Abstract
Free full text

Pneumococcal nasopharyngeal carriage in children <5 years of age visiting the pediatric emergency room in relation to PCV7 and PCV13 introduction in southern Israel
ABSTRACT
The 7-valent and the 13-valent pneumococcal conjugate vaccines (PCV7 and PCV13, respectively) were introduced to the Israeli National Immunization plan in July 2009 and November 2010, respectively. Our aim was to assess pneumococcal conjugate vaccines (PCVs) uptake and dynamics in serotype-specific pneumococcal nasopharyngeal (NP) carriage in children <5 years old in southern Israel, during the immediate 5 y following PCV introduction. This was an ongoing, prospective, population-based, active surveillance, from July 2009 through December 2014. PCVs uptake and NP cultures were obtained daily from children seen at the Pediatric Emergency Room for any reason. Overall, 10,702 vaccine status and 7,610 NP swabs were obtained. Both PCV7 and PCV13 uptake were high, reaching ˜90% by July 2012 and December 2013, respectively. All-pneumococcal carriage rates significantly declined by 10%, from 54.3% in the early-PCV7 period, to 49.1% in the PCV13 impact period. The respective declines for PCV7, 6A and additional PCV13 serotypes carriage rates were 76%, 90% and 66%. In contrast, non-PCV13 serotypes carriage rates increased significantly throughout the study by 71%. All-pneumococcal carriage rates in children <12 months old decreased significantly by 15%, with similar trends observed in other age groups. Initially, all-pneumococcal carriage rates were 45.7%, and 61.9% in Jewish and Bedouin children, respectively (P < 0.001), with a significant 17% reduction throughout the study observed only in Bedouins. While early carriage rates were higher in unvaccinated children compared to vaccinated children, PCV impact on carriage were similar in both groups. In conclusion, a relatively moderate decline in pneumococcal carriage rates, facilitated by a substantial decrease of vaccine-serotypes and increase of non-vaccine serotypes was observed in the immediate period following PCVs introduction in southern Israel.
Abbreviations
- NP
- nasopharyngeal
- PCV
- pneumococcal conjugate vaccines
- NIP
- national immunization plan
- IPD
- invasive pneumococcal disease
- VT
- vaccine serotype
- PCV13
- 13-valent pneumococcal conjugate vaccine
- PCV7
- 7-valent pneumococcal conjugate vaccine
- LRTI
- lower respiratory tract infection
- PER
- pediatric emergency room
- VT7
- PCV7 serotypes
- VT5
- 6 additional PCV13 serotypes excluding 6A
- SUMC
- Soroka University Medical Center
- VT6
- 6 additional PCV13 serotypes
- VT13
- PCV13 serotypes
Introduction
Streptococcus pneumoniae is a leading cause of childhood morbidity and mortality, and is a leading bacterial cause of invasive disease, pneumonia, and acute otitis media in children.1 It is estimated that S. pneumoniae infections, mainly pneumonia and invasive disease, are responsible for approximately 500,000 deaths annually among children <5 years of age, worldwide. The majority of these deaths occur in developing countries.2 Furthermore, hospitalization for pneumonia and use of medical services for otitis media constitute a considerable economic burden, particularly among the very young population (<5 years old).3,4
Nasopharyngeal (NP) carriage of S. pneumoniae precedes disease and is the source of pneumococcal spread in the community.5,6 As asymptomatic pneumococcal carriage is essential for the development of pneumococcal disease, it is not surprising that the peak age for colonization, transmission and disease coincides with early childhood.6 The spread of S. pneumoniae beyond the nasopharynx (promoting pneumococcal disease) depends upon several factors including the virulence of the organism/specific serotype, the host immunocompetence, and the existence of preceding viral infection.7 Pneumococcal NP carriage rates vary in different settings worldwide.8-10 This variation may derive, at least in part, from demographic, ethnical and cultural differences, with higher carriage rates observed in populations suffering from poverty, over-crowding, exposure to smoking and poor hygienic conditions,10-12 as well as history of breast feeding, day-care center attendance and antibiotic treatment at the individual and societal levels.7
