Abstract
Free full text

Wound repair and regeneration: Mechanisms, signaling, and translation
Abstract
The cellular and molecular mechanisms underpinning tissue repair and its failure to heal are still poorly understood, and current therapies are limited. Poor wound healing after trauma, surgery, acute illness, or chronic disease conditions affects millions of people worldwide each year and is the consequence of poorly regulated elements of the healthy tissue repair response, including inflammation, angiogenesis, matrix deposition, and cell recruitment. Failure of one or several of these cellular processes is generally linked to an underlying clinical condition, such as vascular disease, diabetes, or aging, which are all frequently associated with healing pathologies. The search for clinical strategies that might improve the body’s natural repair mechanisms will need to be based on a thorough understanding of the basic biology of repair and regeneration. In this review, we highlight emerging concepts in tissue regeneration and repair, and provide some perspectives on how to translate current knowledge into viable clinical approaches for treating patients with wound-healing pathologies.
INTRODUCTION
Traumatic injury is the leading cause of mortality in Europe and the United States (http://www.worldlifeexpectancy.com). In addition to trauma, millions of surgical wounds are created annually in the course of routine medical care in the United States and Europe (1). Facilitating the healing of these unintended and deliberate injuries and minimizing the aesthetic impact on the patient and maximal restoration of tissue function remains a central concern of clinical care. Although minor injuries in healthy individuals generally heal well, larger injuries or the presence of a variety of physiological or common disease states including age, infection, diabetes/vascular disease, and cancer can negatively affect the healing process in ways that are currently poorly understood (Fig. 1). Moreover, mechanisms underlying pathological scarring, including hypertrophic scarring and more extreme keloid formation, are elusive, and efficient treatment options are currently missing (2).

The repair response can be disturbed by a multitude of local and systemic factors leading to diverse wound-healing pathologies. (A) Medial aspect of lower leg with venous leg ulcer (VLU). (B) Diabetic foot ulcer (DFU). (C) Lateral aspect of lower leg with an arterial ulcer. (D) Pressure sore. (E) Hypertrophic scar after thyroid surgery. (F) Keloid.
Demographically, the number of patients suffering from chronic wounds and impaired healing conditions is reaching epidemic proportions and will become even more burdensome in both human health and economic terms (1, 3). Incomplete understanding of the underlying molecular basis of tissue repair and its failure, as well as a lack of preclinical animal models that properly recapitulate human conditions (4), has led to a lack of therapies for treating nonhealing wounds or for speeding up the repair of acute wounds and reducing scar formation. Clinical research is hampered by a multimorbid and complex patient population, contributing to a paucity of high-quality and large-scale clinical trials for even demonstrating the efficacy of current products. In addition, heterogeneous reimbursement politics and an increasingly cautious climate for industrial investment, limited research funding sources (4), and lack of public awareness have also contributed to slow progress. Hence, there is a strong medical and social need to improve therapeutic approaches enhancing the endogenous tissue regenerative capacity.
As the outer barrier, the skin is the organ most challenged by a range of external stress factors, resulting in frequent cell and barrier damage. As such, skin has developed a set of complex mechanisms to protect itself and to restore tissue integrity when damaged, without resulting in septicemia. Experimentally, because of its accessibility, skin is one of the best organs in which to study response mechanisms to tissue damage and during repair. Findings from such research in skin have contributed to the unraveling of novel fundamental principles in regenerative biology, which have relevance to function of other epithelial-mesenchymal tissues, such as intestine, lung, and liver. Here, we will focus on studies performed in skin, but refer to their implications in other organ systems.
UNMET CLINICAL NEEDS: CHRONIC SKIN WOUNDS AND SCARS
Wound healing after skin injury involves extensive communication between the different cellular constituents of the diverse compartments of the skin and its extracellular matrix (ECM). In normal physiological conditions, restoration of a functional epidermal barrier is highly efficient (Fig. 2), whereas postnatal repair of the deeper dermal layer is less so and results in scar formation with a substantial loss of original tissue structure and function. When the normal repair response goes awry, there are two major outcomes: either an ulcerative skin defect (chronic wound) or an excessive formation of scar (hypertrophic scar or keloid) (Figs. 1 and and33).

Illustrations show molecular and cellular mechanisms pivotal for progression of wound healing. Early stages of wound healing include hemostasis and activation of keratinocytes and inflammatory cells. The intermediate stage involves proliferation and migration of keratinocytes, proliferation of fibroblasts, matrix deposition, and angiogenesis. Late-stage healing involves remodeling of ECM, resulting in scar formation and restoration of barrier. This spatiotemporal process is tightly controlled by multiple cell types that secrete numerous growth factors, cytokines, and chemokines (listed below) to achieve closure and functional restoration of the barrier.
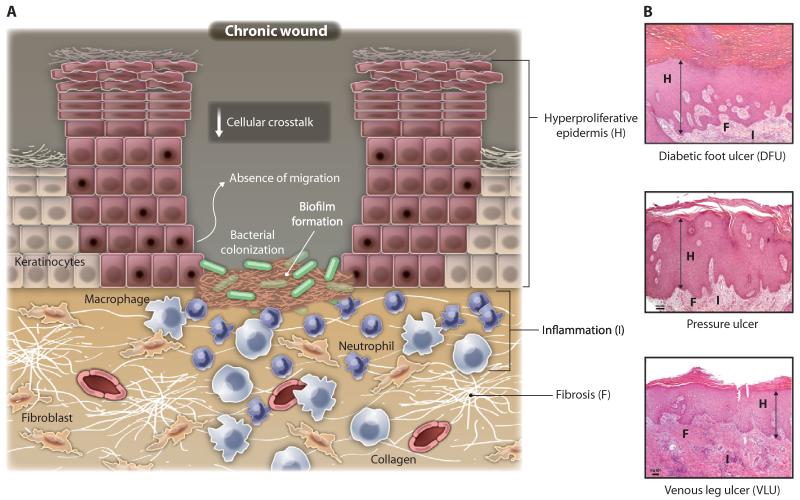
Illustrations show molecular and cellular mechanisms that are impaired in chronic wounds. (A) Chronic wounds show hyperproliferative and nonmigratory epidermis, unresolved inflammation, presence of infection, and biofilm formation. Although there is an increase in inflammatory cells (neutrophils and macrophages), not all are properly functioning. Uncontrolled proteases interfere with essential repair mechanisms. Some fibroblasts become senescent. In chronic wounds, there is a reduction of angiogenesis, stem cell recruitment and activation, and ECM remodeling compared with normal wound healing (Fig. 2). (B) Histologies representing characteristics of a diabetic foot (DFU), venous stasis (VLU), and pressure ulcers. Although different in etiology, these chronic wounds show common cellular features depicted in (A): H, hyperproliferative epidermis; F, fibrosis; I, increased cellular infiltrate (inflammation).
Chronic wounds are defined as barrier defects that have not proceeded through orderly and timely reparation to regain structural and functional integrity. In principle, any skin lesion has the potential to become chronic, and hence, chronic wounds are classified on the basis of their underlying cause. Vascular insufficiency, diabetes mellitus, and local-pressure effects are the major causes and categories of nonhealing skin wounds, although systemic factors, including compromised nutritional or immunological status, advanced age, chronic mechanical stress, and other comorbidities, contribute to poor wound healing (Fig. 1).
Most chronic skin wounds occur on the lower extremities. VLUs are the most common form of leg ulcers with an increasing incidence among the elderly of up to 3 to 4% (5) (Figs. 1A and and3).3). VLUs are the most severe symptom of chronic venous disease (5). The molecular mechanisms behind chronic venous disease and venous hypertension leading to severe lipodermatosclerotic, structural, and functional alterations of the lower leg, and ultimately skin ulceration, remain unknown, although recent findings provide several theories including persistent inflammation, interruption of keratinocyte migration, and misregulated signaling and/or expression of specific microRNAs (6-9).
