies suggest that findings are more robust when individuals at high risk for depression are targeted (youth with T1D are at increased risk) and when offered to older teens (PRP T1D is targeted to high school students), underscoring the importance of our targeted population.25,30,40,41
PRP teaches cognitive-behavioral and social problem-solving skills in a group format.26,33,33 The cognitive risk factors implicated in depression and targeted in PRP are: linking beliefs, feelings, and behaviors; identifying one’s own cognitive framework/thinking style; the impact of one’s thinking styles on decision-making; and challenging negative thinking by evaluating the accuracy of one’s beliefs. The behavioral risk factors implicated in depression and targeted in PRP are: problem-solving techniques such as negotiating, assertiveness, and decision-making; and coping skills such as relaxation techniques and seeking social support. Depression prevention occurs via promoting resilience, which consists of four key constructs: a sense of hopefulness, an optimistic explanatory style, effective coping strategies, and positive problem solving skills. PRP promotes resilience by teaching one to challenge hopeless thoughts, think flexibly and accurately about challenges, develop adaptive problem-solving strategies, and use social supports. The cognitive-behavioral and the social-problem solving components are complementary and integrated throughout the prevention program.
In addition to the effectiveness of PRP in building resilience skills, evidence suggests that such skills serve as a protective mechanism for important health outcomes. Studies in adults have linked pessimistic explanatory styles (a primary target of PRP) and poor health outcomes. Individuals with pessimistic explanatory styles have a higher risk of death after a cancer diagnosis42 and after a coronary event.43,44 Pessimistic explanatory styles are also linked to a passive approach to health needs, leading to worse outcomes in middle adulthood.45,46 In contrast, optimistic explanatory styles are associated with positive health outcomes, resulting in less illness, fewer physician visits, more exercise, and healthier eating.43 Since PRP targets explanatory style, which is modifiable, it can decrease pessimistic explanatory styles and improve health behaviors and health outcomes. The current study is the first to test an adaptation of PRP for teenagers with a chronic disease, directly test the link between resilience and health outcomes, and do so in a randomized, controlled trial. This design allows for a direct examination of the causal effects of explanatory style on health outcomes.
3.2 Recruitment Procedures
Recruitment occurred through IRB-approved mailings, in-clinic recruiting, and postings on hospital websites. The study was registered on the clinicaltrials.gov website (NCT01490619). Telephone screening took place to ensure participants met the inclusion criteria:14–18 years old, diagnosis of T1D according to ADA criteria for at least 1 year, daily insulin dosing of at least 0.5 units per kilogram per day, fluent in English, and provide assent to participate. Exclusion criteria included: other chronic, physical disease or condition except for celiac or thyroid disease, diagnosis of major depressive disorder, current treatment with an antidepressant, diagnosis of major mental disorder (e.g., bipolar disorder, psychosis, anorexia nervosa), diagnosis of developmental disorder (e.g., mental retardation, autism, Asperger’s), or ward of the state. Written informed consent was obtained from the teenager’s primary caregiver and written informed assent was obtained from the teenagers. Home visits were then scheduled to collect baseline data, including the K-SADS (a diagnostic interview for depression) to insure that the teenager was not clinically depressed and not receiving and antidepressant treatment. Of the 668 families who contacted the study staff, 264 teens were enrolled and randomized. One hundred sixty nine teens did not meet eligibility requirements (50 were in treatment for depression). One hundred forty four families decided not to participate after initial contact due to concerns about time commitment or travel, and 79 teens and/or their families were unreachable after initial contact with us. Nine families contacted the study staff after recruitment had ended. Three participants were younger siblings of enrolled and randomized participants; they participated in assessments and study groups of their siblings, but their data were not used.
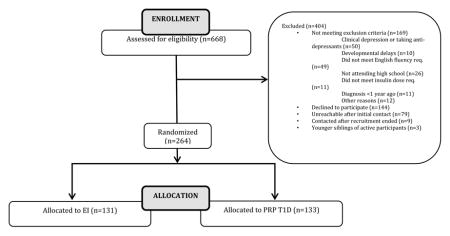
3.3 Randomization
Once enrolled, teens were randomized to either PRP T1D or EI and stratified on A1c (< 8.5% or > 8.5%). Randomization was based on randomized block sizes of 4 and 8 to ensure equal allocation to each treatment arm. An investigator held the randomization and no staff with direct contact with participants through assessments or interventions saw assignments before they were revealed. One hundred thirty three teenagers were randomized to PRP T1D and 131 were randomized to EI. There were no differences in age, gender, race, age at diagnosis, method of insulin delivery or hemoglobin A1c (See Table 1 for baseline characteristics) between the two groups.