The introduction of the pneumococcal conjugate vaccines (PCVs) to the national immunization plans (NIP) worldwide resulted in substantial rate reductions of various pneumococcal disease end-points, including invasive pneumococcal disease (IPD),13,14 pneumonia,15-18 and otitis media,3 including unvaccinated age groups and individuals.17-21
Pneumococcal conjugate vaccines protect against carriage among vaccinated individuals and therefore prevent transmission to unvaccinated children and susceptible adults. This indirect (herd) protection increases PCV impact, both in terms of population health gain and economic value.19,20 The reduction observed in pneumococcal disease rates in the unimmunized (indirect protection) is mainly attributed to the reduction in nasopharyngeal carriage of S. pneumoniae serotypes included in the vaccine (VT) in vaccinated children.9,20,21 Reduction in pneumococcal nasopharyngeal colonization is achieved by preventing new acquisitions, and there is currently no evidence of a direct vaccine impact on the time it takes to clear a pneumococcal colonization episode.22,23
In contrast to the impressive impact of PCVs on different pneumococcal diseases, with the potential of rapidly achieving near eradication of vaccine serotype disease in the vaccinated age group,3,14 vaccine impact on overall pneumococcal carriage was less prominent.8-10 This lesser effect was observed in many studies,6,7,19,24 however, over time, vaccine impact on VT-carriage reached a magnitude similar to that observed with IPD.10,19 In contrast to IPD, replacement of NP carriage of VT serotype by non-VT serotypes occurred rapidly and extensively, leading to increased colonization and transmission of non-VT strains.10,19,25
The 13-valent pneumococcal conjugate vaccine (PCV13) introduction resulted in lower acquisition and prevalence of NP colonization than the 7-valent pneumococcal conjugate vaccine (PCV7) for the majority of additional PCV13 serotypes as well as serotypes 6C and 19F, and it was comparable to PCV7 with regard to all other common serotypes.1,25 Further beneficial impact of PCVs, beyond reduction of disease and carriage rates was reduction in antibiotic use and in pneumococcal antibiotic resistance.8,26-28
Evidently, PCV impact on pneumococcal disease and carriage rates depends also on the individually established vaccine efficacy, vaccine uptake, adherence to plans.29 and potentially vaccine dosing regimens.9,30
PCV7 was licensed in Israel in mid-2007, and was used sporadically until 2009. In July 2009, PCV7 was introduced to the NIP (administered at age 2, 4, and 12 months) with a catch-up campaign in all children born since July 2007. In November 2010, PCV13 gradually replaced PCV7, without a further catch-up, as was described previously.3
In southern Israel, the children population consists of 2 distinct ethnic groups, Jewish and Bedouin children, who differ considerably with regard to their socio-economic and cultural characteristics, and who have minimal contact during childhood.3,31 In general, the Bedouin population live under lower socioeconomic conditions with more crowded living conditions, a greater proportion of children <5 years, and a lower proportion of elderly persons, as well as a more rapid population growth than the Jewish population. Prior to vaccination, higher rates of pneumococcal nasopharyngeal carriage, respiratory infections, and IPD were reported among the Bedouin population.3,14
The aims of this prospective study were: 1) To evaluate PCV uptake in children <5 years old in southern Israel; and 2) to assess the dynamics in the overall and serotype-specific pneumococcal NP carriage during the immediate 5 y following PCV introduction.
Results
During the study period (July 2009 through December 2014), 7,610 NP swabs were obtained; 3,110 (40.9%) and 4,500 (59.1%) from Jewish and Bedouin children, respectively (Fig. S1). Vaccine status was obtained from 10,702 children; 5,299 (49.5%) and 5,403 (50.5%) from Jewish and Bedouin children, respectively.
Our study population was younger than the region overall population <5 years old, with a median age of 13 months vs. Thirty months, respectively. The proportion of children <1 year of age was 47.1% vs. ~20%, respectively. Moreover, our study population consisted of ~60% Bedouin children and ~40% Jewish children, compared with ~50% in each group in the overall region population <5 years old.
During the study period, lower respiratory tract infections (LRTI) rates were stable, with ~20% of all pediatric emergency room (PER) visits attributed to LRTI, in each of the study years.