Atherosclerotic disease represents the second most common underlying cause for nonhealing skin wounds of the lower leg. Arterial ulcerations are a consequence of reduced arterial blood supply resulting in tissue hypoxia and tissue damage (Fig. 1D). Diabetes mellitus is the most common metabolic disease associated with impaired wound-healing conditions. Currently, the prevalence of type 2 diabetes is 6.4% in the world population and is anticipated to increase to close to 8% in the year 2030 (10). It is not clear to what extent impaired healing is due to direct effects of insulin deficiency or its sequelae, including hyperglycemia, hyperlipidemia, peripheral neuropathy, and/or obesity. The most common clinical indication of impaired wound healing associated with diabetes is the DFU (Figs. 1B and and3).3). Among diabetic patients, 2 to 3% will develop a foot ulcer each year, and 15% will develop a foot ulcer during their lifetime.
Pressure ulcers (Figs. 1E and and3)3) are areas of tissue necrosis caused by unrelieved pressure to soft tissue compressed between a bony prominence and an external surface for a prolonged period of time. Major etiologic factors involved in their development are biomechanical forces (confined pressure, shearing forces, and friction), moisture, and local ischemia. Pressure ulcers particularly affect multimorbid and elderly patients, especially those that are bed- or wheelchair-bound. In spite of high mortality rates, predominantly for advanced-stage pressure ulcers, there is no efficacious therapy yet approved for their treatment.
Perhaps, it is now the time to recognize that chronic wound repair is a mortal disease similar to cancer (11). Although not an immediately obvious concept, the 5-year mortality rate for patients suffering from DFU or ischemic ulcers is much higher than that of prostate or breast cancer (11-13). In addition, the 5-year mortality rate for patients with diabetic-related amputations is about 50% (13).
Scarring and tissue fibrosis
One of the mysteries in the field of tissue regeneration and repair is the heterogeneity among diverse organisms: some organisms perfectly regenerate injured tissues and organs, whereas others replace the damaged tissue by a pathological connective tissue defined as scar. In humans, perfect tissue regeneration has only been described in fetal skin (14). Postnatal human epidermis, gut epithelium, and the hematopoietic system represent tissues that maintain the highest regenerative capacity. Furthermore, there are anecdotal reports of fingertip regeneration in young children (15). In all soft tissues and organs comprising connective tissue, the parenchymal tissue can be replaced by the deposition of excessive ECM, leading gradually to tissue fibrosis and, ultimately, to loss of organ function. The initial tissue damage can result from multiple acute or chronic stimuli, including autoimmune reactions, infections, or mechanical injury.
In human skin, two categories of pathological scarring after injury are distinguished: hypertrophic scars and keloids (Fig. 1, C and F). Hypertrophic scars develop after surgery, trauma, particularly burns, or spontaneously in predisposed patients. In contrast to hypertrophic scars, keloids extend beyond the margins of the original tissue damage, do not tend to regress spontaneously (hypertrophic scars generally regress within 6 months), and show a genetic predisposition. Furthermore, keloids and hypertrophic scars can be histologically differentiated by their diverse arrangement of collagen fibers, presence of α-smooth muscle actin (αSMA)–positive myofibroblasts, and extent of angiogenesis (16). Epidemiological data on the occurrence of pathological scars are not well documented, although it is widely accepted that darker-skinned populations have a higher occurrence of keloids than lighter-skinned populations (17). Some reports suggest that pathological scarring occurs most commonly in puberty. Scarring can cause functional disability, for example, if extended over a joint, or may cause patient discomfort and psychological stress.
Both hypertrophic scars and keloids are a major therapeutic challenge for surgeons and dermatologists. Although multiple treatment regimens are practiced, including silicone gel sheeting, pressure therapy, corticosteroids, cryotherapy, 5-fluorouracil, laser therapy, and radiation, none of these are optimal and effective, and therapeutics based on molecular targets remain elusive (2). Novel therapies for the treatment of cutaneous pathological scarring may be extrapolated from clinical trials targeting fibrosis in other organs including lung, liver, or kidney (18). Although tissue-specific features of fibrogenesis appear to exist, there is increasing appreciation of common pathways of fibrosis that are conserved among tissues, including transforming growth factor–β (TGFβ), connective tissue growth factor (CTGF), interleukin-4 (IL-4), IL-13, platelet-derived growth factor (PDGF), and osteopontin (19).
WOUND-HEALING PATHOLOGY
The molecular mechanisms that lead to impairment of wound healing are poorly understood. The complexity of the wound-healing process, which involves many cell types locally at the site of the wound as well as systemically (Fig. 2), along with comorbidities represent hurdles in identifying therapeutic targets and in clinical trial design. An added difficulty for this field of research is the paucity of animal models that precisely correlate with the human condition, which leads to difficult transition from experimental models and pre-clinical studies to clinical trials. Current state of knowledge is in its infancy and is based on integration of data resulting from analysis of human wound samples (predominantly from patients with VLUs and DFUs) and from data collected from animal models.
Future investigations will need to unravel to what extent the microenvironment of chronic wounds with different underlying etiologies may overlap in common pathomechanisms. Furthermore, based on current knowledge, it is unlikely that animal models that perfectly recapitulate the complex network of pathological factors in chronic human wounds will be developed in the near future (Fig. 3) (20). Instead, we speculate that animal models targeting specific pathological pathways will provide a more focused approach to gain knowledge on selected mechanisms that play a role in the development of chronic wounds. Humanized mouse models may provide a particularly useful approach to bridge the gap between bench and bedside (21).
The inflammatory response
Natural (acute) wound healing proceeds through several largely overlapping phases that involve an inflammatory response and associated cellular migration, proliferation, matrix deposition, and tissue remodeling (Fig. 2). Interruption or deregulation of one or more phases of the wound-healing process leads to nonhealing (chronic) wounds (Fig. 3). Injury leads to immediate activation of the clotting cascade, which, through the assembly of a fibrin clot, assures hemostasis and provides the basic matrix architecture to initiate the invasion and recruitment of inflammatory and other cells. Platelets trapped in the clot also release growth factors and chemokines into the local wound environment. The relevance of platelets and their products for successful tissue reconstitution is reflected in the clinical use of platelet-rich plasma to promote healing (22) and impaired healing in preclinical models recapitulating platelet disorders (23).
In parallel with hemostasis, the early inflammatory response mobilizes local and systemic defense responses to the site of the wound (Fig. 2). Inflammation is prolonged in chronic wounds (Fig. 3), and it is believed that these wounds might be trapped in a chronic inflammatory state that fails to progress (24). Specifically, recent investigations of chronic wound tissue and fluid indicate a continual competition between inflammatory and anti-inflammatory signals leading to a misbalanced environment for proper wound healing to occur (25, 26).
It has been shown that increased proinflammatory cellular infiltrates composed largely of neutrophils and macrophages contribute to delayed healing in chronic ulcers (27, 28). As a result, deregulation of several key proinflammatory cytokines, such as IL-1β and tumor necrosis factor–α (TNFα), prolong the inflammatory phase and delay healing (26, 29). IL-1β and TNFα are increased in chronic wounds, and this increase has been shown to lead to elevated metalloproteinases that excessively degrade the local ECM and thus impair cell migration (30). Recent studies have implicated the inflammasome, a multiprotein complex of the innate immune system responsible for activation and release of IL-1β from several skin cell types, in the development of chronic wounds (31, 32). In addition, continued presence of a high bacterial load in wounds results in a sustained influx of pro-inflammatory cells and increased inflammation also leading to delayed healing (Fig. 3) (33-35). A more detailed understanding of the mechanisms governing the inflammatory response and its resolution is needed.
Infection
Wound infection is likely to be a contributing factor in either development or maintenance of a chronic wound. All wounds are colonized to some degree, and a major role of the inflammatory phase of wound healing is to bring microbes down to steady-state levels that can be tolerated and cleared by healthy tissues. This is aided by the skin—the epidermis in particular—up-regulating and secreting antimicrobial peptides early in response to barrier damage and exposure to microbes (Fig. 2) (36, 37). Furthermore, in these polymicrobial wound communities, individual species may alter virulence and quantity as well as formation of a biofilm, which further impedes the efficacy of the “host response” and thus delays repair (33) (see Microbiome section).