TABLE 1
| VARIABLE | OVERALL (N=264) | EI GROUP (N=131) | PRP T1D GROUP (N=133) |
|---|---|---|---|
|
| |||
| AGE (years) | 15.7±1.1 | 15.7±1.1 | 15.7±1.1 |
|
| |||
| GENDER (N, % FEMALE) | 158 (60%) | 79 (60%) | 79 (60%) |
|
| |||
| RACE/ETHNICITY (N, %) | |||
| White, not of Hispanic Origin | 178 (68%) | 88 (68%) | 90 (69%) |
| African American, not of Hispanic Origin | 33 (13%) | 16 (12%) | 17 (13%) |
| Hispanic/Latino | 26 (10%) | 13 (10%) | 13 (10%) |
| Asian American/Pacific Islander | 6 (2%) | 3 (2%) | 3 (2%) |
| Reported as Multi-Racial/Ethnic | 17 (6%) | 10 (8%) | 7 (5%) |
| Did not Report | 4 (1%) | 1 (<1%) | 3 (1%) |
|
| |||
| DURATION OF T1D (years) | 6.9±4.0 | 6.5±3.9 | 7.3±4.2 |
|
| |||
| A1C (%) | 9.1 (1.9) | 9.1 (2.0) | 9.1 (1.9) |
|
| |||
| METHOD OF INSULIN DELIVERY (% pump) | 68% | 67% | 70% |
|
| |||
| FAMILY INCOME (N, %) | |||
| Less than $50,000 | 39 (15%) | 17 (13%) | 22 (17%) |
| $50,000 – $75,000 | 38 (15%) | 18 (14%) | 20 (16%) |
| $75,000 – $100,000 | 43 (17%) | 21 (17%) | 22 (17%) |
| $100,000 – $150,000 | 50 (20%) | 26 (21%) | 24 (19%) |
| More than $150,000 | 63 (25%) | 33 (25%) | 31 (25%) |
| Did not report | 19 (8%) | 12 (10%) | 7 (6%) |
|
| |||
| FAMILY STRUCTURE (N, % with two parents in home) | 210 (80%) | 112 (85%) | 98 (74%) |
|
| |||
| EDUCATIONAL LEVEL OF PRIMARY CAREGIVER (N, % with college degree) | 158 (60%) | 74 (56%) | 84 (63%) |
3.4 Intervention
PRP T1D was led by master’s level clinicians whose specific training included20 hours per week for three months learning the manual, delivering it to test subjects, and role-playing scenarios with the research team. Interventionists were supervised by the study PIs. To maintain treatment fidelity, all intervention sessions were audio-taped. The PIs listened to 20% of all sessions to insure that content was delivered in keeping with the study protocol. No significant deviations from either the intervention or control group protocols were noted. In addition, retraining of all interventionists (both intervention and control group leaders) occurred once per year for two years to maintain treatment fidelity. Interventionists had an Instructor’s Guide to help them proceed through PRP T1D session topics and promote group discussion and interaction throughout. PRP T1D consistent of 9 bi-weekly sessions, each lasting between 90 and 120 minutes. As described above in the conceptual framework, the topics for the PRP T1D program included: 1) Resilience through connecting adversity, beliefs and consequences, 2) Catching errors in logic and understanding thinking errors, 3) Learning self-disputing skills by understanding explanatory styles and creating alternative explanations, 4) Learning self-disputing skills by evaluating the evidence and generating alternatives, 5) Learning to put beliefs into perspective by understanding the worst case, best case and most likely scenarios, 6) Learning relaxation and focusing techniques, 7) Applying resilience skills in real-time situations, 8) Learning assertiveness skills in social communication, and 9) Reviewing and consolidating resilience skills. All participants were given a Student Workbook that reviewed the concepts from each session and also contained homework that reinforced the concepts discussed each week.