Pcv uptake
PCV uptake ≥2 doses in children 12–23 months old
PCV7 vaccination status was obtained from 2,787 children 12–23 months old, from July 2009 through December 2014. The proportion of children who had received ≥2 doses of PCV7 was 14.5% in the period between July and September 2009, increasing to ~95% in the period between January and March 2011, thereof declining as PCV13 was introduced, reaching 0% by December 2012. The proportion of children who had received ≥2 doses of PCV13 was 1.3% and 10.0% in the period between July 2009 and June 2011, respectively, increasing to ~95% since July 2012 (Fig. 1).
PCV uptake ≥3 doses in children 24–35 months old
PCV7 vaccination status data were obtained from 1,424 children 24–35 months old, during July 2009 through December 2014. The proportion of children who had received ≥3 doses of PCV7 was 1.5% in the period between July and September 2009, increasing to 86.0% in the period between April and June 2012, then declining as PCV13 was introduced, reaching 0% by December 2013. The proportions of children who had received ≥3 doses of PCV13 were 0.0% and 7.0% in the period between July 2009 and September 2012, respectively, increasing to 19.1% since December 2012, and ~90% since December 2013 until the end of the surveillance.
Both PCV7 and PCV13 uptake rates were similar in Jewish and Bedouin children (data not shown).
PCV7 serotypes (VT7), 6A, 6 PCV13 serotypes excluding 6A (1, 3, 5, 6A and 7F, VT5) and all pneumococcal carriage in children <5 years old
The proportion of children carrying VT7, 6A and VT5 declined by 76%, 90% and 66%, respectively, from the early PCV7 period to the PCV13 impact period. These reductions were observed in a 2-step manner, with VT7 rates declining significantly both in the PCV7 and the PCV13 periods, while 6A and VT5 rates did not vary significantly in the PCV7 period and later declined in the PCV13 period (Table 1, Fig. 2, Fig. S2).
Table 1.
Pneumococcal carriage rates and odds ratios (ORs) in children < 5 y presenting to the pediatric emergency room, southern Israel - All children.
| VT7 | 6A | VT5 | Non-13VT | Overall pneumococcal carriage | |
|---|---|---|---|---|---|
| Nov 2009 – Jun 2010 (early PCV7) | 16.80% | 5.10% | 8.10% | 24.40% | 54.30% |
| Jul 2010 – Jun 2011 (PCV7 impact) | 9.60% | 4.40% | 8.50% | 31.70% | 54.20% |
| Jul 2011 – Jun 2012 (early PCV13, interim) | 5.90% | 2.00% | 5.60% | 35.70% | 49.10% |
| Jul 2012 – Jun 2013 (early PCV13, interim) | 4.60% | 1.00% | 3.90% | 38.00% | 47.50% |
| Jul 2013 – Jun 2014 (PCV13 impact) | 4.00% | 0.50% | 2.80% | 41.80% | 49.10% |
| Jul 2014 – Dec 2014 | 3.40% | 0.10% | 2.10% | 36.00% | 41.60% |
| OR early PCV7 vs. PCV7 impact periods | 0.57 (0.47–0.70) | 0.86 (0.61–1.21) | 1.05 (0.81–1.36) | 1.30 (1.15–1.48) | 0.99 (0.93–1.07) |
| OR PCV7 impact vs. PCV13 impact periods | 0.42 (0.31–0.57) | 0.12 (0.06–0.26) | 0.33 (0.23–0.47) | 1.32 (1.20–1.45) | 0.91 (0.84–0.97) |
| OR early PCV7 vs. PCV13 impact periods | 0.24 (0.18–0.32) | 0.10 (0.05–0.23) | 0.34 (0.24–0.50) | 1.71 (1.51–1.94) | 0.90 (0.84–0.98) |
| (Overall impact) |

Pneumococcal carriage in children <5 years old attending pediatric emergency room, southern Israel, November 2009 through December 2014.
In contrast, non-PCV13 serotypes (VT13) rates increased significantly throughout the study by 71%.
All pneumococcal carriage rates significantly declined by 10%, from 54.3% of all tested children in the early PCV7 period to 49.1% in the PCV13 impact period.
All pneumococcal carriage by age groups and ethnicity
As the age distribution of our study population differed from that of the entire <5 years old distribution (with the former constituting 47% of children <1 year old compared with ~20% in the latter) we analyzed carriage rate throughout the study in different age groups.