Proteases
The failure of chronic wounds to heal properly has been attributed, in part, to deregulation of proteases and their inhibitors. Unlike the acute wound-healing process, where tissue proteases are normally under tight regulation, it appears that in a chronic wound, disruption of the production and activation of proteases plays a role in wound pathogenesis. The proteolytic unbalance may be a consequence of the aforementioned faulty regulation of inflammation and/or microbial contamination. It has been shown that matrix metalloproteinases (MMPs), such as collagenase and gelatinases A and B, are elevated in chronic wound fluids when compared to acute wound fluid (25, 38). Last, in chronic wounds, there is a marked increase in serine proteases that degrades matrix components, including fibronectin, as well as various key growth factors, all of which are required for remodeling of the ECM and cell growth (38-41).
Stem cells
To date, epidermal stem cells of the skin have been shown to occupy at least three distinct niches: the bulge of the hair follicle, the base of the sebaceous gland, and the basal layer of the epidermis (42). Recent reports have shown that local adipocyte progenitor cells (43) as well as melanocyte progenitors (44) contribute also to the wound repair process. Recruitment of bone marrow and endothelial progenitors to the site of injury is coordinated by specific chemokines (Fig. 2), which have been shown to be depleted in conditions that contribute to compromised healing response (Fig. 3), such as aging and diabetes (29, 45). Furthermore, frequent cycling of epidermal stem cells in patients with chronic wounds can lead to depletion of local stem cell populations (46). Compromised function of local and systemic stem cells and progenitors may play a considerable role in pathology of chronic wounds. Hence, stem cell modulation is becoming one of the most explored potential therapeutic strategies.
Angiogenesis and vasculogenesis
Blood vessel growth is an essential component of tissue repair, as vessels support cells at the wound site with nutrition and oxygen. Both angiogenesis (sprouting of capillaries from existing blood vessels) and vasculogenesis (mobilization of bone marrow–derived endothelial progenitors) contribute to new blood vessel formation during tissue repair (47). Inadequate local angiogenesis is considered a very likely contributor to the impaired healing of DFUs (3). Proteins with antiangiogenic properties, such as myeloperoxidase, exhibit higher expression levels in chronic wounds of diabetic patients as compared to acute wounds, whereas angiogenic stimulators, such as extracellular superoxide dismutase, are generally decreased (48). Reduced angiogenesis leads to elevated cell death, as revealed by expression of the late apoptotic cell marker annexin A5, which is exclusively found in diabetic wound exudates and is believed to be reporting a shortage of wound nutritional supply (48). Similar findings were reported in samples derived from human VLUs (25). Functional and ultrastructural analyses of the microcirculation in the wound edge of VLUs have revealed microvascular alterations indicating endothelial cell damage that may, in turn, lead to slow and insufficient capillary growth (49).
Deprivation of proangiogenic factors, such as members of the vascular endothelial growth factor (VEGF) family, by proteolytic degradation and subsequent interference with their bioactivity in the hostile chronic wound microenvironment has been suggested as a critical underlying cause (41, 50). In pressure ulcers, chemokine (C-X-C motif) ligand 9 expression levels fail to increase as they do in healthy, healing wounds (51), leading to inhibition of endothelial cell chemotaxis at the proliferative stage of angiogenesis and subsequently aberrant angiogenesis (52). An ability to modulate the fine balance between pro- and antiangiogenic molecules might lead to novel therapeutics for induction of angiogenesis in a chronic wound.
Senescence
Cellular senescence has been implicated in pathological tissue repair (Fig. 3), although there is controversy as to the mechanism (53). One hypothesis is that cells, fibroblasts in particular, become prematurely senescent within a chronic wound setting. Premature aging in fibroblasts derived from VLUs was found to be telomere-independent (54) and to vary across wounds reflecting migratory capacity (55). Senescence caused by oxidative stress might also drive uncontrolled fibroblast proliferation and keloid formation (56). Senescent fibroblasts and keratinocytes secrete MMPs 2, 3, and 9, and might therefore exert an antifibrotic effect (57). Moreover, senescent keratinocytes have been documented to secrete the antiangiogenic factor maspin (58), which may also be detrimental to repair. Conversely, senescent cells are also known to express cell surface–bound IL-1α, IL-6, and IL-8 (59)—all of which play key roles in wound repair.
CURRENT TREATMENTS AND THEIR LIMITATIONS
The past decade has witnessed a growth in rational chronic wound management and some new developments in wound dressings, including attempts to incorporate recombinant growth factors and live cells. Notably, it is primarily the local wound environment that has been considered as the key target for therapeutic strategies, regardless of the underlying causative, systemic disease. Dressings provide the most favorable environment for successful healing by protecting the local wound site from further trauma while providing moisture and absorbing excess exudate. More complex dressings may have additional biological properties, such as being antibacterial, attracting circulating cells, stimulating local cells to migrate and proliferate, as well as stimulating appropriate matrix deposition. However, owing to the limited clinical evidence on acceleration of wound closure, current wound dressings can be considered only as adjunctive means to provide an optimal local healing milieu.
Various “biological active” therapeutic attempts, mainly the delivery of local growth factors to enhance clinical management of chronic wounds, have been attempted but shown limited success so far (60). For example, topically applied recombinant human granulocyte macrophage colony-stimulating factor (rhGM-CSF) and granulocyte colony-stimulating factor (G-CSF) had positive effects on wound healing in small (20 patients), randomized, controlled studies involving chronic venous and DFUs (61). Larger, randomized, controlled, double-blinded trials are needed to confirm the early observations of promising clinical outcomes.
Another growth factor, PDGF, has proven effective in stimulating healing in patients. In 1997, becaplermin, a recombinant human PDGF (rhPDGF-BB), was approved by the U.S. Food and Drug Administration (FDA) for treatment of DFUs. rhPDGF-BB was shown to improve healing in randomized clinical trials involving DFUs to decrease amputations, and to be beneficial in patients with advanced-stage pressure ulcers (62). Although rhPDGF-BB is an approved therapeutic modality for DFUs, large, randomized control clinical trials are needed to test the efficacy of this growth factor in other types of ulcers. In attempts to accelerate healing in patients with chronic ulcers, one has to keep in mind the risks that can be associated with use of factors that promote epithelial migration, angiogenesis, matrix deposition, and other processes that are dysregulated in chronic wounds. For example, in 2008, the FDA included a “black box warning” of increased mortality rates secondary to malignancy in patients after treatment with three or more dispensings (http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2008/ucm116909.htm), although a follow-up study indicated that the cancer death risk decreased and was no longer statistically significant (63).
Several other growth factors that have multiple functions in wound healing have entered the clinical testing arena. For example, fibroblast growth factor 2 [FGF-2; or basic FGF (bFGF)] influences granulation tissue formation, epithelialization, and tissue remodeling and has shown variable results in randomized control clinical trials with favorable outcomes for burn and pressure ulcer healing (64). Furthermore, keratinocyte growth factor-1 (KGF-1; also known as FGF-7), which targets epidermal cells, has been reported to promote healing of skin wounds in mice (65). KGF-1 received FDA approval in 2004 and was marketed as palifermin or Kepivance for the prevention of severe oral mucositis in patients with hematological malignancies receiving high-dose chemotherapy (66).
With the discovery of VEGF more than 30 years ago and with its envisioned potential for vascular therapy, it was presumed to be the solution for therapeutic angiogenesis (67). Although preclinical testing in diverse animal models showed promise in stimulating the growth of functional vascular structures, initial clinical trials investigating VEGF-A as a proangiogenic factor in various ischemic conditions have failed. To date, VEGF has not been convincingly used in the clinic to stimulate the growth of functional vessels (68). Local treatment of chronic neuropathic DFUs in humans with recombinant VEGF-A165 (telbermin) revealed positive trends, suggesting biological activity for incidence and time of complete ulcer healing (69), but additional studies will be needed to characterize the safety and efficacy of VEGF-A.
Most currently applied and FDA-approved cellular products for regenerative therapies in the clinic use primary cells and are based on the discovery by Rheinwald and Green in the 1980s, showing that human epidermal keratinocytes could be cultured and rapidly expanded in vitro using a feeder layer of murine fibroblasts (70). In parallel to keratinocyte studies, diverse efforts have been directed toward the development of dermal substitutes that have qualities similar to native skin (71). Several companies manufacture “living skin equivalents” of autologous and allogeneic primary cells harvested from explant material. During the last two decades, many of these products have received FDA approval for the treatment of large and diseased skin defects that are refractory to conventional therapy and that have been shown to be effective in a limited number of clinical trials [reviewed in (72)].