Educational Intervention
Adolescents randomized to receive EI received 9 bi-weekly sessions, matching the dose and attention of the PRP T1D condition. Sessions were delivered by certified diabetes educators who received intensive training by the study PIs, with re-training occurring once per year for two years to maintain treatment fidelity. Nurse educators had an Instructor’s Guide to help them proceed through the EI topics and promote group discussion throughout. The topics included: 1) A diabetes overview, including differences between T1 and T2 diabetes as well as symptoms and treatment of out of range numbers; 2) A review of general nutrition for teenagers, strategies for carbohydrate counting, and managing challenging foods; 3) Why exercise is a recommended aspect of diabetes care, its impact on blood sugars, and strategies for preventing low blood sugars; 4) A review of the different types and actions of insulin, the importance of site rotation and strategies for storage; 5) Strategies for blood glucose monitoring and pattern management; 6) Strategies for prevention, detection and treatment of acute complications; 7) A review of diabetes technology including insulin pumps and continuous glucose monitors; 8) A review of current research on the cure for diabetes; and 9) Reviewing and consolidating diabetes education skills.
3.5 Measures
Data are collected both on-line and in patient’s homes at baseline (0 months), post-intervention (4.5 months), and during the surveillance period (8, 12, 16, 28 and 40 months). Measures assess two primary outcomes (depressive symptoms and glycemic control) and two mechanisms of change (resilience and adherence). The assessment battery is listed in Tables 2 and and3.3. From a general perspective, the rationale guiding the selected measures includes: 1) measuring both general and diabetes-specific domains ise vital for a comprehensive examination of the teen’s functioning; 2) using reliable, valid, and easy-to-administer measures protects participants, reduces burden, and provides greater likelihood of understanding these complex relationships in statistical analyses; and 3) process variables, outcomes, and covariates are important to assess in analyses. To keep participant burden at a manageable level and to increase retention rate, all families were invited to complete the questionnaires on a secured web-site that could be accessed while with the research study staff, or while at home, work, in a public library or while in clinic. Metabolic control was assessed by collecting a small sample of blood from the participant and sending it to the Diabetes Diagnostic Laboratory at the University of Missouri (http://www.diabetes.missouri.edu/). This laboratory served as the reference laboratory for the DCCT and NHANES III and IV studies.
Table 2
Primary Set of Measures, All Completed By Adolescent
| Measure | Construct Measured / Relevant Points | Reliability in Study Sample |
|---|---|---|
| PRIMARY OUTCOME: Depressive Symptoms | ||
| Children’s Depression Inventory 47 | Widely used measure of depressive symptoms in youth. Total score and 5 subscale scores can be generated. | Cronbach’s alpha = 0.87 |
| PRIMARY OUTCOME: Glycemic Control | ||
| HbA1c measurement | Small sample of capillary blood is obtained during visit with kit provided by central laboratory. “Gold standard” measure of glycemic control. | N/A |
| MECHANISM OF CHANGE: Resilience (five indicators) | ||
| Resiliency Scales for Children and Adolescents | Assesses perceived mastery of everyday activities and social relatedness with peers and family. Total resilience score and subscale scores on relatedness and mastery. | Total score alpha = 0.97 Relational score alpha = 0.94 Mastery score alpha = 0.95 |
| Diabetes strengths and resilience measure | Assesses perceived competence for managing the T1D regimen, adapting to unpredictability of diabetes and seeking help and support for diabetes challenges. | alpha=.89 |
| Hopelessness Scale for Children 48 | Assesses degree of hopelessness. | alpha = 0.71 |
| Automatic Thoughts Questionnaire 49– 51 | Assesses maladaptive thinking styles and automatic negative thoughts. | alpha = 0.97 |
| Coping Efficacy Questionnaire 52 | Assesses coping skills, specifically satisfaction with handling past problems and anticipated effectiveness in handling future problems | alpha = 0.90 |
| Social Problem Solving Inventory 53 | Assesses social problem solving, awareness of ability to solve problems and efficacy of problem solving. | alpha for scales = 0.67 to 0.82; total score alpha = 0.72 |
| Diabetes Problem Solving Inventory 7 | Assesses diabetes-specific problem solving. It is a structured interview. Coding manual used for scoring. | N/A |