Pneumococcal carriage rates in the early-PCV7 period were 54.4%, 54.6%, 54.1% and 53.5% in children <12 , 12–23, 24–35 and 36–59 months old (P = non-significant for all comparisons between the age groups). By the PCV13 impact period, carriage rates in children <12 months of age had decreased significantly by 15%. Comparable reduction trends were noted, but did not reach statistical significance in the other age groups (Table 2).
Table 2.
Pneumococcal carriage rates and odds ratios (ORs) in children <12 , 12–23, 24–35, 36–59 months old presenting to the pediatric emergency room, southern Israel - All children.
| <12m n = 3,579 | 12–23m n = 1, 955 | 24–35m n = 989 | 36–59m n = 1, 087 | |
|---|---|---|---|---|
| Nov 2009 – Jun 2010 (early PCV7) | 54.40% | 54.60% | 54.10% | 53.50% |
| Jul 2010 – Jun 2011 (PCV7 impact) | 55.00% | 56.00% | 53.90% | 47.70% |
| Jul 2011 – Jun 2012 (early PCV13, interim) | 51.20% | 48.00% | 47.50% | 45.80% |
| Jul 2012 – Jun 2013 (early PCV13, interim) | 51.60% | 50.80% | 41.10% | 49.90% |
| Jul 2013 – Jun 2014 (PCV13 impact) | 46.10% | 50.10% | 49.90% | 47.20% |
| Jul 2014 – Dec 2014 | 40.10% | 48.10% | 30.60% | 43.20% |
| OR early PCV7 vs. PCV7 impact periods | 1.01 (0.91–1.12) | 1.03 (0.90–1.17) | 1.00 (0.82–1.21) | 0.89 (0.72–1.10) |
| OR PCV7 impact vs. PCV13 impact periods | 0.84 (0.75–0.93) | 0.90 (0.78–1.03) | 0.93 (0.77–1.12) | 0.99 (0.81–1.22) |
| OR early PCV7 vs. PCV13 impact periods (Overall impact) | 0.85 (0.75–0.95) | 0.92 (0.79–1.06) | 0.92 (0.75–1.14) | 0.89 (0.72–1.10) |
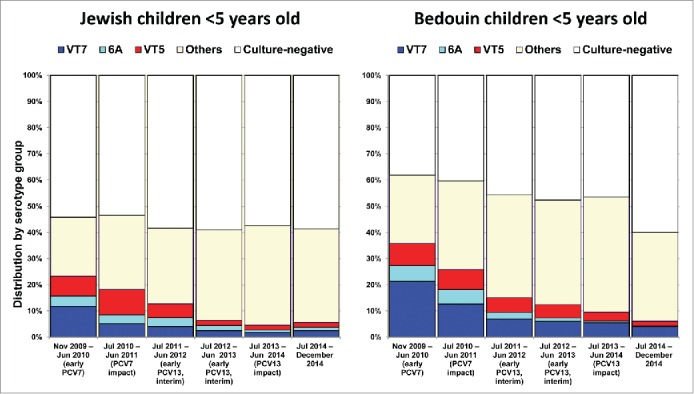
In the early PCV7 period, all-pneumococcal carriage rates were 45.7%, and 61.9% in Jewish and Bedouin children <5 years old, respectively (P < 0.001). By the PCV7 impact period, all-pneumococcal carriage rates had not changed significantly (Fig. 3, Table 3). However, by the PCV13 impact period (compared with the early PCV7 period), all-pneumococcal carriage rates had decreased non-significantly by 8% in the Jewish population and significantly by 17% in the Bedouin population.
Table 3.
