Abstract
Free full text

TDP-43 stage, mixed pathologies, and clinical Alzheimer’s-type dementia
Abstract
TDP-43 proteinopathy may lower the threshold for clinical expression of Alzheimer-type dementia. James et al. investigate the relationship between TDP-43 and Alzheimer’s dementia in a large cohort of community-dwelling older adults. Mixed Alzheimer/TDP-43 pathology is associated with increased likelihood of clinical Alzheimer-type dementia proximate to death, independent of other pathologies.
Abstract
Hyperphosphorylated transactive response DNA-binding protein 43 (TDP-43, encoded by TARDBP) proteinopathy has recently been described in ageing and in association with cognitive impairment, especially in the context of Alzheimer’s disease pathology. To explore the role of mixed Alzheimer’s disease and TDP-43 pathologies in clinical Alzheimer’s-type dementia, we performed a comprehensive investigation of TDP-43, mixed pathologies, and clinical Alzheimer’s-type dementia in a large cohort of community-dwelling older subjects. We tested the hypotheses that TDP-43 with Alzheimer’s disease pathology is a common mixed pathology; is related to increased likelihood of expressing clinical Alzheimer’s-type dementia; and that TDP-43 pathologic stage is an important determinant of clinical Alzheimer’s-type dementia. Data came from 946 older adults with (n = 398) and without dementia (n = 548) from the Rush Memory and Aging Project and Religious Orders Study. TDP-43 proteinopathy (cytoplasmic inclusions) was present in 496 (52%) subjects, and the pattern of deposition was classified as stage 0 (none; 48%), stage 1 (amygdala; 18%), stage 2 (extension to hippocampus/entorhinal; 21%), or stage 3 (extension to neocortex; 14%). TDP-43 pathology combined with a pathologic diagnosis of Alzheimer’s disease was a common mixed pathology (37% of all participants), and the proportion of subjects with clinical Alzheimer’s-type dementia formerly labelled ‘pure pathologic diagnosis of Alzheimer’s disease’ was halved when TDP-43 was considered. In logistic regression models adjusted for age, sex, and education, TDP-43 pathology was associated with clinical Alzheimer’s-type dementia (odds ratio = 1.51, 95% confidence interval = 1.11, 2.05) independent of pathological Alzheimer’s disease (odds ratio = 4.30, 95% confidence interval = 3.08, 6.01) or other pathologies (infarcts, arteriolosclerosis, Lewy bodies, and hippocampal sclerosis). Mixed Alzheimer’s disease and TDP-43 pathologies were associated with higher odds of clinical Alzheimer’s-type dementia (odds ratio = 6.73, 95% confidence interval = 4.18, 10.85) than pathologic Alzheimer’s disease alone (odds ratio = 4.62, 95% confidence interval = 2.84, 7.52). In models examining TDP-43 stage, a dose-response relationship with clinical Alzheimer’s-type dementia was observed, and a significant association was observed starting at stage 2, extension beyond the amygdala. In this large sample from almost 1000 community participants, we observed that TDP-43 proteinopathy was very common, frequently mixed with pathological Alzheimer’s disease, and associated with a higher likelihood of the clinical expression of clinical Alzheimer’s-type dementia but only when extended beyond the amygdala.
Introduction
Hyperphosphorylated transactive response DNA-binding protein 43 (TDP-43, encoded by TARDBP) is a pathological protein associated with frontal temporal lobar degeneration (FTLD) and amyotrophic lateral sclerosis (ALS) (Neumann et al., 2006). It has recently been shown to be common in older brains (Geser et al., 2010; Arnold et al., 2013), is reported to be present in at least one in five and up to two in three brains with pathologically confirmed Alzheimer’s disease (Amador-Ortiz et al., 2007; Uryu et al., 2008; Tremblay et al., 2011), and may progress through the brain in a stereotypical manner in patients with Alzheimer’s disease pathology (Hu et al., 2008; Josephs et al., 2014a, 2016). In subjects with pathologically confirmed Alzheimer’s disease, TDP-34 was associated with greater memory loss and hippocampal atrophy (Josephs et al., 2008, 2014b). In other studies in patients both with and without pathological Alzheimer’s disease, TDP-43 was associated with greater cognitive impairment and dementia (Nelson et al., 2010; Robinson et al., 2011; Wilson et al., 2013). Taken altogether, this suggests that TDP-43 may lower the threshold for the clinical expression of Alzheimer’s-type dementia in much the same way that other common comorbid pathologies have been shown to contribute to the clinical expression of Alzheimer’s disease. However, the relationship between TDP-43 and the clinical expression of Alzheimer’s-type dementia has not been systematically investigated in a large cohort of community-dwelling older subjects both with and without Alzheimer’s disease neuropathology, and with and without dementia.