Given the complexity of the multicellular tissue repair response (Fig. 2), it is not surprising that therapeutic delivery of a single factor and/or cellular component often only achieves limited efficacy in stimulating healing of chronic wounds. Furthermore, recent studies indicate that cells within the chronic wound may lack other aspects of the molecular milieu that enable an appropriate response to growth factor stimuli, such as down-regulation of epidermal growth factor receptor (EGFR) and TGFβ receptors as well as SMADs in nonhealing tissue of chronic ulcers (45, 73). Other issues, including the need to discover the most efficacious sustained delivery protocol, the optimal dosing, and frequency of delivery, are all obstacles to overcome before we realize the full therapeutic benefits of these new agents.
In summary, despite a clear, unmet clinical need, research in wound healing has not yielded new, science-led therapies for more than a decade. Multifactorial etiology, lack of funding for research into mechanisms, limitations of preclinical models, and requirements for complete closure as the only primary outcome in clinical trials all contribute to the slow development. The likelihood of a single therapy to be highly efficacious is low. The challenge lies in developing combination therapy approaches, identifying the appropriate timing of delivery for each of the components, and overcoming the prohibitive costs of clinical trials for products containing more than one biologically active compound. For example, a product that stimulates angiogenesis followed by a compound that stimulates matrix deposition and epithelial migration might be optimal.
In addition to new therapies, new animal models are desperately needed that properly mimic human acute and chronic wound pathologies. Currently, we can learn many lessons from the animal kingdom, especially from animals that naturally regrow lost limbs and heal adult tissues effectively. We focus on some of the most promising such lessons below.
LESSONS FROM THE ANIMAL KINGDOM
Tissue repair is universal across all multicellular organisms (Fig. 4); thus, conserved mechanisms may be identified in models more experimentally tractable than humans and subsequently extrapolated to the clinic. Because of similarities to human skin, pig models of wound healing were used in the early days for investigating repair mechanisms (74), and remain a popular model for preclinical trials of potential therapeutics (75). However, their poor genetic tractability, complicated anesthesia and surgical procedures, cost, and housing issues have seen rodent models, largely mice, take over as the predominant model for investigating the fundamental cell and molecular mechanisms underlying mammalian tissue repair.
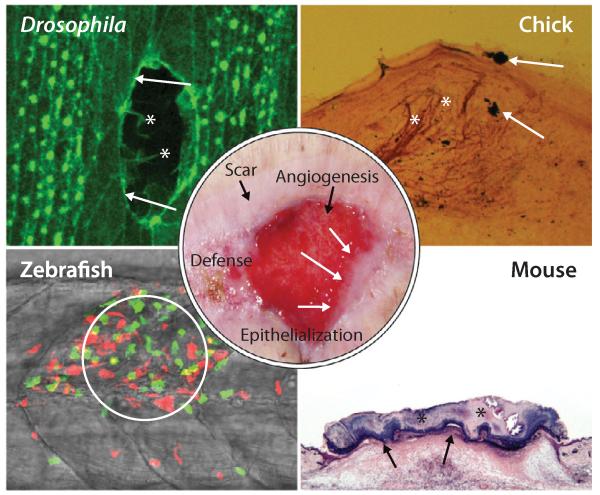
Activation of the immune response, angiogenesis, innervation, epithelialization, and scar formation are fundamental repair processes and are conserved between human (center, abdominal wound) and other multicellular organisms, which offer particular advantages for experimental investigation. Drosophila are highly genetically tractable and at embryonic stages are translucent, enabling live imaging of wound reepithelialization. This laser-wounded Drosophila embryo is expressing green fluorescent protein (GFP):actin (green) that reveals the cytoskeletal machineries of leading-edge epithelial cells in real time (arrows, actin cable; asterisks, filopodia). Zebrafish larvae are also translucent and have been used to investigate the dynamics of the wound inflammatory response in transgenic fish, such as this wounded fish expressing GFP: neutrophils (green) and red fluorescent protein (RFP):macrophages (red) to show how leukocytes are recruited to a wound (circled). The chick is a lesser used model, but has the advantage of easy accessibility during embryonic stages (in ovo, rather than in utero). A silver-stained section from a chick-limb wound reveals the cutaneous hyperinnervation (asterisks) triggered by wounding; the wound site is marked by carbon black particles (arrows). Rodents are the most frequently used models for wound healing studies despite having looser skin than humans. A hematoxylin and eosin (H&E)–stained section of a 3-day excisional wound on the dorsum of a mouse reveals the stage when a scab is still present (asterisks) and the inflammatory response apparent beneath the almost repaired epithelium (arrowheads) as it migrates at the interface between scab and granulation tissue.
What mice tell us about wound reepithelialization
Much of what we know about the cell and gene players in wound healing and the relative time courses of the various phases of skin repair come from studies in mice. Transgenic and knockout mouse studies over the last two decades have provided opportunities to investigate the functions of more than 100 genes that are potentially important for one or more aspects of skin healing (76). Because the most overt sign of a chronic wound is failure of wound reepithelialization (Fig. 4), a better understanding of how acute wounds normally reepithelialize may help develop procedures for “kick-starting” the same process in a nonhealing (chronic) wound. To this end, several mouse wound transcriptome studies have been performed (77, 78).
Gene changes occur in the wound edge epithelium, extending back up to 70 rows of cells from the cut wound edge (79). The earliest gene up-regulations are classic immediate early genes, including Ap-1, Fos, and Jun (80), and the Krox zinc finger transcription factors (81), which presumably function as part of the transcriptional activation machinery for the several hundred genes that are subsequently up-regulated in these cells and enable a surge in cell proliferation and associated epidermal migration of a leading tongue of keratinocytes at the interface between the scab and healthy wound granulation tissue. It has become clear that some of these late-activated genes, such as Egfr, are kept silent by histone methylation marks deposited by the polycomb family of epigenetic regulators. However, the polycombs are down-regulated, and these marks are removed soon after wounding so the silenced genes are then available for transcription (82).
Some of the changes needed for forward migration of wound edge keratinocytes involve alterations in the cell-matrix adhesions. These adhesions previously spot-welded the skin cells to the basement membrane, but in wound healing must enable migration over a new wound-specific, fibrin-rich matrix. Several integrins must be switched off to detach from the basement membrane, and others now become essential for wound migration. For example, keratinocyte-specific knockout of β1 integrins in mice leads to severe retardation in wound reepithelialization (83). Cell-cell junctions also must be modified. A recent study showed how the desmosomal junctions linking the advancing wound keratinocytes become looser and calcium-dependent, and that this switch is likely to be protein kinase C α (PKCα)–dependent because Pkca−/− mice failed to alter these adhesions and exhibited delayed healing (84). Proteases, in particular several MMPs, are needed to somehow clip or loosen the link between integrins and collagen as the epidermis advances over the wound substrutum (85).
All of these changes are required for cell migration. However, it seems that reepithelialization will not commence without the additional activation signals of several driving growth factors, including hepatocyte growth factor (HGF) and one or more members of the FGF and EGF families. Indeed, keratinocyte-specific knockout of c-met (HGF receptor) or FGF receptors 1 and 2 (86, 87) or global knockout of EGFR led to retarded reepithelialization in mice (88).
Generally, epidermal cells are lost after any skin injury, and they must be replaced by cell proliferation, which occurs largely in an epithelial zone further back than the migrating epidermal tongue (65). The contribution to new keratinocytes by stem cells and the source of such stem cells are not entirely clear. Labeled cells from the stem cell–dense bulge region of adjacent hair follicles are seen to move up and, at least initially, can populate the denuded territory (Fig. 2) (89), but a more permanent source of new keratinocytes appears to originate from non-bulge region follicular cells (90). Previously, it was believed that if a wound extended deeper than the roots of hair and sweat glands, then these structures would not regenerate; but this dogma is now in doubt because of new studies in mice in which hair follicles arose de novo from nonfollicular epidermis, in a Wnt-dependent manner that recapitulated embryonic appendage formation (91).