Pneumococcal carriage rates and odds ratios (ORs) in Jewish and Bedouin children <5 years old presenting to the pediatric emergency room, southern Israel - All children.
| Jewish children n = 3, 110 | Bedouin children n = 4, 500 | p value | |
|---|---|---|---|
| Nov 2009 – Jun 2010 (early PCV7) | 45.70% | 61.90% | <0.001 |
| Jul 2010 – Jun 2011 (PCV7 impact) | 46.00% | 59.70% | <0.001 |
| Jul 2011 – Jun 2012 (early PCV13, interim) | 40.30% | 54.20% | <0.001 |
| Jul 2012 – Jun 2013 (early PCV13, interim) | 41.60% | 55.20% | <0.001 |
| Jul 2013 – Jun 2014 (PCV13 impact) | 42.10% | 51.20% | 0.002 |
| Jul 2014 – Dec 2014 | 42.00% | 41.60% | 0.91 |
| OR early PCV7 vs. PCV7 impact periods | 1.01 (0.89–1.14) | 0.96 (0.89–1.05) | ns |
| OR PCV7 impact vs. PCV13 impact periods | 0.92 (0.80–1.04) | 0.86 (0.79–0.93) | ns |
| OR early PCV7 vs. PCV13 impact periods (Overall impact) | 0.92 (0.80–1.06) | 0.83 (0.75–0.91) | ns |
Pneumococcal carriage in children 7–11, 12–23 and 24–59 months old: vaccinated vs. unvaccinated
To analyze carriage rates in vaccinated and unvaccinated children, we divided the entire study duration into 2 main periods: November 2009 through June 2011 and July 2011 through June 2014 (due to relatively small numbers of unvaccinated children in the different age groups).
In the period between November 2009 and June 2011, VT7 and all-pneumococcal carriage rates were significantly higher in unvaccinated children compared to vaccinated children in all 3 age groups (with the exception of children 24–59 months for all-pneumococcal carriage). In contrast, the 6 additional PCV13 serotypes (1, 3, 5, 6A, 7F and 19A; VT6) carriage rates were similar when comparing vaccinated and unvaccinated children in all 3 age groups. Non-VT13 carriage rates were higher in vaccinated compared to unvaccinated children 24–59 months old, but were similar in children 7–11 and 12–23 months old (Fig. 4, Table S1).

Pneumococcal carriage in children 7–11, 12–23 and 24–59 months old attending pediatric emergency room, southern Israel: vaccinated vs. unvaccinated.
In the period between July 2011 and June 2014, VT7 and VT6 carriage rates were reduced similarly in all 3 age groups in both vaccinated and unvaccinated children, with statistical significance reached in vaccinated children 7–11 and 12–23 months old and unvaccinated children 24–59 months old (due to small numbers in other age groups). Similarly, a trend for increase in non-VT13 carriage rates was observed in all 3 age groups, in both vaccinated and unvaccinated children.
Discussion
Following the introduction of PCVs to the Israeli NIP, vaccine uptake reached levels of ~90% in both Jewish and Bedouin children of southern Israel, within <18 months. This was associated with 76%, 90% and 66% reduction in carriage of VT7, 6A and VT5, respectively, and 71% increase in non-PCV13 serotypes carriage. The overall (all-pneumococcal) carriage was reduced significantly by 10% in children <5 years old attending the Soroka University Medical Center (SUMC) PER.
Not surprisingly, PCV Impact on carriage took place in 2 phases. Initially, following the introduction of PCV7, only VT7 carriage rates were reduced significantly (by 43%), while non-vaccine serotypes rates increased, resulting overall in a non-significant change in carriage rates. Later, following PCV13 introduction, significant declines of VT7, 6A and VT5 carriage rates were observed, along with a further increase in non-13VT.
Despite a marked reduction in VT13 serotype carriage, the impact of PCVs on all-pneumococcal carriage is less pronounced than that observed for IPD and other pneumococcal diseases.8–10 This lesser effect was observed mainly due to the increase of non-PCV13 serotypes carriage, replacing vaccine-serotypes carriage. This replacement in the NP niche was reported to occur rapidly and extensively, leading to increased colonization and transmission of non-VT strains.6,7,19,24 Notably, this universal observation of replacement in carriage was associated with an overall marked reduction in pneumococcal disease, indicating that most replacement serotypes are less invasive strains with a lower disease potential.10,19,25,32,33
While overall-pneumococcal carriage rates were stable following PCV introduction in several reports,7,24,34 a similar reduction to that observed in our study was found in a rural South African community 35 and among American Indian children <5 years old.36,37 In France, PCV introduction resulted in a significant reduction in overall-pneumococcal carriage among children with otitis media,28 and children with pneumonia, but not in healthy children [personal communication, Robert Cohen]. Thus, it is possible that pneumococcal carriage in crowded populations or in children seen in clinics for various reasons (including respiratory infections) has been somewhat reduced (by~10%) following PCVs introduction, while this has not been clearly seen among other populations. Further studies with a long-term follow-up are needed to ascertain PCVs impact on overall pneumococcal carriage.
