Abstract
Background
The management of biochemical failure (BF) following external beam radiotherapy (EBRT) for prostate cancer is controversial, due to both the heterogeneous disease course following a BF and a lack of clinical trials in this setting.Objective
We sought to characterize the natural history and predictors of outcome for patients experiencing BF in a large cohort of men with localized prostate cancer undergoing definitive dose-escalated EBRT.Design, setting, and participants
This retrospective analysis included 2694 patients with localized prostate cancer treated with EBRT at a large academic center. Of these, 609 experienced BF, defined as prostate-specific antigen (PSA) nadir + 2 ng/ml. The median follow-up was 83 mo for all patients and 122 mo for BF patients.Intervention(s)
All patients received EBRT at doses of 75.6-86.4 Gy.Outcome measurements and statistical analysis
The primary objective of this study was to determine predictors of distant progression at the time of BF. Cox proportional hazards models were used in univariate and multivariate analyses of distant metastases (DM), and a competing risks method was used to analyze prostate cancer-specific mortality (PCSM).Results and limitations
From the date of BF, the median times to DM and PCSM mortality were 5.4 yr and 10.5 yr, respectively. Shorter posttreatment PSA doubling time, a higher initial clinical tumor stage, a higher pretreatment Gleason score, and a shorter interval from the end of radiotherapy to BF were independent predictors for clinical progression following BF. Patients with two of these risk factors had a significantly higher incidence of DM and PCSM following BF than those with zero or one risk factor. The main limitations of this study are its retrospective nature and heterogeneous salvage interventions.Conclusions
Clinical and pathologic factors can help identify patients at high risk of clinical progression following BF.Patient summary
In this report, we look at predictors of outcome for patients with prostate cancer recurrence, as determined by prostate-specific antigen (PSA) levels, following radiation treatment. We found that the approximate median times to distant metastasis and death from prostate cancer for patients in this situation were 5 yr and 10 yr, respectively. Furthermore, we found that patients with a rapid increase in PSA levels following treatment, a short time to PSA recurrence, invasion of extraprostatic organs, or a high Gleason score had worse outcomes.Free full text

The Natural History and Predictors of Outcome Following Biochemical Relapse in the Dose Escalation Era for Prostate Cancer Patients Undergoing Definitive External Beam Radiotherapy
Abstract
Background
The management of biochemical failure (BF) following external beam radiotherapy (EBRT) for prostate cancer is controversial, due to both the heterogeneous disease course following a BF and a lack of clinical trials in this setting.
Objective
We sought to characterize the natural history and predictors of outcome for patients experiencing BF in a large cohort of men with localized prostate cancer undergoing definitive dose-escalated EBRT.
Design, setting, and participants
This retrospective analysis included 2694 patients with localized prostate cancer treated with EBRT at a large academic center. Of these, 609 experienced BF, defined as prostate-specific antigen (PSA) nadir + 2 ng/ml. The median follow-up was 83 mo for all patients and 122 mo for BF patients.
Intervention(s)
All patients received EBRT at doses of 75.6–86.4 Gy.
Outcome measurements and statistical analysis
The primary objective of this study was to determine predictors of distant progression at the time of BF. Cox proportional hazards models were used in univariate and multivariate analyses of distant metastases (DM), and a competing risks method was used to analyze prostate cancer–specific mortality (PCSM).
Results and limitations
From the date of BF, the median times to DM and PCSM mortality were 5.4 yr and 10.5 yr, respectively. Shorter posttreatment PSA doubling time, a higher initial clinical tumor stage, a higher pretreatment Gleason score, and a shorter interval from the end of radiotherapy to BF were independent predictors for clinical progression following BF. Patients with two of these risk factors had a significantly higher incidence of DM and PCSM following BF than those with zero or one risk factor. The main limitations of this study are its retrospective nature and heterogeneous salvage interventions.
Conclusions
Clinical and pathologic factors can help identify patients at high risk of clinical progression following BF.
Patient summary
In this report, we look at predictors of outcome for patients with prostate cancer recurrence, as determined by prostate-specific antigen (PSA) levels, following radiation treatment. We found that the approximate median times to distant metastasis and death from prostate cancer for patients in this situation were 5 yr and 10 yr, respectively. Furthermore, we found that patients with a rapid increase in PSA levels following treatment, a short time to PSA recurrence, invasion of extraprostatic organs, or a high Gleason score had worse outcomes.
1. Introduction
Prostate cancer is the most common noncutaneous malignancy in men and the second leading cause of cancer death in the USA amongst men [1]. Radiotherapy is a cornerstone of the curative management of this disease, and is employed in approximately 25% of patients younger than 65 yr and in 40% of patients aged 65 yr or older [2]. Dose-escalated external beam radiotherapy (EBRT) is by far the most common radiation modality currently employed [3], and when delivered either with or without concomitant androgen deprivation therapy (ADT), it achieves excellent cure rates for localized prostate cancer. Nevertheless, a significant minority will eventually relapse [4,5].
Elevation of the prostate-specific antigen (PSA) above a defined threshold, known as biochemical failure (BF), is almost universally the earliest harbinger of relapsed disease following EBRT, and may manifest years prior to clinical recurrence [6]. However, although BF signifies a failure of primary treatment, the natural history of biochemically recurrent prostate cancer is extraordinarily heterogeneous, spanning the spectrum from indolent disease that remains persistently clinically undetectable to, more rarely, aggressive recurrences that are rapidly lethal. In combination with a conspicuous lack of randomized controlled trials investigating management strategies in this setting, this makes the management of BF one of the most challenging situations faced by urologic oncologists [7].
Although multiple studies have examined the natural history of biochemically recurrent prostate cancer following radical prostatectomy (RP) [8–10], the natural history of BF following radiotherapy is less well characterized, especially in the dose escalation era. We, therefore, investigated the natural history and predictors of outcome in a cohort of patients treated with EBRT who subsequently developed BF.