Cellular contribution to granulation tissue and dermis replacement
Many genes are up-regulated in mice in the fibroblast at the edge of a wound. It was assumed previously that these cells were the sole source of the wound granulation tissue and that they became activated by exposure to various growth factor signals, which triggered their proliferation and migration in synchrony with the advancing epidermis. A portion of these cells transform into the contractile specialist, the myofibroblast cell, after exposure to TGFβ and mechanical loading signals (92). It now seems probable that at least a subset of fibroblasts within wound granulation tissue are not derived locally but rather originate from a bone marrow–derived, mesenchymal stem cell (MSC) pool. By tracking fluorescent MSCs after intravenous injection into mice, several groups have reported up to 30% of total contribution to the wound fibroblast population (93-95). The translational aspect of this imaging is the potential for stem cell–mediated therapies for improving impaired healing, which has been tested in rodents (96) and in humans (97). For cell therapy, timing may also be important, as a recent study in mice showed that two populations of stem cells exist in the wound: one superficially, critical for hair development, and one deeper, forming the lower dermis, and possibly an early source of locally derived wound fibroblasts (98).
The complexity of inflammation during skin repair
Studies of repair in embryonic and fetal mice and also human patients undergoing fetal surgery have indicated that, before the onset of a wound inflammatory response, immature tissues are capable of scarless healing (99, 100). Inflammation might therefore be a cause of wound fibrosis. Indeed, mice lacking the ETS family transcription factor PU.1 (and thus not able to generate any leukocytic lineages) are able to heal wounds effectively as neonates—and do so without subsequent scarring, unlike their wild-type littermates (101).
Many mouse studies have been designed to individually test the function of most immune cell lineages in the wound repair process. Not all are in full agreement, but the current consensus is that early-recruited neutrophils largely deal with killing invasive microorganisms at any breach of the skin barrier (102), whereas macrophages are needed for clearing apoptotic neutrophils and orchestrating early wound closure events, but also emit signals that cause later scarring (102, 103). By contrast, mast cells appear to play only fine-tuning roles during wound repair, because their genetic depletion leads to almost entirely normal healing (104, 105). Other immune cell lineages are less well studied and may only become involved in the repair process if it becomes chronic. Currently, very little is known about the role of adaptive immunity in the normal mammalian wound-healing process, but one study of murine γδ T cells suggests that these may be vital for recognizing keratinocyte “damage” signals and releasing key growth factors for epidermal migration (106).
Inflammatory cell recruitment and activation are a consequence of many signals that occur at the wound site. Some of the earliest signals include factors released by degranulating platelets (23) and by damage- and pathogen-associated molecular patterns (DAMPs and PAMPs, respectively) where cells are damaged and microbes gain access (107). All of these signals are potential therapeutic targets for modulating the initial wound inflammatory response in humans. A newly discovered and clinically relevant signaling pathway derives from wound biomechanics: as a wound gapes open and then begins to contract, focal adhesion kinase/extracellular signal–regulated kinase (FAK-ERK) leads to activation of chemokine ligand 2 release by wound fibroblasts, which, in turn, draws in a larger inflammatory response (108). Blocking any of these steps leads to reduced scarring in mice (108) and supports the theory that inflammation is the primary driver of scarring at the wound site.
TGFβ1 is almost certainly one of the growth factors downstream of the wound inflammatory response, and knockdown of this signaling axis has been shown to reduce scarring (109). What remains unclear is how inflammation-triggered molecular changes in wound fibroblasts—which include up-regulation of osteopontin (110)—lead to deposition and bundling of collagen fibers in ways that cause pathological scarring.
Unexpected roles of wound genes and pathways
The mouse genes and signaling pathways discussed here are likely regulators of the wound-healing process. In addition, the experimental and genetic opportunities provided by mice have revealed some surprising players. In early differential display screens, a reactive oxygen species (ROS)–detoxifying enzyme, peroxiredoxin 6, was seen to be up-regulated by wound edge keratinocytes (111). This is logical because peroxiredoxin 6 is part of the “tolerance” machinery that enables wound cells to protect themselves from harmful ROS activities, which are increased during the wound inflammatory response. Mouse studies have also highlighted systemic hormones in healing. Falling estrogen levels, for instance, may be the principal cause of age-related healing impairment in both males and females (112). Similarly, social isolation leading to reduced cortisol levels and associated lowering of KGF and VEGF at the wound site lead to significantly retarded healing (113). These findings in mice are relevant to clinical translation, particularly for treating elderly patients.
As with any model, murine wound healing is a not a perfect mirror of what happens as human skin repairs itself. Mouse skin is loose and not attached to the underlying deep tissues, so the balance of contributions by reepithelialization and connective tissue contraction is different from human skin, where the epidermal wound edge is indirectly associated with the deeper wound tissues (Fig. 2). Another clear difference is that human skin is much less hairy than its murine equivalent. Thus, what is true for murine hair–derived stem cells and hair regrowth may only partially translate to clinical wound-healing scenarios. Indeed, the pattern and stage of hair growth in different regions of the mouse skin can significantly affect rates of skin healing in mouse tissue (114).
Also important to note is that many models of pathological wound healing in mice, although accurately mirroring some of the systemic causes of impaired healing—for example, the diabetic (db/db) mouse—seldom make allowance for other important associations, such as age and wound microbial load. There is a strong case to be made for improving such models by including these additional influencing factors so that data can be more usefully extrapolated to the clinic (20). Despite the limitations of mouse models, they have been instrumental in pre-clinical testing, leading to clinical trials and approval of products such as Regranex (PDGF-BB) (62, 115, 116).
New wound-healing models: Drosophila and zebrafish
Although mice are more amenable to genetic investigations than humans, studies of gene function are still time-consuming and expensive, and it is not feasible, at least for individual laboratories, to perform major genome-wide screens with mice. These limitations have encouraged wound-healing studies in Drosophila (117) and zebrafish (118) (Fig. 4). Although they have their own limitations, Drosophila and zebrafish models offer translucency for live imaging and even greater genetic tractability than mice, to study fundamental tissue repair mechanisms not possible in humans.
In the Drosophila embryo, movies of hemocytes (the fly equivalent of macrophages) that are mutant for, or expressing dominant negative versions of, each of the Rho family small GTPases (guanosine triphosphatases) have revealed roles for these regulators of the cytoskeleton as hemocytes undergo the rapid wound inflammatory response (119). The same may be true for neutrophils and macrophages migrating to human wounds; in vitro studies hint that this is the case. Although the advancing wound epidermis is hidden beneath a scab in mouse wounds, the simpler fly epidermis can be live-imaged, revealing dynamic cytoskeletal machineries, including lamellipodial and filopodial protrusions that enable bonding of epidermal wound edges together at the end of the healing process (120).
A recent study in Drosophila showed that there are active cell shape changes and junctional alterations several rows back from the wound edge, moving attention away from the front row of cells, which have been considered the key players (121). Several screens have been performed on embryo and larval Drosophila wound models to identify differentially expressed genes and mutants that suffer impaired healing (122, 123); some of these are unique to flies, but others have highlighted conserved transcriptional activator pathways, including wnt and Grh (124), that have been shown to extrapolate to mammalian repair (125, 126) and may become candidate therapeutic targets.
Translucent zebrafish larvae offer a phylogenetic step up from Drosophila, with greater parallels to human repair machinery. For example, rather than a single immune cell lineage, as in Drosophila, they have equivalents of all our innate immune cells. Currently, the most exciting insight from zebrafish studies of wound inflammation has been that ROS can serve as immediate damage attractants to draw immune cells to wounds (127). Zebrafish are also beginning to offer clues to the endogenous mechanisms for resolution of inflammation. For example, neutrophils may be partly responsible for their own resolution by clearance of the attractants that first drew them to the wound (128).
In addition to studies of inflammation, there are now new models of skin healing in adult zebrafish that reveal considerable parallels with mammalian wound repair, including similar, yet faster reepithelialization and transient scarring, all driven by conserved signaling pathways (129). This will provide further opportunities to use zebrafish for high-throughput, small-molecule drug screens as an initial filter for testing potential therapeutics to improve healing in the clinic.
Lessons from regenerating organisms
Several vertebrate groupings not only repair wounds but also regenerate complete organs and appendages. Zebrafish can grow back missing fins, whereas salamanders are famously able to regenerate whole limbs (130). Of course, the ultimate goal of researching these “regenerating” models is to replicate such capacities in humans, to grow back lost limbs or to replace regions of necrotic heart tissue, for instance. However, are there also clues as to how we might enhance normal wound-healing mechanisms?