We previously showed that co-occurrences of Alzheimer’s disease with other common dementia-related neuropathologies, i.e. mixed pathologies, increase the odds of developing dementia before death (Schneider et al., 2007), and that mixed pathologies account for most cases of clinical Alzheimer’s-type dementia (Schneider et al., 2009b). Thus far, the field of dementia research has primarily focused on the pathologies of three diseases: Alzheimer’s disease, cerebrovascular disease, and Lewy body disease. But these three disease pathologies account for less than half of the variance in age-related cognitive decline (Boyle et al., 2013). More recently, hippocampal sclerosis, defined as severe gliosis and neuronal loss in the hippocampus and subiculum, has been increasingly studied in dementia and has been linked to Alzheimer’s disease (Attems and Jellinger, 2006); and is often studied in combination with TDP-43, as most cases with hippocampal sclerosis have TDP-43 pathology (Amador-Ortiz et al., 2007; Nelson et al., 2011; Zarow et al., 2012; Nag et al., 2015). Conversely, many cases of TDP-43 proteinopathy do not have hippocampal sclerosis. Here we perform a comprehensive study of TDP-43 pathology, Alzheimer’s disease pathology, and clinical Alzheimer’s-type dementia in a large autopsy cohort derived from a community cohort. We tested the hypotheses that TDP-43 mixed with Alzheimer's disease pathology is one of the most highly prevalent mixed pathologies; that it increases the odds of expressing clinical Alzheimer's-type dementia independent of pathological Alzheimer's disease, cerebrovascular disease (infarcts and small vessel disease–specifically arteriolosclerosis), Lewy body, and hippocampal sclerosis pathology; that it is more strongly related to clinical Alzheimer's-type dementia than the presence of pathological Alzheimer's disease alone; and that the TDP-43 pathological stage is an important determinant of clinical Alzheimer's-type dementia. Data came from two clinicopathological cohorts of ageing and dementia, the Memory and Aging Project and the Religious Orders Study, involving community-dwelling participants without dementia at study entry who agreed to annual cognitive testing and organ donation at death.
Materials and methods
Subjects
We used clinical and neuropathological data from autopsied participants of two clinical-pathologic cohorts of ageing, the Religious Orders Study (Bennett et al., 2011) and the Rush Memory and Aging Project (Bennett et al., 2012, 2013). Participants in the Religious Orders Study, which began in 1994, are older Catholic nuns, priests and brothers from across the USA. The Rush Memory and Aging Project started in 1997 and involves older lay subjects recruited from retirement communities and subsidized housing facilities across the Chicago metropolitan area. Both studies recruit participants without known dementia at baseline who agreed to annual clinical evaluations and organ donation at the time of death. Each participant signed an informed consent and an Anatomical Gift Act form. The studies were approved by the institutional review board of Rush University Medical Center. Currently, more than 3100 subjects have enrolled in both studies. Follow-up rates exceed 90% of survivors. During follow-up, 1514 participants died and 1307 (86%) have undergone autopsy. Neuropathological data were available on 1275 participants at the time of analysis. Of participants with neuropathological data, 975 had a full assessment for TDP-43 pathology at the time of analysis. Subjects who had a full assessment for TDP-43 pathology were on average 3 years older at time of death (P < 0.001), had 1.1 years less education (P < 0.001), scored 1.4 points lower on the Mini-Mental State Examination (MMSE) proximate to death (P = 0.013), and were more likely to be female (67.9% versus 56.6%, P = <0.001) than subjects who had been autopsied but did not yet have a full TDP-43 assessment. These differences are due to the fact that a full TDP-43 assessment was more likely to be completed on more recently autopsied participants, and there are slight differences in characteristics of subjects recruited into the study in recent years compared to the beginning of the study. Older autopsied brains are being retrospectively assessed for TDP-43 and eventually the entire cohort will be assessed. Because we wanted to isolate clinical Alzheimer’s-type dementia from other dementia types, 15 subjects who received a final clinical diagnosis of non-Alzheimer’s-type dementia proximate to death (see ‘Clinical evaluation’ section) were excluded from analyses; another 13 were excluded because they did not yet have a definitive final clinical diagnosis, and one subject with a pathologic diagnosis of FTLD was excluded, leaving 946 people for analyses.