In the current study, we evaluated the dynamics of pneumococcal carriage post PCV introduction by age groups, ethnicity and vaccination status as well. Firstly, in the early PCV7 period, all-pneumococcal carriage rates were high (~54%) compared with other populations worldwide,6,7,24 and were similar in all 4 age groups. All-pneumococcal carriage rates were reduced following PCVs introduction, showing a gradual effect. By the PCV13 impact period, all-pneumococcal carriage rates had been reduced significantly by 15% in children <12 months old, with a similar non-significant (possibly due to lower sample size) trend observed in other age groups.
Secondly, throughout the study (and separately for each period), carriage rates were higher in Bedouin than in Jewish children. Furthermore, while PCV7 introduction did not result in significant reduction in carriage rates in both groups, PCV13 introduction resulted in a significant and more impressive reduction in carriage rates in the Bedouin population only, while in Jewish children only a mild reduction trend was observed. The differences in carriage rates could be attributed to overcrowding, bigger families and possibly other factors more common in the Bedouin population.12,38 Similarly, the more impressive vaccine impact observed in the Bedouin population may suggest a faster achievement of herd protection in overcrowded populations.
Thirdly, while early carriage rates were higher in unvaccinated children compared to vaccinated children, PCV impact on VT7, VT6 and other pneumococcal serotype carriage were similar throughout the study and in different age groups. The striking similarity in trends between the 2 groups (vaccinated and unvaccinated) indicates a substantial herd protection achieved soon after PCV introduction. Additionally, as expected, in vaccinated children, PCVs had a more pronounced impact on vaccine-serotypes decline and non-VT increase, compared with the impact on unvaccinated children.
The overall pneumococcal carriage rate observed in our study population (children <5 years old) was ~50%. This rate is comparable to rates found in our region in an ongoing study (initiated in 2011) in healthy children, where pneumococcal carriage rates in healthy children were 27%, 52%, 58% and 60% in children 0–3, 6–12, 12–23 and 24–35 months old, respectively (unpublished data).
The major strength lies in its being a long-term, ongoing, prospective surveillance of both PCVs uptake and pneumococcal nasopharyngeal carriage in the same region where all children are treated in a single medical center. The daily, continuous obtainment of NP cultures and vaccine status data enables better appreciation of carriage dynamics post PCV introduction.
There are several limitations in our study: The first is the lack of pre-PCV carriage data; instead, baseline data in our study were those of the early post-PCV7 introduction period. Hence the results presented here may represent an underestimate of the true vaccine impact. The second is that children seen in our study are those who presented to the PER, with various health problems, including high rate of respiratory diseases. Therefore, the carriage measured here may not represent the complete picture of healthy children in the community. The third limitation is the lack of data regarding antibiotic treatment given to children in the study prior to NP culture obtainment.
Currently, data in the medical literature are scarce regarding differences in pneumococcal carriage rates between healthy and sick (e.g. respiratory infections) children. Our study population consists of children presenting to the PER with various indications. Furthermore, this population differed from that of the entire region population since it is younger and includes a higher proportion of Bedouin children.
Thus, it is plausible that the carriage rates observed in children presenting to the PER differ from those of healthy children. However, carriage rates are the highest in respiratory diseases,34 and LRTI rates were constant throughout the study. Furthermore, we conducted separate analyses for carriage rates in the different age groups and ethnicity and found similar trends in all analyses comparisons.
In conclusion, through an active surveillance of PCVs uptake and NP carriage we were able to observe a relatively moderate decline in pneumococcal colonization, facilitated by a substantial decrease of VT and increase of non-VT.