2. Patients and methods
2.1. Patient selection and pretreatment evaluation
From 1991 to 2008, 2694 patients with localized prostate adenocarcinoma were treated with EBRT at doses ≥75.6 Gy at Memorial Sloan Kettering Cancer Center; these patients formed the study cohort. Risk groups were defined according to the National Comprehensive Cancer Network (NCCN) risk-stratification system [11]. Institutional review board approval was granted prior to data collection.
2.2. Treatment
EBRT techniques have been described previously [5]. Briefly, patients underwent computed tomography (CT)–based simulation using an Aquaplast mold for immobilization. EBRT was delivered in 1.8-Gy daily fractions utilizing 15-MV photons. Elective pelvic lymph-node radiation was not administered to any patient in accordance with our institution’s policy during that time. Patients with progressive recurrent cancer underwent salvage treatment at the discretion of the treating physician (Supplementary Table 1). Six patients progressed while receiving adjuvant ADT, and 110 patients received one or more doses of ADT prior to clinical recurrence.
2.3. Endpoints
BF for this study was defined, according to the Phoenix definition, as a posttreatment PSA measurement 2 ng/ml greater than the posttreatment PSA nadir [12]. Additionally, any patient treated with salvage ADT for a rising PSA was considered to have experienced BF for the calculation of PSA recurrence-free survival (PSA-RFS) amongst the entire 2694-patient cohort, but was excluded from the subsequent analyses determining predictors of outcome following BF if they did not meet the Phoenix BF definition. The interval to BF (IBF) was defined as the time from completion of radiotherapy to BF. Distant metastasis (DM) was defined as metastasis in the bones, visceral organs, or lymph nodes outside of the pelvis. All DMs were either pathologically confirmed, demonstrated a clear radiographic response to ADT, or continued to grow as the PSA increased in the setting of castration-resistant disease. Prostate cancer–specific mortality (PCSM) was defined as a death clearly attributable to prostate cancer progression, or death from unknown causes in the setting of castration-resistant metastatic disease. Time to BF and overall follow-up time was calculated from the end of radiotherapy. Time to DM and time to PCSM were calculated from the date of BF.
2.4. Calculation of PSA doubling time
The posttreatment PSA doubling time (PSA-DT) calculation was performed using all PSA values from the date of recording the nadir PSA value to the date of BF. PSA-DT was calculated as the reciprocal of the slope of the ordinary least-squares regression line of the base 2 logarithm of PSA versus time in patients with at least three PSA measurements from nadir to BF. In this study, 145 of the 609 patients with BF had fewer than three PSA measurements during this time interval, and they were excluded from multivariate analyses including PSA-DT as a variable. For PSA measurements <0.05 ng/ml, the PSA was set to 0.05 ng/ml in order for the logarithm of the PSA to result in a finite value.
2.5. Statistical methods
The primary objective of this study was to identify predictors of DM from the time of BF. The Kaplan-Meier method was used to estimate actuarial probabilities of PSA-FS, DM, and overall survival. Univariate and multivariate analyses for these endpoints were performed using a Cox proportional hazards model. For PCSM, the cumulative incidence was calculated using a competing-risks analysis, and comparisons of PCSM across subgroups were performed using Gray’s k-sample test. The Fine and Gray method was employed for multivariate analyses of PCSM. To calculate optimal cut-off points for PSA-DT and IBF, nine arbitrary cut-off points occurring at nine deciles for each variable were tested using maximally selected chi-square statistics. The optimal cut-off point was selected as the decile value yielding the lowest p value for time to DM following BF after correcting for multiple hypothesis testing [13,14]. Given the difficulty in adjusting for or interpreting the effects of salvage therapy on outcome [15], the timing and nature of these interventions were not considered for this analysis, other than the inclusion of salvage ADT as a time-dependent covariate in our analysis of our primary endpoint, time to DM following BF. All statistical analyses were performed using R version 2.14.1 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
Baseline clinical, pathologic, and treatment characteristics are detailed in Table 1. The median follow-up from the end of radiotherapy was 83 mo for the entire cohort and 122 mo for patients with BF. Neoadjuvant ADT was given to 30.2%, 48.5%, and 79.1% of patients with NCCN low-, intermediate-, and high-risk disease, respectively. The estimated 8-yr PSA-RFS was 90.3%, 77.3%, and 57.1% for these respective risk groups (Fig. 1).

Prostate-specific antigen (PSA) recurrence-free survival in patients with National Comprehensive Cancer Network low-, intermediate-, and high-risk prostate cancer undergoing dose-escalated external beam radiotherapy.