| MECHANISM OF CHANGE: Behavioral Adherence (two indicators) | ||
| Self-Care Inventory54 | Assesses level of adherence to diabetes self-care recommendations over the previous 1 – 2 months. | alpha = 0.77 |
| Blood glucose monitoring frequency | Downloads of meters to get an estimate of BGM frequency. BGM frequency is a strong, objective indicator of adherence.48,49 | N/A |
Table 3
Additional Measures for Screening, Exploratory Outcomes, and Covariates
| Measure | Construct Measured / Relevant Points | Reliability / Validity |
|---|---|---|
| SCREENING: Identify major depressive disorder and screen out | ||
| K-SADS diagnostic interview 55 | Mood disorders section. Provides designation whether adolescent has major depressive disorder. | N/A |
| EXPLORATORY PSYCHOLOGICAL OUTCOMES: Anxiety, diabetes-specific distress, family conflict | ||
| State-Trait Anxiety Inventory 5,56 | Anxiety measure with state (current) and trait (dispositional) symptoms. Previously used in adolescents with T1D. | State alpha = 0.93 Trait alpha = 0.93 |
| Problem Areas In Diabetes–Teen version 57,58 | Measure of diabetes-specific emotional distress in adolescents with diabetes and their parents. | teen-reported alpha = 0.95 parent-reported alpha = 0.96 |
| Diabetes Family Conflict Scale59 | Measure of parent-adolescent conflict around diabetes-specific tasks. | alpha = 0.91 for youth and 0.87 for caregivers |
| COVARIATES | ||
| Services for Children and Adolescents 60 | Comprehensive interview documents services used across mental health, primary care, school, and community settings. This covers service uptake by teens and caregivers. Developed at NIMH and used extensively in clinical trials. | N/A |
| Center for Epidemiologic Studies – Depression 61 | Completed by caregivers as an index of caregiver mental health. Prior research shows depression prevention efforts are less effective when the teen’s caregiver is depressed. 55 | alpha = 0.87 |
| DKA, Hospitalizations, Severe Hypoglycemia | Obtained at clinic visit via participant report and chart review. Measures other diabetes-specific health outcomes. | N/A |
| Family Information Survey | Caregiver completed. All relevant demographic characteristics are collected (e.g. age, gender, ethnicity, family composition). | N/A |
3.6 Baseline Data
Demographic and Clinical Characteristics
As seen in Table 1, we recruited a racially diverse sample, with 30% of our sample identifying as a racial or ethnic minority group member. Characteristics across family structure and education are similar to what would be expected in the larger population, but also reflect wider variability. This may be due to recruiting in two metropolitan areas with different costs of living. In addition, our sample has a high rate of insulin pump use (68%), however, this is consistent with the T1D exchange data.
Participation Rates
Of the 264 adolescents enrolled and randomized, more than 86% completed at least 6 of the 9 sessions. There were no differences in completion between the PRP T1D group and the EI group. Two hundred thirty five of the 264 remain actively participating (88%). Nine of the 264 adolescents dropped out and do not want to be contacted by study staff (3.4%), and 22 of the 264 stopped participating during the course of the intervention sessions but are continuing to complete the online SNAP survey system for data collection (8.3%).
Primary Outcomes
At baseline, our sample’s scores on the Children’s Depression Inventory was below the national norms, suggesting that we did, indeed, recruit a non-depressed group of adolescents for our depression prevention intervention. Our sample is consistent with national norms with respect to metabolic control for teenagers in high school (See Table 4).
Table 4
Baseline Characteristics, Primary Outcomes:
| VARIABLE | OVERALL | EI Group | PRP T1D Group | NORMS |
|---|---|---|---|---|
| Depression (CDI) | 7.7 (6.2) | 7.9 (6.3) | 7.6 (6.1) | 9.09 (7.04) Higher=more depressed |
| HbA1c | 9.1 (1.9) | 9.1 (2.0) | 9.1 (1.9) | 9.0 (1.7) T1D exchange Higher=worse metabolic control |
Mechanisms of Change
At baseline, our sample appears highly resilient. They have adaptive cognitions, are hopeful about the future, are able to engage in typical levels of social and diabetes-specific problem solving for youth their age, have similar levels of diabetes-specific distress as their peers, and report high levels of adherence to their diabetes regimen. One note about Table 5 values is that 4 of the 264 participants did not complete every survey in the baseline assessment battery. The remaining 260 completed every survey item. The total amount of missing data is less than 1% and no values were imputed.