Methods
Setting
The study population consisted of all children <5 years old in southern Israel, presenting to the PER for any reason. In 2009 and 2013, 71,600 and 79,100 children <5 years, respectively, lived in southern-Israel (the Negev region), of whom ~50% were Jewish, and ~50% were Moslem Bedouins.39
The SUMC is the only general hospital in the region, where >95 % of the Negev children are born and treated, enabling population-based studies. The hospital includes the only PER in the entire region, and it serves the entire population of the southern region of Israel.3,31,40
Study design
This is an ongoing, prospective, population-based, active surveillance, initiated in July 2009 for vaccine uptake evaluation and November 2009 for NP pneumococcal carriage.
Each working day, NP culture was obtained from the first 4 Jewish and 4 Bedouin children <5 years old, resident of the Negev region, presenting at the PER for any reason, whose parents agreed to their enrollment. After obtaining a written informed consent and NP swab, PCV vaccination dates and the nature of the vaccine (PCV7 or PCV13) were recorded by the study team from data received directly from the vaccination center, together with selected ethnic and demographic data, obtained from the parents. The study was approved by the SUMC Ethics Committee.
If <8 children were available for NP carriage evaluation, vaccine status evaluation, but not carriage, was obtained from randomly selected hospitalized children. Children not residing in the Negev region were excluded. In cases where multiple swabs were obtained from a child during the same month, only the first culture was included. The return rate for data obtained from vaccinating centers was >95 % for vaccine status and approximately 75% of the patients offered enrollments consented.
Nasopharyngeal cultures and laboratory testing
Nasopharyngeal cultures
Nasopharyngeal samples were obtained using a flexible dacron-tipped swab, introduced through the nostrils and advanced until resistance was found. These swabs were inoculated into modified Stewart transport medium (Medical Wire and Equipment Co., Ltd., Corsham, England) and processed within 16 hours at the SUMC Clinical Microbiology Laboratory. Material from swabs was plated on Columbia agar with 5% sheep blood and 5.0 µg/mL gentamicin, and incubated aerobically at 35°C in a CO2-enriched atmosphere for 48h. This method was used in our previous studies and yielded a high rate of positive cultures.22,26,41,42
The presumptive identification of S. pneumoniae was based on the presence of α-hemolysis and the inhibition by optochin, and the identity of the bacteria present was confirmed by a positive slide agglutination test result (Phadebact; Pharmacia Diagnostics). One S. pneumoniae colony per plate was then subcultured, harvested, and kept frozen at −70°C for further testing.
Serogrouping and serotyping
Serogrouping and serotyping of S. pneumoniae were performed by the Quellung reaction, using serum samples produced by the Statens Seruminstitut.41 Isolates that had negative reactions to all pooled serum samples and to omni serum were considered nontypeable.
All isolates testing positive by the Quellung reaction to serotype 6A were further characterized by polymerase chain reaction testing to differentiate between 6A and 6C, as previously described.42,43
Serotypes 4, 6B, 9V, 14, 18C, 19F, 23F were classified as VT7. Serotype 6A was analyzed individually since it is included in PCV13 but also cross reacts with serotype 6B included in PCV7. Serotypes 1, 3, 5, 7F and 19A were classified as VT5. All other serotypes were classified as non-PCV13 serotypes.
Vaccine uptake evaluation
Estimates of PCV7 coverage before 2009 were based on sales figures provided by the distributor.14,44 In the current study, for each of the children, the vaccination status and vaccination date were recorded by the study stuff. Vaccination status included data on PCVs, diphtheria toxoid, tetanus toxoid and acellular pertussis vaccine (DTaP, or any of its combinations) and measles, mumps and rubella (MMR) with or without varicella vaccine. Records of data included number of doses administered and date of administration. For PCVs, the type of vaccine (PCV7 or PCV13) was also recorded.
Missing data were obtained from the child's primary physician and the Ministry of Health vaccines registry.
To compare dynamics in pneumococcal carriage rates between vaccinated and unvaccinated children, we defined vaccinated children 7–59 months old (with subdivision analysis conducted for 3 age groups; 7–11, 12–23 and 24–59 months old) as those who received ≥2 doses of PCV (PCV7 or PCV13). Unvaccinated children were defined as those who did not receive any PCV.
Statistical analysis
Analysis of contingency data was conducted by the χ2 test or Fisher, as appropriate. Continuous variables were compared using the Student's t-test. A P value of <0 .05 was considered statistically significant.