Table 1
Baseline clinical characteristics of patients receiving dose-escalated external beam radiotherapy for localized prostate cancer
| Parameter | All patients | Patients with biochemical failure | ||
|---|---|---|---|---|
|
| ||||
| n | % | n | % | |
| Patients | 2694 | – | 609 | – |
| Median follow-up (mo) | 83 | – | 122 | – |
| Age | – | – | – | – |
 Median (yr) Median (yr) | 69 | – | 67.6 | – |
 ≤70 yr ≤70 yr | 1531 | 56.8 | 390 | 64.0 |
 >70 yr >70 yr | 1163 | 43.2 | 219 | 36.0 |
| Clinical stage | – | – | – | – |
 ≤T1c ≤T1c | 1307 | 48.5 | 188 | 30.9 |
 T2a T2a | 548 | 20.3 | 107 | 17.6 |
 T2b-c T2b-c | 532 | 19.7 | 161 | 26.4 |
 T3a T3a | 121 | 4.5 | 44 | 7.2 |
 T3b–T4 T3b–T4 | 186 | 6.9 | 109 | 17.9 |
| Gleason score | – | – | – | – |
 ≤6 ≤6 | 1083 | 40.2 | 162 | 26.6 |
 3 + 4 = 7 3 + 4 = 7 | 760 | 28.2 | 141 | 23.2 |
 4 + 3 = 7 4 + 3 = 7 | 416 | 15.4 | 136 | 22.3 |
 8 8 | 268 | 9.9 | 101 | 16.6 |
 9–10 9–10 | 167 | 6.2 | 69 | 11.3 |
| PSA | – | – | – | – |
 Median Median | 8.07 | – | 8.07 | – |
 ≤10 ng/ml ≤10 ng/ml | 1685 | 62.5 | 285 | 46.8 |
 >10 ng/ml >10 ng/ml | 1009 | 37.5 | 324 | 53.2 |
| % Positive cores | – | – | – | – |
 <50% <50% | 1375 | 51.0 | 206 | 33.8 |
 ≥50% ≥50% | 761 | 28.2 | 249 | 40.9 |
 Unknown Unknown | 558 | 20.7 | 154 | 25.3 |
| NCCN risk | – | – | – | – |
 Low Low | 590 | 21.9 | 58 | 9.5 |
 Intermediate Intermediate | 1289 | 47.8 | 230 | 37.8 |
 High High | 815 | 30.3 | 321 | 52.7 |
| Radiation dose | – | – | – | – |
 Median Median | 81 Gy | – | 81 Gy | – |
 Mean Mean | 82.2 Gy | – | 82.2 Gy | – |
 75.6 Gy 75.6 Gy | 467 | 17.3 | 170 | 27.9 |
 79.2–82.8 Gy 79.2–82.8 Gy | 1162 | 43.1 | 244 | 40.1 |
 86.4 Gy 86.4 Gy | 1065 | 39.5 | 195 | 32.0 |
| ADT | – | – | – | – |
 No No | 1245 | 46.2 | 275 | 45.2 |
 Yes Yes | 1449 | 53.8 | 334 | 54.8 |
 Median duration (mo) Median duration (mo) | 6 | – | 6 | – |
ADT = androgen deprivation therapy; NCCN = National Comprehensive Cancer Network; PSA = prostate-specific antigen.
In total, 609 of the 2694 patients (22.6%) undergoing EBRT experienced BF according to the Phoenix definition during the study follow-up. Amongst these patients, the median follow-up from the date of BF was 57 mo. For the subset of patients with BF, the median time from BF to clinically detected DM was 5.4 yr, with an estimated 5-yr post-BF DM rate of 47% (Fig. 2A). Similarly, the median time to PCSM was 10.5 yr from the date of BF (Fig. 2B). The 5-yr cumulative incidence of PCSM following BF was 18%.
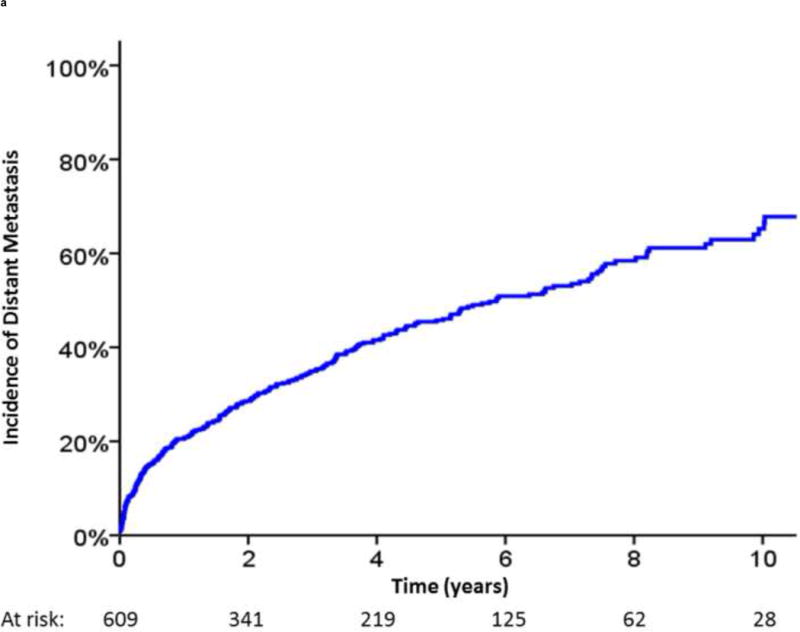
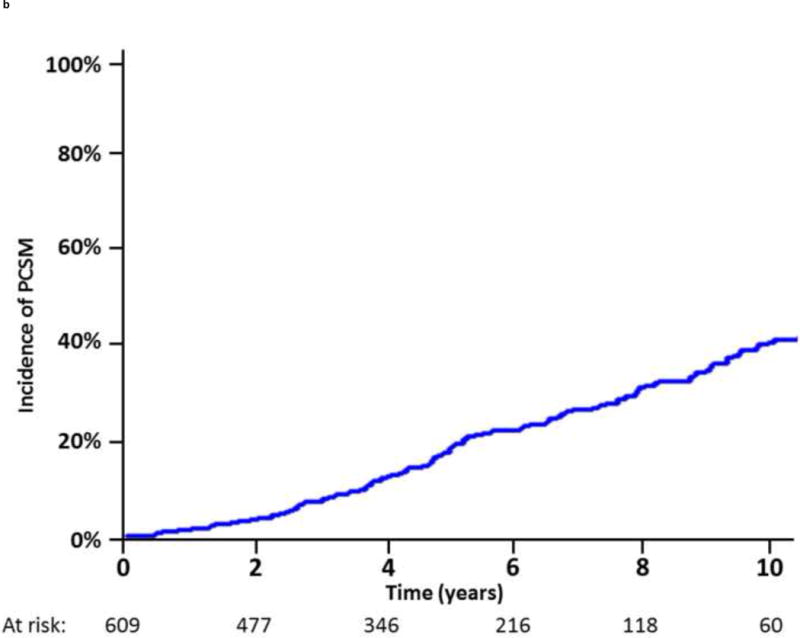
Incidence of (A) distant metastasis and (B) prostate cancer–specific mortality following biochemical failure.