One intriguing contributor to the limb regenerative process is a source of nerves whose ensheathing Schwann cells are known to release an early pulse of signals, including the secreted newt anterior gradient (nAG) protein (of which there are mammalian orthologs). The release of nAG from nerves appears to kick-start expression of nAG in the wound epithelium (131). Without this initial nerve signal, the wound stump heals, but no limb grows back in newts. Little is known about the role of nerves during skin healing, although studies in the chick embryo suggest a reciprocal positive association between nerves and wound repair (Fig. 4) (132), and several clinical anecdotes hint that poor innervation makes for poor healing.
Just as inflammation appears to play key roles in mammalian skin healing, there is also evidence for a link between the wound inflammatory response and regenerative capacity, but the mechanism is unclear. For example, the refractory period in tadpole development (when these organisms are unable to regenerate a tail) coincides with a spike in immune cell presence (133); yet, recent studies in both adult salamander limb and zebrafish fin regeneration indicate that an innate immune cell influx, particularly of macrophages, preceding blastema formation is an essential component of regeneration (134, 135).
FROM REPAIR MECHANISMS TO CLINICAL APPROACHES
Despite unraveling key mechanisms and players in physiological and pathological tissue repair, these findings have not yet led to a substantial improvement in patient care. Translating novel technologies and concepts in the field of tissue repair into standardized therapies has several challenges. When considering therapeutic strategies to restore diseased or damaged tissues, it is crucial to realize that most wound-healing pathologies are due to a combination of underlying systemic disease with regional/anatomical factors that cause tissue stress, an ulcerative lesion, and/or scar formation (Figs. 1 to to3).3). The best treatment approach for wound healing is to normalize the underlying (systemic) cause and simultaneously administer local treatment. Similarly, at the wound site, a combination of therapies may be required because it is unlikely that replacing a single tissue component, growth factor, ECM scaffold, or cell type will be optimal on its own. Rather, a comprehensive understanding of how these different components act together in time and space to successfully restore tissue function will be required.
Interactions between ECM and growth factors
Traditionally, the ECM was considered to be an inert, space-filling material providing mechanical support and tissue integrity. However, in recent years, it has become clear that the matrix also provides a bioactive structure that fundamentally controls cell behavior through chemical and mechanical signals (136). The diverse role of the ECM in organ function is probably best revealed by observing mutant gene defects in human disease, alongside the systematic analysis of ECM functions in genetically modified model organisms (137). These studies have revealed that ECM controls organ development and subsequent function through cell anchorage, integrin-mediated activation and signaling, transduction of mechanical forces, and the sequestering, release, and activation of soluble growth factors. For example, Marfan syndrome, a connective tissue disorder, is caused by mutations in the gene that encodes fibrillin-1, leading to reduced levels of extracellular fibrillin-rich microfibrils, which normally act as a TGFβ reservoir. Thus, although caused by a mutation in an ECM molecule, selected disease manifestations of Marfan syndrome reflect disturbances in TGFβ signaling (138).
Several ECM-based therapeutic systems for tissue repair and regeneration have reached the clinic or are in clinical trials [reviewed in (72)]. Collagen- or fibrin-based products are the most established ECMs being used clinically to guide regeneration of different tissues, including skin, heart valves, trachea, muscle, and tendon (71, 139). These products are used as carriers for transplanted cells, as acellular scaffolds, or as an immediate coverage for large trauma- or disease-associated skin defects. Many of these products have shown efficacy for the treatment of difficult-to-heal skin wounds (140). However, most of the clinical studies on collagen- and fibrin-based materials have not been controlled (for example, ECM alone without cellular component) or have been compared only to standard wound dressings, and their mechanisms of healing action remain speculative.
To advance the field of wound healing, it will be important to understand the relative efficacy of currently available products and how they work. By extrapolating from principles of developmental morphogenesis, an engrafted fibrin matrix is an appropriate natural material that can be modified to respond to the dynamic requirements of the repair microenvironment in both time and space (141-143). In experimental models of bone or skin repair, it has been shown that covalent attachment of growth factors and recombinant fibronectin fragments into a fibrin scaffold can provide spatiotemporally controlled release of the growth factor and enhance growth factor–matrix interactions that ultimately significantly reduce the morphogen concentrations required for effective tissue generation (142). These studies have also confirmed the essential role of the ECM for efficient delivery of growth factors for induction of blood vessel growth (141, 143). Timely restoration of blood vessel supply in therapeutic tissue repair approaches remains an unresolved need in all aspects of regenerative medicine.
Myriad synthetic materials are being explored preclinically for diverse reparative approaches, typically as three-dimensional microenvironments to mimic the features of natural ECM (144). The major challenge in developing synthetic biomaterials for clinical applications is reproducing the complexity of form and dynamics of function of the wound microenvironment (144). Early developments focused on materials where only a few biological moieties were integrated; now, so-called hybrid materials provide multiplexed signaling in a temporal sequence, recapitulating the complexity of the regenerative tissue environment. Poly(ethylene glycol) (PEG) is a commonly used synthetic component of these hybrid systems because of its favorable biocompatibility and chemical properties. Bioactive components have been integrated into PEG-based hydrogel matrices, including heparin, cyclic RGD (Arg-Gly-Asp) adhesion peptides, and growth factors (145). PEG-based matrices that are responsive to physical (light) or chemical (enzymes) stimuli have been created to reproduce the dynamics of the reparative process, by modulating the local milieu in time and space (146).
Future studies will need to prove the functionality of these complex synthetic materials for encouraging new tissue growth in vivo, under physiological and pathological wound conditions. Nevertheless, engineering synthetic biomaterials opens up avenues for investigating the systematic and independent variation of biomolecular and mechanical features of wound healing. In this regard, biomaterials research could provide a better understanding of how the ECM and its mechanical forces affect cell invasion, growth, and differentiation (147-149). Thus, although synthetic biomaterials are currently simplified mimics of natural ECM, the capacity to manipulate and direct fundamental cell functions and to apply this knowledge to tissue growth and repair will be a cornerstone for the future of regenerative medicine.
Cell-based therapies
The currently applied and FDA-approved cellular products for regenerative therapies in the clinic use primary human cells. Primary cells have obvious limitations; yields and proliferation rates are low, and for some tissues—for example, neurons, heart, and muscle—cells do not divide at all. With advances in stem cell biology as well as techniques to isolate, expand, and engraft stem cells, a new and exciting era has opened for cellular therapy in regenerative medicine.
Over the past decade, stem cells from bone marrow, adipose tissue, adult blood, cord blood, epidermis, and hair follicle have been investigated in numerous preclinical studies and a few clinical pilot studies. Along these lines, clinical studies have shown that bone marrow– and adipose tissue–derived MSCs can augment the repair process when applied locally to chronic skin wounds in patients (96, 150). A recent clinical trial reported improved wound healing and increased mechanical skin stability in children with recessive dystrophic epidermolysis bullosa (RDEB) after allogeneic bone marrow or umbilical cord blood transplantation (151).
Currently, there is no FDA-approved stem cell product on the market for the treatment of skin wounds. We can perhaps learn lessons from other organ systems, such as the heart. Acute myocardial infarction and chronic ischemic heart disease have been targets for numerous phase 1/2 clinical trials using stem cells for tissue regeneration and repair. Those studies have proven that cardiac stem cell therapy is relatively well tolerated and feasible (152). For conclusions regarding the efficacy of cardiac stem cells, we will need to wait for completion of ongoing, large-scale, phase 3 trials. Similarly, the tissue repair field will learn from the final outcomes of ongoing clinical trials applying bone marrow MSCs in the treatment of articular cartilage defects, bone defects, or idiopathic pulmonary fibrosis.
Recent developments in reprogramming skin and other differentiated cells into induced pluripotent stem cells (iPSC) provide a new cell source that can potentially be used for therapy. It has been shown that human skin equivalents can be created entirely from fibroblasts and keratinocytes generated from patients’ iPSCs, and that healthy cells can be generated from reprogrammed cells from patients with RDEB (153, 154). More recently, revertant keratinocytes of a junctional epidermolysis bullosa patient with compound heterozygous COL17A1 mutations used an iPSC approach to generate genetically corrected keratinocytes (155). In addition, iPSCs have been shown to ameliorate diabetic polyneuropathy (a neuropathic disorder associated with diabetes mellitus) in mice (156), and to activate an angiogenic response in mice with hindlimb ischemia (157).