To assess changes in carriage rate, data are presented graphically as continuous semiannual rates. To estimate the potential PCVs impact, we divided the study period into 5 sub-periods: (1) early-PCV7 period: July 2009 – June 2010 (however, collection of NP data started in November 2009 only); (2) PCV7 impact period: July 2010–June 2011; (3) early-PCV13 period (interim): July 2011–June 2012; (4) early-PCV13 period (interim): July 2012–June 2013; and (5) PCV13 impact period: (July 2013 – June 2014). Data from July 2014 through December 2014 are also shown, but were not analyzed, since they represent only half an epidemiologic year. Rates during early-PCV7, PCV7 impact and PCV13 impact periods were compared, and odds ratios (ORs with 95% confidence intervals) were calculated.
Disclosure of Potential Conflicts of Interest
Prof. David Greenberg has received grants from Merck Sharp & Dohme; has been a scientific consultant for GlaxoSmithKline, Merck Sharp & Dohme and Pfizer and is a speaker for GlaxoSmithKline, Merck Sharp & Dohme and Pfizer. Prof. Ron Dagan has received grants/research support from Pfizer and Merck Sharp & Dohme; has been a scientific consultant for Genocea, MeMed, Merck Sharp & Dohme, and Pfizer, Merck Sharp & Dohme and a speaker for GlaxoSmithKline and Pfizer.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
Articles from Human Vaccines & Immunotherapeutics are provided here courtesy of Taylor & Francis
Full text links
Read article at publisher's site: https://doi.org/10.1080/21645515.2015.1095414
Read article for free, from open access legal sources, via Unpaywall:
https://www.tandfonline.com/doi/pdf/10.1080/21645515.2015.1095414?needAccess=true
Citations & impact
Impact metrics
Citations of article over time
Article citations
Prevalence and serotype distribution of nasopharyngeal carriage of Streptococcus pneumoniae among healthy children under 5 years of age in Hainan Province, China.
Infect Dis Poverty, 13(1):7, 19 Jan 2024
Cited by: 2 articles | PMID: 38238873 | PMCID: PMC10797996
Dynamics of Antibiotic Resistance of Streptococcus pneumoniae in France: A Pediatric Prospective Nasopharyngeal Carriage Study from 2001 to 2022.
Antibiotics (Basel), 12(6):1020, 06 Jun 2023
Cited by: 5 articles | PMID: 37370339 | PMCID: PMC10295685
The COVID-19 pandemic as an opportunity for unravelling the causative association between respiratory viruses and pneumococcus-associated disease in young children: a prospective study.
EBioMedicine, 90:104493, 28 Feb 2023
Cited by: 15 articles | PMID: 36857965 | PMCID: PMC9970381
Remote Versus In-person Outpatient Clinic Visits and Antibiotic Use Among Children During the COVID-19 Pandemic.
Pediatr Infect Dis J, 41(8):636-641, 13 Jul 2022
Cited by: 2 articles | PMID: 35544725 | PMCID: PMC9281428
Nasopharyngeal Carriage in Children After the Introduction of Generalized Infant Pneumococcal Conjugate Vaccine Immunization in Germany.
Front Med (Lausanne), 8:719481, 13 Sep 2021
Cited by: 12 articles | PMID: 34589501 | PMCID: PMC8473806
Go to all (27) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
PCV13-vaccinated children still carrying PCV13 additional serotypes show similar carriage density to a control group of PCV7-vaccinated children.
Vaccine, 35(6):945-950, 11 Jan 2017
Cited by: 8 articles | PMID: 28087146
The rise and fall of pneumococcal serotypes carried in the PCV era.
Vaccine, 35(9):1293-1298, 01 Feb 2017
Cited by: 39 articles | PMID: 28161425
Pneumococcal carriage and invasive disease in children before introduction of the 13-valent conjugate vaccine: comparison with the era before 7-valent conjugate vaccine.
Pediatr Infect Dis J, 32(2):e45-53, 01 Feb 2013
Cited by: 50 articles | PMID: 23080290
Pneumococcal serotype evolution in Western Europe.
BMC Infect Dis, 15:419, 14 Oct 2015
Cited by: 77 articles | PMID: 26468008 | PMCID: PMC4606906
Review Free full text in Europe PMC