Univariate analysis performed in the 609 patients with BF showed that PSA-DT, IBF, a Gleason score of 8–10, stages T2a or T3b–T4, and neoadjuvant ADT predicted for decreased time to DM, PCSM, and any death following BF (Table 2). A multivariate analysis in the subset of 464 patients with calculable PSA-DT showed that only a Gleason score of 8–10, tumor stage T2a, tumor stage T3b–T4, and PSA-DT were independent predictors of decreased time to DM, PCSM, and any death following BF. IBF was an independent predictor of DM, but not PCSM or death following BF. The inclusion of salvage ADT as a time-dependent covariate in our multivariate model did not significantly change these results in terms of predictors of DM following BF (Supplementary Table 2).
Table 2
Univariate and multivariate analysis of predictors of distant metastasis (DM), prostate cancer–specific mortality (PCSM), and overall survival (OS) from time of biochemical failure
| Time to DM | Time to PCSM | OS | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Univariate analysis | ||||||
 Gleason score Gleason score | ||||||
  ≤6 ≤6 | 1.0 (reference) | – | 1.0 (reference) | – | 1.0 (reference) | – |
  3 + 4 3 + 4 | 1.06 (0.66–1.70) | 0.81 | 1.95 (0.97–3.91) | 0.056 | 1.46 (0.92–2.32) | 0.1 |
  4 + 3 4 + 3 | 2.11 (1.36–3.30) | 0.0008 | 2.65 (1.34–5.26) | 0.004 | 1.56 (0.98–2.48) | 0.055 |
  8–10 8–10 | 3.75 (2.50–5.64) | <0.0001 | 4.72 (2.52–8.83) | <0.0001 | 2.01 (1.32–3.08) | 0.001 |
 Tumor stage Tumor stage | ||||||
  ≤T1c ≤T1c | 1.0 (reference) | – | 1.0 (reference) | – | 1.0 (reference) | – |
  T2a–c T2a–c | 1.82 (1.24–2.66) | 0.0017 | 2.00 (1.10–3.62) | 0.02 | 1.56 (1.02–2.36) | 0.034 |
  T3a T3a | 1.85 (0.98–3.51) | 0.054 | 1.32 (0.44–3.93) | 0.62 | 1.38 (0.68–2.78) | 0.36 |
  T3b–T4 T3b–T4 | 3.46 (2.26–5.28) | <0.0001 | 3.01 (1.61–5.61) | 0.0004 | 1.87 (1.16–3.00) | 0.008 |
 Log PSA Log PSA | 1.14 (1.01–1.28) | 0.028 | 1.09 (0.92–1.29) | 0.31 | 0.99 (0.87–1.13) | 0.94 |
 Neoadjuvant ADT Neoadjuvant ADT | 1.94 (1.43–2.63) | <0.0001 | 1.59 (1.06–2.39) | 0.023 | 1.45 (1.06–1.98) | 0.019 |
 Age Age | 1.00 (0.98–1.02) | 0.77 | 0.99 (0.96–1.02) | 0.5 | 1.02 (1.00–1.05) | 0.025 |
 Radiation dose Radiation dose | 1.00 (0.74–1.37) | 0.99 | 1.33 (0.89–2.00) | 0.16 | 1.09 (0.79–1.51) | 0.79 |
 IBF IBF | 0.79 (0.73–0.85) | <0.0001 | 0.80 (0.70–0.90) | 0.0002 | 0.93 (0.87–1.00) | 0.036 |
 PSA-DT PSA-DT | 0.97 (0.96–0.98) | <0.0001 | 0.97 (0.96–0.99) | 0.0005 | 0.99 (0.98–1.00) | 0.02 |
| Multivariate analysis | ||||||
 Gleason score Gleason score | ||||||
 ≤6 ≤6 | 1.0 (reference) | – | 1.0 (reference) | – | 1.0 (reference) | – |
 3 + 4 3 + 4 | 0.99 (0.60–1.63) | 0.96 | 1.69 (0.85–3.40) | 0.13 | 1.31 (0.81–2.10) | 0.26 |
 4 + 3 4 + 3 | 1.55 (0.98–2.47) | 0.06 | 2.11 (1.03–4.34) | 0.038 | 1.30 (0.79–2.13) | 0.29 |
 8–10 8–10 | 2.53 (1.62–3.94) | <0.0001 | 3.61 (1.87–6.94) | <0.0001 | 1.61 (1.01–2.57) | 0.043 |
 Tumor stage Tumor stage | ||||||
 ≤T1c ≤T1c | 1.0 (reference) | – | 1.0 (reference) | – | 1.0 (reference) | – |
 T2a–c T2a–c | 1.68 (1.13–2.50) | 0.01 | 1.98 (1.05–3.72) | 0.03 | 1.52 (0.99–2.33) | 0.051 |
 T3a T3a | 1.71 (0.89–3.31) | 0.10 | 0.97 (0.27–3.48) | 0.96 | 1.25 (0.61–2.59) | 0.53 |
 T3b–T4 T3b–T4 | 2.44 (1.54–3.87) | 0.0001 | 2.53 (1.26–5.10) | 0.008 | 1.72 (1.03–2.86) | 0.034 |
 Log PSA Log PSA | 0.96 (0.83–1.08) | 0.42 | 0.99 (0.82–1.20) | 0.96 | 0.95 (0.82–1.09) | 0.44 |
 Neoadjuvant ADT Neoadjuvant ADT | 0.97 (0.66–1.40) | 0.85 | 0.71 (0.42–1.20) | 0.16 | 1.11 (0.76–1.62) | 0.58 |
 Age Age | 1.00 (0.98–1.02) | 0.96 | 0.99 (0.96–1.02) | 0.39 | 1.02 (1.00–1.05) | 0.034 |
 Radiation dose Radiation dose | 1.17 (0.83–1.667) | 0.36 | 1.55 (0.96–2.52) | 0.08 | 1.14 (0.80–1.63) | 0.45 |
 IBF IBF | 0.90 (0.83–0.98) | 0.01 | 0.90 (0.78–1.04) | 0.16 | 0.98 (0.90–1.07) | 0.64 |
 PSA-DT PSA-DT | 0.98 (0.97–0.995) | 0.008 | 0.98 (0.96–0.999) | 0.035 | 0.99 (0.98–1.01) | 0.31 |
ADT = androgen deprivation therapy; BF = biochemical failure; CI = confidence interval; IBF = interval to biochemical failure; HR = hazard ratio; PSA = prostate-specific antigen; PSA-DT = PSA doubling time.