Table 5
Baseline Characteristics: Mechanisms of Change
| VARIABLE | OVERALL | CONTROL | TREATMENT | NORMS |
|---|---|---|---|---|
| Relational Resilience | 98.7 (14.4) | 99.0 (14.8) | 98.4 (14.1) | 50.45 (9.75) Higher = better |
| Mastery Resilience | 80.2 (12.7) | 80.4 (11.3) | 80.0 (14.0) | 51.3 (9.65) Higher=better |
| Diabetes Resilience | 49.0 (7.9) | 49.0 (7.2) | 48.9 (8.6) | Higher=better |
| Coping Efficacy | 24.7 (4.2) | 24.8 (4.0) | 24.6 (4.4) | 21.87 (3.18) Higher=better |
| Adaptive Cognitions | 49.3 (21.1) | 49.2 (20.3) | 49.4 (21.9) | 68.5 (29.7) Higher=worse |
| Hopelessness | 2.8 (2.5) | 2.6 (2.3) | 2.9 (2.7) | 5.2 (3.2) Higher=worse |
| Social Problem Solving Inventory (total standard score) | 104.1 (13.4) | 103.6 (12.5) | 104.6 (14.3) | 100 (15) Higher=better |
| Diabetes Problem Solving Inventory–Hypoglycemia Score | 7.7 (1.5) | 7.6 (1.6) | 7.8 (1.5) | 6.5 (1.7) for 9– 14 year olds (only norms available) |
| Diabetes Problem Solving Inventory–Hyperglycemia Score | 7.8 (1.5) | 7.8 (1.5) | 7.9 (1.6) | 6.5 (1.7) for 9– 14 year olds (only norms available) |
| Self-Care Inventory | 54.1 (7.8) | 54.7 (7.1) | 53.4 (8.5) | 37.9 (6.6) Higher=better |
| BGM frequency (daily average past 2 weeks) | 3.7 (2.4) N=236 | 3.5 (2.2) N=116 | 3.88 (2.5) N=120 | |
| Diabetes specific emotional distress (Youth) | 73.1 (26.7) | 74.2 (25.0) | 72.1 (28.3) | 65.0 (25.9) Higher=worse |
| State Trait Anxiety Inventory (State) | 33.0 (10.7) | 33.2 (10.3) | 32.9 (11.2) | 30.0 (5.0) Higher=more anxiety |
| State Trait Anxiety Inventory (Trait) | 37.6 (10.7) | 37.9 (11.0) | 37.2 (10.4) | 32.1 (7.0) Higher=more anxiety |
| Diabetes specific emotional distress (Parent) | 75.1 (27.0) | 76.0 (27.3) | 74.2 (26.9) | 74.0 (27) Higher=worse |
| Diabetes Family Conflict (youth) | 27.7 (7.1) | 27.6 (6.0) | 27.7 (8.1) | 24.4 (5.0) Higher=more conflict |
| Parent CESD | 8.7 (7.9) | 8.4 (7.3) | 9.0 (8.5) | 11.7 (4.6) Higher=more depressed |
| Diabetes Family Conflict (Parent) | 27.4 (6.0) | 27.4 (5.9) | 27.4 (6.2) | 24.0 (3.9) Higher=more conflict |
Overall, the sample recruited for this randomized controlled trial seems psychologically well-adjusted. The teenagers show low symptoms of depression or anxiety, high levels of resilience and adaptive cognitions, and high levels of social and diabetes-specific problem-solving. With respect to diabetes-specific outcomes, they have similar metabolic control to the normative population and report similar levels of diabetes--specific emotional distress as the normative group. Adherence also appears to be similar to same-aged peers, particularly for youth largely using insulin pumps. Similarly, the parents seem psychological well-adapted, with low levels of depressive symptoms and low levels of baseline diabetes-specific family conflict. In sum, the study sample appears well suited for a depression prevention trial given absence of significant psychological distress on both the family and individual levels.