Cell therapies will continue to contribute to regenerative medicine in the 21st century. However, fundamental questions regarding the optimal cell populations for treatment of a chronic condition (for example, multipotent versus pluripotent), favorable route and time point of cell delivery (after injury, for example), the cells’ mode of action, survival and integration of transplanted cells, and whether cells can establish and maintain identity in new microenvironment still need to be addressed (158). Beside these biological questions, safety issues and complex regulatory requirements provide additional major challenges in the advancement of developing cell-based therapies for wound healing (159).
Meaningful clinical endpoints and study design
From our early understanding of the pathomechanisms involved in inhibition of healing, it became clear that targeting a single molecule or a cellular process will not work alone; rather, combinatorial treatment approaches are needed. Ultimately, the most important step in bringing therapy to patients rests on successful clinical trials. The development and approval process of treatment modalities for wound healing has seen a large number of drug/biologic failures, with only one drug (Regranex) and two biologic devices (Apligraf and Dermagraft) having obtained approval for efficacy from the FDA in the past 15 years.
Clinical trial design in the area of chronic wounds faces multiple challenges (160). Heterogeneity of patients and their wounds (even within the same category of chronic wound) indicates a need for better diagnostic stratification of patients who are being included in clinical trials. Defining inclusion and exclusion criteria is another challenge, because patients with chronic wounds have underlying chronic illnesses and are often undergoing additional systemic therapies, raising the question of drug-drug interaction and drugs’ multiple pharmacodynamic actions. For example, “hard-to-heal” wound inclusion criteria range from wounds that are large and long-standing (>1 year) and have been treated with all available therapies without success, to wounds that show <40% closure in 4 weeks of standard of care. Such inclusion/exclusion criteria will also greatly influence patient recruitment and consequently duration and expense of the trial. Variability of wound healing “standard of care” between clinics, academic centers, private sector, regions of the city, country, or parts of the world all contribute to difficulty in obtaining cumulative, large data sets from multicenter trials.
There is an ongoing debate in the field regarding defining endpoints. Complete (100%) closure, rate of closure, reduction in size, recurrence, and “surrogate endpoints” (early changes in wound size that are intended to predict the true, meaningful clinical endpoint) are all potential primary and/or secondary outcomes (161). One has to compare with the field of cancer and ask: How many cancer treatments would be available today if complete cure was the only accepted outcome of clinical trials? A more unified approach in standardizing protocols and defining endpoints—beside 100% closure—in clinical trial design is desperately needed. A better understanding of local patient population, demographics, and socioeconomics will be important in clinical trial design.
The wound-healing community, including The Wound Healing Society (http://woundheal.org/), the American Association for Advancement of Wound Care (http://aawconline.org/), the Canadian Association of Wound Care (http://www.cawc.net), and the Mexican Association for Comprehensive Wound Care (http://www.amcichac.com), is currently in the process of formulating consolidated guidelines for each of the common types of ulcers. It is anticipated that these guidelines will lead to standardized, evidence-based clinical protocols. In a further collaborative effort, the Wound Healing Society and the American Association for the Advancement of Wound Care have initiated discussions with the FDA to develop expanded, clinically relevant, evidence-based primary endpoints for clinical trials.
UNANSWERED QUESTIONS AND EMERGING FOCUS AREAS
There are several emerging focus areas in wound healing where new tools or biological insights are beginning to open up novel areas of research that may have an impact on future wound-healing therapeutics and translation. These are discussed briefly below.
Microbiome
Because skin is directly exposed to environmental factors, it exhibits a complex, diverse, and dynamic microflora (162). Wounding therefore exposes deeper tissues to surface-resident and external microbes. It will be important to learn which wound microbiome compositions are conducive to the normal wound-healing process, because this may instruct new therapeutics for patients with nonhealing wounds [reviewed in (163)]. Microbiota changes during human skin barrier disruption have shown interesting dynamics, with an early but short-lived microbiome consisting of microbial constituents from surrounding superficial layers; this was subsequently replaced by bacteria populations from deeper layers of the stratum corneum (164). Changes in microbial communities, in turn, stimulated expression of epidermal antimicrobials and inflammatory molecules, underscoring crosstalk between microorganisms and the host (164).
Polymicrobial presence (bioburden) has been well documented in chronic wounds and can play a major role in impaired wound healing, even in the absence of clinical signs of infection (Fig. 3) (163, 165). These microbial communities are believed to exist predominantly in the form of a biofilm (166), the composition of which depends on the type of chronic wound; the most prevalent genera in chronic wounds are Staphylococcus, Pseudomonas, and Corynebacterium. In addition, obligate anaerobes Bacteroides, Peptoniphilus, Finegoldia, Anaerococcus, and Peptostreptococcus spp. have also been identified [reviewed in (163)]. The overuse of antibiotics may even increase the prevalence of particular microbes, such as Pseudomonadaceae (167).
The diversity of the chronic wound microbiome may be substantially reduced in comparison to that of neighboring healthy skin (168), but a recent study using animal models of wound infections demonstrated that polymicrobial infection with Staphylococcus aureus and Pseudomonas aeruginosa led to more prominent inhibition of epithelialization than infection with a single species (33), suggesting that the complex chronic wound microbiome may have even more of a detrimental effect on wound closure. More accurate microbial diagnostics using swabs and biopsies from patients’ wounds will serve as guiding tools for more customized therapeutic approaches (Fig. 5A).
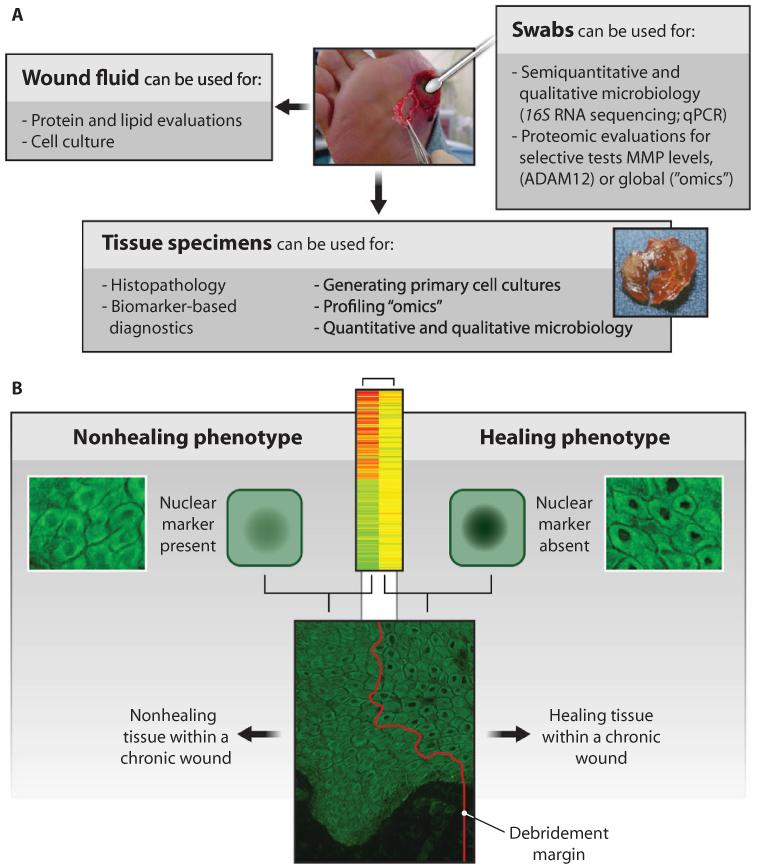
(A) Various types of biological materials can be collected from a chronic wound, including wound fluids, swabs, and tissue specimens. These fluids, cells, and tissues can support cellular and molecular analyses to better understand the chronic wound pathology and to identify biomarkers of wound healing and impairment. (B) Example analyses using tissue specimens. Genomic profiling and immunohistochemistry revealed distinct profiles of healing capacity. A biomarker of a healing phenotype, decreased nuclear β-catenin, has been identified in human wounds (178). Such methods can be used to identify margin of debridement (red line).