Additionally, given the clinical utility of specific cut-off points in clinical medicine, we investigated the optimal cut-off points for PSA-DT and IBF using maximally selected chi-square statistics. For PSA-DT, we found that the 10th percentile, 3.2 mo, and for IBF, the 30th percentile, 2.9 yr, yielded the optimal cut-off points in our dataset. In the 5 yr after BF, patients with a PSA-DT ≤3.2 months experienced a significantly higher risk of DM (92.6% vs 35.5%; hazard ratio [HR], 5.09 [95% CI, 3.54–7.32]; p < 0.0001) and PCSM (34.4% vs 11.7%; HR, 3.65 [95% CI, 2.26–5.91]; p < 0.0001) compared with those patients with a PSA-DT >3.2 mo (Fig. 3A and and3B).3B). Similarly, patients with an IBF ≤2.9 yr had increased DM (68.3% vs 32.7%; HR, 2.675 [95% CI, 2.11–3.38]; p < 0.0001) and PCSM (28.3% vs 11.1%; HR, 2.45 [95% CI, 1.76–3.43]; p < 0.0001) at 5 yr after BF compared with those patients with a longer IBF (Fig. 3C and and3D3D).
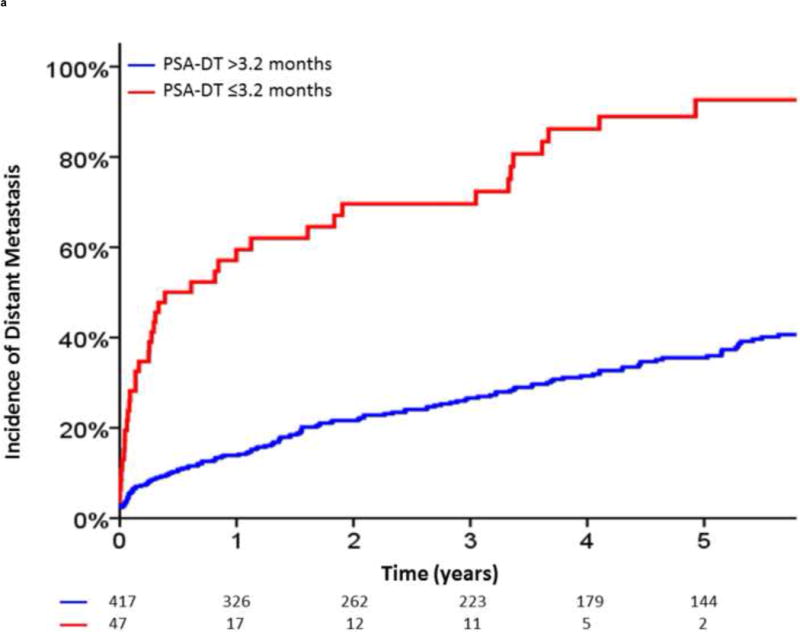
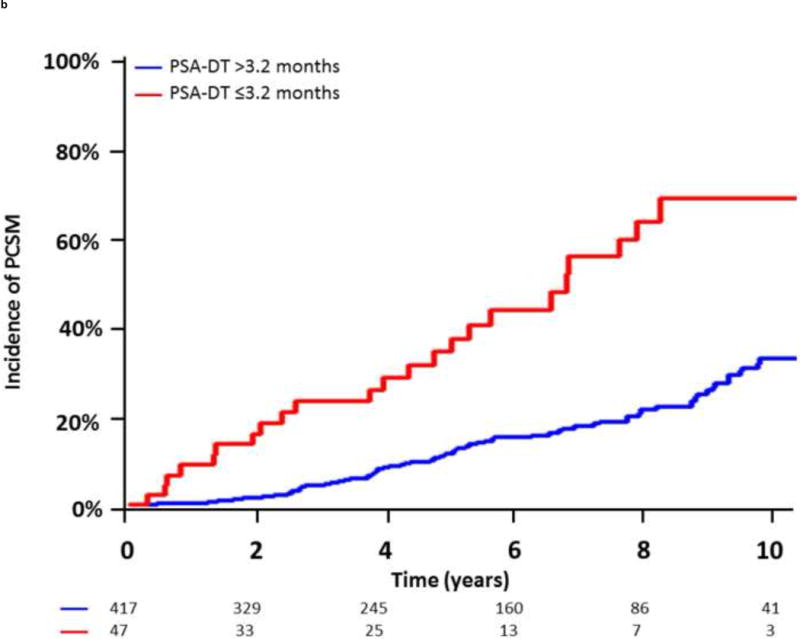
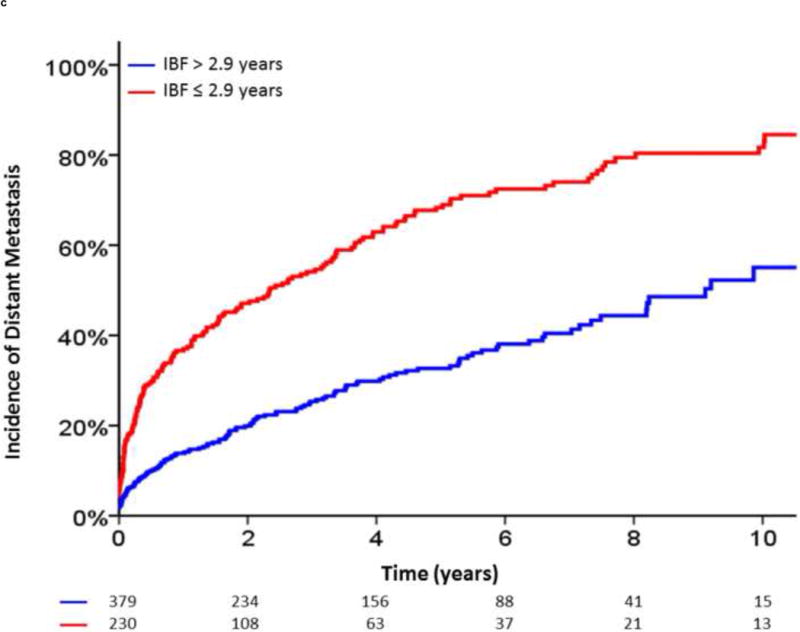
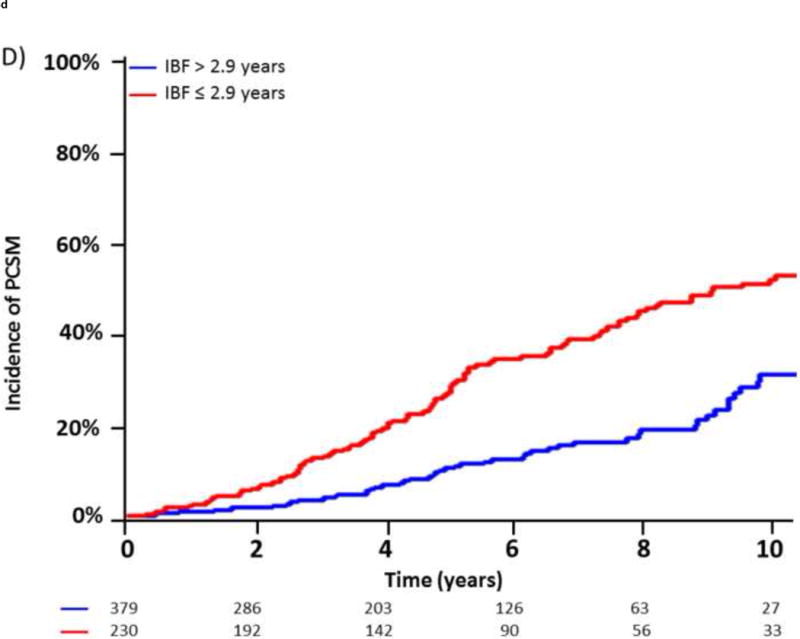
Incidence of distant metastasis and prostate cancer–specific mortality following biochemical failure, stratified by (A,B) a prostate-specific antigen doubling time (PSA-DT) cut-off point of 3.2 mo or (C,D) an interval to biochemical failure (IBF) cut-off point of 2.9 yr.