3.7 Planned Statistical Analyses and Sample Size
Our data analysis plan relies on a structural equation modeling framework to simultaneously allow for the examination of (a) individual change over time, (b) group change over time, and (c) mechanisms of change for the treatment effects of interest. Because we will have seven time points at study completion, we will be able to plot the course of both depressive symptoms and glycemic control at the individual and group level over time using longitudinal models for change (see, e.g., Singer & Willett, 2003)62, examining the plausibility of both linear and nonlinear trajectories for our outcomes over the 40-month study time span. We expect that treatment effects comparing our two treatment arms on these outcomes will manifest themselves at different times. Specifically, we expected to see significant differences on resilience outcomes between groups immediately post-intervention. We further expected that the changes in resilience will lead to depression prevention and will be observed first at 8 months due to the significant separation of trajectories of depressive symptoms between the groups; EI symptoms will increase while PRP T1D will stay the same. Depression prevention will lead to improved adherence, observed at 12 months. As depression, a major barrier to improved adherence is prevented, movement toward improved glycemic control will be observed at the next time point, 16 months. Maintenance of these effects will be observed at 28 months. The collection of our posited mechanisms of change and outcomes over multiple time points will also allow for the examination of state-of-the-art longitudinal mediation models (see, e.g., Cole & Maxwell, 2003 63) to examine the plausibility and strength of the mechanisms of interest.
Our sample size is sufficient for this approach as it is predicated upon several important considerations. First, we are conducting intent-to-treat analyses that employ an analysis of covariance on the posttest where the pretest is the covariate, an effect size of 0.32σ at 8 months for CDI total score (based on the results of the PRP), and correlations of 0.6 between (1) the pretest and the 8-month CDI total score and (2) the pretest and the 12-month follow-up measure for A1c (both correlations being conservative estimates, based on our own prior research referenced earlier). Second, we assumed an attrition rate of 15% across the study, 80% power, and an α2-sided = 0.025 because there are two primary endpoints. Furthermore, this sample size is sufficient because it does not account for the multiple measurement occasions obtained for depressive symptoms and A1c, which will increase statistical power through their inclusion in the statistical models.
3.8 Discussion
The purpose of this paper is to describe the study design, intervention, and the baseline characteristics of teenagers who participated in an intervention designed to prevent depression through a resilience promoting curriculum. Overall, our sample consists of remarkably resilient youth who report a high level of adherence to their diabetes regimen tasks and who are psychiatrically healthy. The parents of the participants also appear to be well-adjusted. While this relatively well-adjusted sample of adolescents may not be an accurate reflection of the entire population of teens with T1D, it may be an accurate representation of teens and families not experiencing psychological distress and willing to sign up for a clinical trial.
Results also highlight similar baseline characteristics and scores between groups. This suggests that randomization worked and that later observed changes on the outcomes of interest can be tracked back to our primary covariate – intervention status. One cautionary note for us to consider is that while study participants do appear to be an appropriate sample for a depression prevention program (i.e., not depressed or psychologically distressed), their high-functioning may also indicate pre-existing resiliency skills. Thus, it is possible that those skills may not dramatically improve during longitudinal examination and instead, these skills may be reinforced with the intervention. As longitudinal data are examined, this will become more clear as mechanisms of change are investigated. With respect to recruitment and enrollment, our participants appear to be representative of T1D population, with the key exception that our sample shows greater ethnic diversity than is typical for youth with T1D. Specifically, while 30% of our sample identified as a racial or ethnic minority, almost 80% of youth in the T1D exchange identify as Non-Hispanic Caucasian.
Our research team engaged in many strategies aimed at maintaining participation in the intervention and reducing attrition. We provided graduated gift-card incentives, we offered home visits to collect data, and we offered multiple sites for families to choose from for participating in the intervention programs, therefore reducing potential barriers to attendance. Moreover, we offered the interventions during Sunday evenings, a time that was most highly requested by participants and their families. We also collected information regarding caregiver and participant work, home and cell phone numbers as well as e-mail addresses to stay in touch over time in addition to obtaining contact information for at least one additional individual who would know how to find the family if they moved. In addition, we created a study website to house the on-line study surveys in a convenient and central location. We also held celebratory parties for all participants (separated by group assignment) at the end of the interventions to keep families informed about our study progress and to thank them for their participation. It is our belief that all of these efforts led to our very small 3.4% drop out rate and our 8.3% rate of families who chose to not fully participate in the intervention sessions but were willing to continue completed study measures over time.