Aging
One feature that most patients with chronic wounds have in common, regardless of their underlying systemic disease, is increased age. Studies in healthy mice and humans have shown that aging attenuates skin repair (169). Delayed wound healing has been used as measure to characterize the aging phenotype of skin (170). It is possible that aging increases risk and, when combined with an additional comorbidity (diabetes, extended pressure, or ischemia), leads to compromised wound healing. Aging or underlying diseases, such as diabetes, may also contribute to changes in skin microbiota, as shown recently in diabetic mice (171), and we do not yet know how these changes influence healing.
Hormones, particularly estrogens and androgens, are contributing factors in aging and have been implicated as regulators of wound healing in experimental models and patients (112, 163, 172, 173). Elderly male patients have the highest incidence of VLUs, and this correlates with a reduced level of estrogen (174). Furthermore, age-related changes can be reversed by the systemic administration of hormone replacement therapy (175) or by topical estrogen in both male and female subjects (176). Yet, the specific molecular mechanisms underlying cellular aging and loss of organ function are still poorly understood (177). With a better understanding of intrinsic aging mechanisms, we may then better understand how aging affects wound healing.
Personalized medicine approaches
Given the diversity of patients, their wound etiologies, and their comorbidities, it is becoming increasingly evident that treatments need to be tailored for a subset of patients or even individual patients. However, personalizing wound-healing therapies will require improved diagnostic and prognostic tools. Collecting various samples from wounds provides important information regarding pathology, microbial composition, gene signatures, and protein and lipid composition for each patient (Fig. 5A). A search for biomarkers that can serve as an objective measure of the different stages of normal (healing) versus pathogenic (nonhealing) tissue (Fig. 5B), and of particular biological process, such as reepithelialization and scarring, is an emerging area of wound-healing research.
To date, although there are several diagnostic biomarkers that identify presence of potential single or multiple factors that might influence clinical outcome, there are still no biomarker “signatures” that can, with high confidence, predict outcome of healing. As depicted in Fig. 5B, the nuclear presence of β-catenin was found to indicate a non-healing cellular phenotype in humans (178). Conversely, its absence indicated a tissue capable of healing. Both cellular phenotypes constituted the wound edge. Thus, by assessing tissue biopsies from patients, one can begin to predict the healing capacity and define the necessary extent of tissue debridement (Fig. 5). Furthermore, a more accurate localization of the healing tissue (phenotype) will help to expose it to stimulators of healing, such as growth factors, ECM components, and cells.
The identification of particular polymicrobial organisms (179) or the observation of high protease levels, such as MMPs or ADAMs (a disintegrin and metalloproteinases), found in the wound bed (180) are already being used to modify treatment plans and improve outcomes. “Omics,” nanotechnology, and imaging will further contribute to advanced diagnostics (Fig. 5A). For example, genomic, proteomic, and lipidomic analyses of human wounds have already improved our understanding of the mechanisms that guide the repair process and its impairment, and have identified several potential biomarkers, such as c-myc and β-catenin (7, 178), S100 proteins and MMPs (48), or glycerophosphocholines, glycerophosphoglycerols, glycerophosphoinositols, and triacylglycerols (181). Similarly, portable confocal imaging, which can provide noninvasive assessment of lesional and nonlesional psoriatic skin (182), is revolutionizing diagnosis and treatment of this disorder. With new initiatives in “big data,” one can envision the integration of genomic, proteomic, and microbiome data, as well as nanomedicine and imaging merging with electronic medical records to formulate patient-specific treatment plans.
LOOKING FORWARD
Over the past decade, considerable insights into the molecular pathways driving the animal healing response and impairment have suggested new therapeutic targets and provided scientific rationale for future clinical trials. To translate these new therapies to the clinic, we need to gain a better understanding of the heterogeneity of patients and their wounds, so as to better diagnose and stratify patient subsets, define meaningful clinical endpoints, and design effective human studies. Recognition of the complexity of the wound-healing process and its diseases as well as acceptance of the seriousness and mortality associated with repair pathologies will be a critical step in these future efforts. Consequently, the combination of our current knowledge in basic biology, the identification of the limits of past clinical trials as well as translational research that includes development of improved animal models, harnessing of new technologies for more accurate imaging, and biomarker-based diagnostics will provide a strong basis to advance viable clinical approaches for treating patients with wound-healing pathologies.
Acknowledgments
We are grateful to members of our laboratories for input and discussions.
Funding: DFG (Deutsche Forschungsgemeinschaft) German Research Foundation (SFB829) (S.A.E.); NRW/EU Ziel 2-Programm Europäischer Fond für regionale Entwicklung (EFRE) 2007-2013 (S.A.E.); the Wellcome Trust (P.M.); Medical Research Council (P.M.); Cancer Research UK (P.M.); British Heart Foundation and Biotechnology and Biological Sciences Research Council (P.M.); NIH grants 5R01NR013881 and 9R01DK098055 (M.T.-C.).
REFERENCES AND NOTES
Full text links
Read article at publisher's site: https://doi.org/10.1126/scitranslmed.3009337
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4973620?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1126/scitranslmed.3009337
Article citations
A microenvironment-modulating dressing with proliferative degradants for the healing of diabetic wounds.
Nat Commun, 15(1):9786, 12 Nov 2024
Cited by: 0 articles | PMID: 39532879 | PMCID: PMC11557877
Metallic nanoparticles: a promising novel therapeutic tool against antimicrobial resistance and spread of superbugs.
Biometals, 24 Oct 2024
Cited by: 0 articles | PMID: 39446237
Review
Application of hydrogel-loaded dental stem cells in the field of tissue regeneration.
Hum Cell, 38(1):2, 22 Oct 2024
Cited by: 0 articles | PMID: 39436502
Review
Microenvironment-responsive nanomedicines: a promising direction for tissue regeneration.
Mil Med Res, 11(1):69, 21 Oct 2024
Cited by: 0 articles | PMID: 39434177 | PMCID: PMC11492517
Review Free full text in Europe PMC
Exploring the Role of miR-132 in Rat Bladders and Human Urothelial Cells during Wound Healing.
Int J Mol Sci, 25(20):11039, 14 Oct 2024
Cited by: 0 articles | PMID: 39456820 | PMCID: PMC11508042
Go to all (1,098) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Cellular and molecular mechanisms of skin wound healing.
Nat Rev Mol Cell Biol, 25(8):599-616, 25 Mar 2024
Cited by: 31 articles | PMID: 38528155
Review
The Role of MSC in Wound Healing, Scarring and Regeneration.
Cells, 10(7):1729, 08 Jul 2021
Cited by: 122 articles | PMID: 34359898 | PMCID: PMC8305394
Review Free full text in Europe PMC
Wound healing related agents: Ongoing research and perspectives.
Adv Drug Deliv Rev, 129:242-253, 01 Mar 2018
Cited by: 26 articles | PMID: 29501699
Review
Inflammation and metabolism in tissue repair and regeneration.
Science, 356(6342):1026-1030, 08 Jun 2017
Cited by: 464 articles | PMID: 28596335
Review
Funding
Funders who supported this work.
Biotechnology and Biological Sciences Research Council
British Heart Foundation (1)
To investigate inflammation in heart repair using zebrafish as the model
Professor Paul Martin, University of Bristol
Grant ID: PG/12/13/29434
Cancer Research UK
Medical Research Council (2)
Investigating the functions and therapeutic potential for Eph receptors and ephrins during wound repair and inflammation
Professor Paul Martin, University of Bristol
Grant ID: G0901822
Modeling of wound repair and inflammation in the Drosophila embryo
Professor Paul Martin, University of Bristol
Grant ID: MR/J002577/1
NIAMS NIH HHS (1)
Grant ID: R21 AR060562
NIDDK NIH HHS (3)
Grant ID: R01 DK098055
Grant ID: 9R01DK098055
Grant ID: RC1 DK086364
NINR NIH HHS (2)
Grant ID: 5R01NR013881
Grant ID: R01 NR013881
Wellcome Trust (2)
Grant ID: 097791/Z/11/Z
Investigating the links between inflammation and fibrosis during tissue repair.
Professor Paul Martin, University of Bristol
Grant ID: 097791