Based on these analyses, we defined unfavorable risk factors for distant progression following BF as a pretreatment Gleason score of 8–10, a pretreatment clinical tumor stage of T3b–T4, a posttreatment PSA-DT of <3 mo, or an IBF <3 yr (Fig. 4A and and4B).4B). In the 464 patients with BF and calculable PSA-DT, those with exactly one of these risk factors (HR, 2.36 [95% CI, 1.64–3.96]; p < 0.001) and those with two or more risk factors (HR, 5.67 [95% CI, 3.96–8.12]; p < 0.001) had a significantly higher risk of clinical progression following BF than those without these risk factors. In addition, patients with exactly one risk factor (HR, 1.94 [95% CI, 1.12– 3.37]; p = 0.018) and patients with two or more risk factors (HR, 4.18 [95% CI, 2.49–7.01]; p < 0.001) had an increased incidence of PCSM following BF.
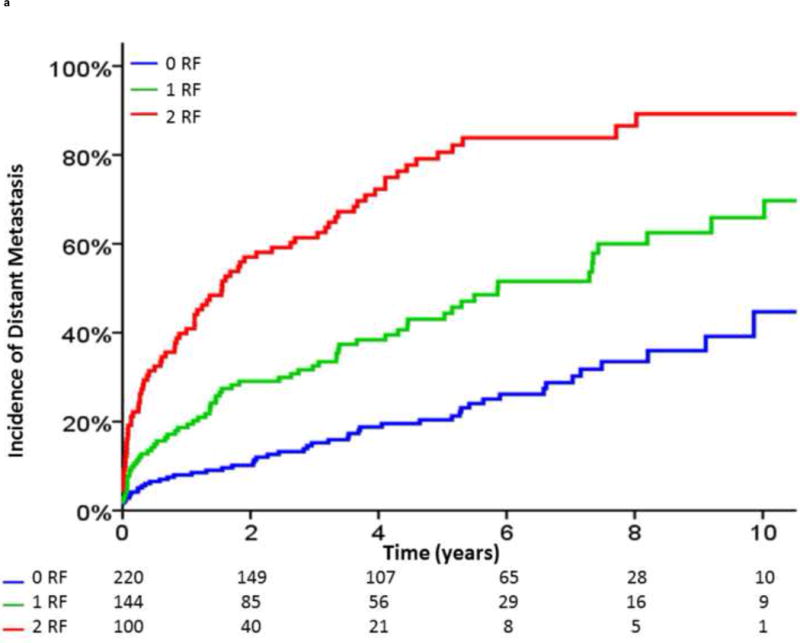
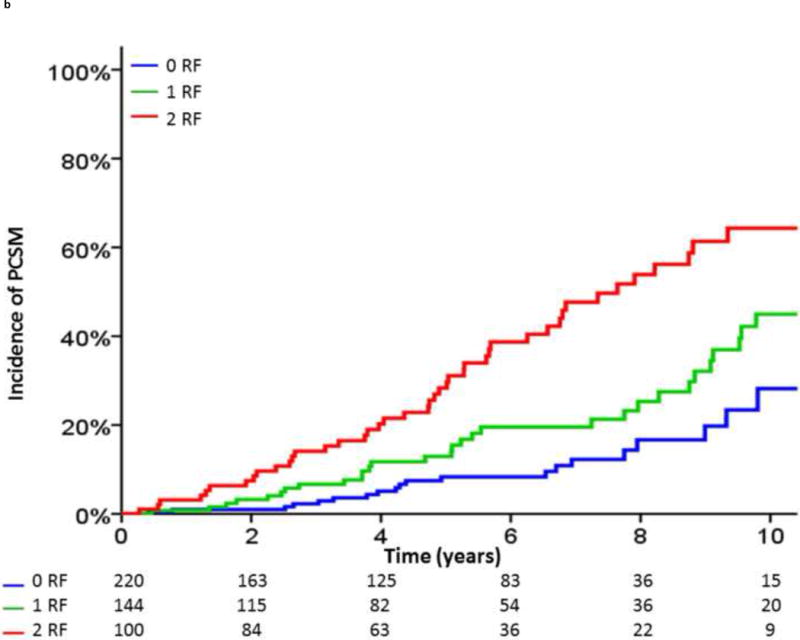
Incidence of (A) distant metastasis and (B) prostate cancer–specific mortality following biochemical failure, stratified by the number of unfavorable risk factors (RF). Unfavorable RFs were defined as a pretreatment Gleason score of 8–10, a pretreatment clinical tumor stage of T3b or T4, a posttreatment prostate-specific antigen (PSA) doubling time of <3 mo, or an interval to biochemical failure of <3 yr.