Our team adapted an empirically supported intervention (PRP) by adding diabetes-specific content and then pilot-testing and integrating feedback in an iterative fashion before launching this multi-site randomized control trial. This innovative intervention offers a unique blend of problem solving and CBT-based components with diabetes-specific examples in a group format. Moreover, our advanced diabetes education comparison group matched both dose and frequency of the PRP-T1D, enabling us to ascribe any observed differences to the intervention itself. As this study was largely framed within a comparative effectiveness approach to clinical trials and we wanted to ensure that participants in the control group received more than standard care and diabetes education they can receive as part of diabetes care, positive differences between intervention and control groups should be even more compelling. In sum, our preliminary data suggest we were successful enrolling, randomizing, and retaining participants. As we proceed with longitudinal follow-up and examination of mechanisms of action as they relate to metabolic and psychosocial outcomes, efforts will continue to retain and systematically evaluate this group of teenagers as they age to emerging adults.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.cct.2016.05.006
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4969210?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.cct.2016.05.006
Article citations
Effectiveness of resilience-promoting interventions in adolescents with diabetes mellitus: a systematic review and meta-analysis.
World J Pediatr, 19(4):323-339, 19 Dec 2022
Cited by: 2 articles | PMID: 36534296 | PMCID: PMC9761642
Review Free full text in Europe PMC
Diabetes Management after a Therapeutic Education Program: A Qualitative Study.
Healthcare (Basel), 10(8):1375, 24 Jul 2022
Cited by: 4 articles | PMID: 35893197 | PMCID: PMC9394246
Resilience Interventions Conducted in Western and Eastern Countries-A Systematic Review.
Int J Environ Res Public Health, 19(11):6913, 05 Jun 2022
Cited by: 4 articles | PMID: 35682495 | PMCID: PMC9180776
Review Free full text in Europe PMC
Do baseline resilience profiles moderate the effects of a resilience-enhancing intervention for adolescents with type I diabetes?
Health Psychol, 40(5):337-346, 01 May 2021
Cited by: 3 articles | PMID: 34152787 | PMCID: PMC8363191
Examining Indirect Effects of Anxiety on Glycated Hemoglobin via Automatic Negative Thinking and Diabetes-Specific Distress in Adolescents With Type 1 Diabetes.
Can J Diabetes, 45(5):473-480, 11 May 2021
Cited by: 7 articles | PMID: 34176611 | PMCID: PMC8239251
Go to all (19) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT01490619
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Preventing Diabetes Distress in Adolescents With Type 1 Diabetes: Results 1 Year After Participation in the STePS Program.
Diabetes Care, 41(8):1623-1630, 19 Jun 2018
Cited by: 36 articles | PMID: 29921624 | PMCID: PMC6054495
Associations of Coping Strategies With Glycemic and Psychosocial Outcomes Among Adolescents With Type 1 Diabetes Experiencing Diabetes Distress.
Ann Behav Med, 58(9):628-633, 01 Aug 2024
Cited by: 0 articles | PMID: 39014980 | PMCID: PMC11305127
Protocol for the Promoting Resilience in Stress Management (PRISM) intervention: A multi-site randomized controlled trial for adolescents with type 1 diabetes.
Contemp Clin Trials, 124:107017, 21 Nov 2022
Cited by: 2 articles | PMID: 36410689 | PMCID: PMC9839528
Resilience in adolescents with type 1 diabetes: An integrative review.
J Pediatr Nurs, 78:e41-e50, 29 Jun 2024
Cited by: 0 articles | PMID: 38945756
Review
Funding
Funders who supported this work.
NIDDK NIH HHS (1)
Grant ID: R01 DK090030
National Institute of Diabetes, Digestive and Kidney Diseases (1)
Grant ID: # R01DK090030