4. Discussion
Characterizing the natural history of patients with BF following EBRT is important for counseling patients regarding prognosis, and for making judicious recommendations for salvage therapies. This study provides, to our knowledge, the largest series describing the natural history and predictors of outcome of biochemically recurrent prostate cancer in a cohort of patients exclusively treated with dose-escalated EBRT, and it adds to the existing literature in several ways. Although the natural history of recurrent prostate cancer has been well characterized after prostatectomy [8–10], the natural history of recurrent prostate cancer following radiotherapy, surprisingly, has not. We found that the median intervals from BF to DM and PCSM were 5.4 yr and 10.5 yr, respectively. Notably, these time intervals are significantly shorter than those described in RP series [8,9], despite the fact that 18% of patients in our study received at least one form of ADT prior to clinical failure. This is most likely due to fundamental differences in the posttreatment PSA kinetics, and in the definition of BF following prostatectomy and EBRT. Following RP, PSA typically reaches a nadir that is undetectable within weeks of surgery, and BF is most commonly considered as a postprostatectomy PSA of 0.2 or 0.4 ng/ml. In comparison, following EBRT, BF is most commonly defined as 2 ng/ml above the postradiation nadir PSA, which typically occurs several years after the completion of radiotherapy [12]. This explains why a large prostatectomy series from Johns Hopkins reported that 45% of BFs occurred within the first 24 mo following treatment [8], whereas only 26% of BFs occurred during this time interval in our series. Therefore, although our data confirm that predefined BF occurs later in the course of disease progression for EBRT-treated patients when compared with RP-treated patients [16], it would not be accurate to conclude that the absolute time from treatment to DM or PCSM is likely to be different between these two modalities.
Our findings also have important implications for salvage therapy after the development of BF following EBRT. Multiple large single- and multi-institutional retrospective series have shown that, following RP, salvage RT is most likely to be effective for patients with lower PSA values, whereas patients with a presalvage PSA of ≥2 ng/ml experience little benefit from salvage radiotherapy [17–20]. Extrapolating from these data, it is likely that waiting until the PSA reaches 2 ng/ml more than the nadir PSA is not the optimal time to consider local salvage therapy following EBRT, and this highlights one of the primary challenges in the management of patients following EBRT: how to best identify those patients that will experience clinical recurrence at the earliest possible time. This is a formidable challenge that is difficult to address with currently available clinical tools, given that PSA thresholds and widely available imaging techniques lack both sensitivity and specificity for identifying early recurrence. Employing prostate MRI or posttreatment prostate biopsies after consecutive PSA increases following a PSA nadir, even before Phoenix-defined BF, would increase early detection of locally recurrent or persistent disease. However, postradiation PSA kinetics are complicated to interpret, given that testosterone recovery following ADT, the PSA bounce phenomenon, infection, sexual activity, and many other factors can all cause increases in PSA levels, unrelated to cancer. Ultimately, future advances in molecular imaging, circulating tumor cells, or other techniques may allow the detection of residual prostate cancer at much early stages in the natural history of the disease with greater specificity, and will allow earlier salvage interventions.
Another challenge of the management of BF is that a rising posttreatment PSA could signify local disease progression, regional relapse, distant spread, or a combination of these three events. Distinguishing between these possibilities, although often extremely difficult, is of tremendous importance for further management recommendations. Patients with locally confined disease are potentially curable with either salvage prostatectomy [21] or salvage brachytherapy [22,23], whereas patients with distant metastatic disease could be spared the morbidity of local salvage therapy. Nevertheless, determining the site of clinical failure in patients with a low burden of disease is challenging with modern diagnostic tools, and novel molecular imaging modalities with greater sensitivity and specificity for detection of micrometastatic prostate cancer should be pursued.
Posttreatment PSA-DT was found to be a strong independent predictor of DM and PCSM following BF in our multivariate model. This is consistent with multiple previous studies showing that posttreatment PSA-DT is a critical predictor of adverse outcome following both standard dose EBRT [24–26] and RP [9,19,27]. Unfortunately, there has been no widely accepted definition of posttreatment PSA-DT with respect to PSA values to include in this calculation. For example, several studies have calculated the PSA-DT using PSA measurements from BF to initiation of salvage therapy [24,25]. However, this definition is not useful for predicting outcome at the time of BF, given that it requires knowledge of future PSA values occurring after BF. To avoid this limitation, we chose to calculate the PSA-DT using all PSA values from the last nonrising PSA to the time of BF, similar to another recent multi-institutional study including patients with BF following EBRT without concomitant ADT [28].
Additionally, we also determined that the most discriminating posttreatment PSA-DT cut-off point for predicting DM following BF was 3.2 mo in our dataset, based on an arbitrary division of posttreatment PSA-DT into deciles. This value is in close agreement with multiple previous studies demonstrating inferior outcomes in patients with a posttreatment PSA-DT <3 mo [24,26,29]. Patients with PSA-DT ≤3.2 mo had remarkably poor outcomes following BF, with 93% experiencing DM and 34% dying from prostate cancer within 5 yr. However, although clearly patients meeting this criterion have aggressive disease and may warrant more aggressive management, only 10% of our cohort fell below this PSA-DT threshold. Therefore, although PSA-DT is a powerful predictor of outcome early clinical progression following BF, other predictors are needed for the 90% of patients with PSA-DT >3 mo.
For this reason, we defined unfavorable risk factors for clinical progression following BF as a pretreatment Gleason score of 8–10, a pretreatment clinical tumor stage of T3b or T4, a posttreatment PSA-DT <3 mo, or an IBF of <3 yr, given that these variables were independent predictors of clinical progression in our multivariate models. Patients with two or more unfavorable risk factors were five times as likely to experience DM, and four times as likely to experience PCSM following BF, as those patients without these risk factors. Therefore, these patients represent a cohort with aggressive disease that may benefit from novel therapeutic strategies and should be the focus of future clinical trials. We further propose a novel risk-stratification system for patients experiencing BF after EBRT, with patients with none, one, or two or more unfavorable risk factors being grouped into low-, intermediate-, and high-risk groups for distant progression.
Several weaknesses of this study warrant further discussion. Most significantly, this is a nonrandomized, retrospective series and, therefore, is subject to the inherent biases of all such studies. Furthermore, no attempt was made to adjust for the salvage therapies received by our cohort, including in the 18% of patients that received ADT prior to clinical progression. Although the impact of salvage therapy on mortality in the setting of BF after EBRT is unclear, differences in its delivery could have impacted our results.
5. Conclusions
In summary, in a relatively large cohort of patients treated with modern dose-escalated EBRT, we found that the median times to DM and PCSM were approximately 5 yr and 10 yr, respectively. Furthermore, patients with a posttreatment PSA-DT less than approximately 3 mo, an IBF of less than approximately 3 yr, a pretreatment Gleason score of 8–10, or pretreatment clinical T3b or T4 disease were at significantly higher risk of clinical progression following BF than those patients without these factors. Ultimately, the main drivers of outcome following BF identified in our series, including PSA-DT, IBF, and Gleason score, are likely surrogates for the underlying genomic and epigenomic aberrations that drive a particular cancer’s progression. Understanding the biology of prostate cancer progression, combined with novel molecular imaging techniques capable of identifying prostate cancer recurrence early in the disease course, may eventually improve the capability of physicians not only to predict clinical outcome following BF but also to actually intervene with therapies that positively impact the clinical outcome.
Supplementary Material
1
2
3
4
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was presented in part at the 2013 Annual American Society for Radiation Oncology (ASTRO) conference in Atlanta, GA, USA.
Author contributions: Michael J. Zelefsky had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: ZZ, Zelefsky.Acquisition of data: ZZ, Spratt, Pei, Romesser.
Analysis and interpretation of data: Zumsteg, Pei, Zhang, Polkinghorn, McBride, Kollmeier, Yamada, Zelefsky.
Drafting of the manuscript: ZZ, Zelefsky.
Critical revision of the manuscript for important intellectual content: ZZ, Zelefsky.
Statistical analysis: Zhang.
Obtaining funding: None.
Administrative, technical, or material support: Pei, Zelefsky.
Supervision: Zelefsky.
Other (specify): None.
Financial disclosures: Michael J. Zelefsky certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.eururo.2014.09.028
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc5002994?pdf=render
Citations & impact
Impact metrics
Article citations
Reirradiation - still navigating uncharted waters?
Clin Transl Radiat Oncol, 49:100871, 02 Oct 2024
Cited by: 0 articles | PMID: 39444538 | PMCID: PMC11497423
Review Free full text in Europe PMC
Neoadjuvant lutetium PSMA, the TIME and immune response in high-risk localized prostate cancer.
Nat Rev Urol, 21(11):676-686, 07 Aug 2024
Cited by: 1 article | PMID: 39112733
Review
Integrative analysis regarding the correlation between collagen-related genes and prostate cancer.
BMC Cancer, 24(1):1038, 22 Aug 2024
Cited by: 0 articles | PMID: 39174928 | PMCID: PMC11342612
Utilizing Liquid-liquid phase separation-related lncRNAs to predict the prognosis and treatment response of PCa.
Discov Oncol, 15(1):352, 16 Aug 2024
Cited by: 0 articles | PMID: 39150479 | PMCID: PMC11329450
Clinical Outcomes and Prognostic Factors in Nonmetastatic Castration-Resistant Prostate Cancer Treated with Androgen Receptor Signaling Inhibitors Therapy.
Cancers (Basel), 16(15):2659, 26 Jul 2024
Cited by: 0 articles | PMID: 39123387 | PMCID: PMC11312153
Go to all (94) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Dose escalation for prostate cancer radiotherapy: predictors of long-term biochemical tumor control and distant metastases-free survival outcomes.
Eur Urol, 60(6):1133-1139, 22 Aug 2011
Cited by: 111 articles | PMID: 21889832 | PMCID: PMC4037155
Impact of biochemical failure on overall survival after radiation therapy for localized prostate cancer in the PSA era.
Int J Radiat Oncol Biol Phys, 52(3):704-711, 01 Mar 2002
Cited by: 66 articles | PMID: 11849793
Prostate-Specific Antigen (PSA) Bounce After Dose-Escalated External Beam Radiation Therapy Is an Independent Predictor of PSA Recurrence, Metastasis, and Survival in Prostate Adenocarcinoma Patients.
Int J Radiat Oncol Biol Phys, 100(1):59-67, 13 Oct 2017
Cited by: 8 articles | PMID: 29254782 | PMCID: PMC7402025
A systematic overview of radiation therapy effects in prostate cancer.
Acta Oncol, 43(4):316-381, 01 Jan 2004
Cited by: 75 articles | PMID: 15303499
Review