Clinical evaluation
The annual clinical evaluation included medical history, neurological examination, and detailed cognitive testing (Wilson et al., 2002, 2005). Diagnostic classification followed a multistep procedure as previously described (Bennett et al., 2005). First, cognitive tests were reviewed by a neuropsychologist. Then an experienced clinician reviewed all records and classified participants using recommendations of the joint working group of the NINCDS-ADRDA (McKhann et al., 1984). Following death, all clinical data were reviewed by an expert neurologist blinded to neuropathology who presented a summary diagnostic opinion on most likely clinical diagnoses proximate to death was made, as described previously (Schneider et al., 2009b). A diagnosis of probable or possible Alzheimer’s dementia (here, ‘clinical Alzheimer’s-type dementia’) required a history of cognitive decline and evidence of impairment in episodic memory and at least one additional cognitive domain. A diagnosis of mild cognitive impairment was given to subjects who were rated as cognitively impaired by a neuropsychologist but who were not rated as demented by the examining neurologist. Details of the clinical diagnoses of Alzheimer’s dementia and mild cognitive impairment have been previously reported (Bennett et al., 2002, 2006a).
Pathologic evaluation
Brain autopsies were mainly performed at Rush as well as other predetermined sites across the USA (Bennett et al., 2011, 2012, 2013). The median clinical interval from last examination to autopsy was 7.7 months, and the median post-mortem interval was 6.6 h. Brains were removed and one hemisphere was fixed for at least 3 days in 4% paraformaldehyde. Hemispheres were cut into 1 cm coronal slabs and assessed for macroscopic infarcts. Regions with infarcts and a core set of brain regions including midfrontal, middle temporal, inferior parietal, anterior cingulate, entorhinal cortex, hippocampus, basal ganglia, thalamus, and midbrain with substantia nigra were embedded in paraffin. Neuropathological diagnoses were made by a board-certified neuropathologist blinded to age and clinical data.
Pathological TDP-43 inclusions
As described previously (Wilson et al., 2013), we investigated TDP-43 pathology in six brain regions, as guided by prior research (Neumann et al., 2006; Higashi et al., 2007; Hu et al., 2008; Arai et al., 2009; Kadokura et al., 2009; Bigio et al., 2010; Geser et al., 2011; Nag et al., 2015): the amygdala (and periamygdalar region, when available), hippocampus CA1/subiculum, hippocampus dentate gyrus, entorhinal cortex, midfrontal cortex, and middle temporal cortex. Immunostaining was done on 6-μm sections using monoclonal antibodies to phosphorylated TDP-43 (pS409/410; 1:100, Ascenion) (Neumann et al., 2009). This stains the pathologically phosphorylated TDP-43 proteins in the inclusions seen in FTLD/ALS and other neurodegenerative diseases, and does not stain the normal nuclear TDP-43. Each region of interest was reviewed for presence and location of TDP-43 cytoplasmic inclusions, and severity was rated on a 6-point scale based on the number of inclusions in a 0.25 mm2 area of greatest density within that region as described previously (Wilson et al., 2013). We created a dichotomous variable of TDP-43 positivity based on the presence of TDP-43 pathology (one or more inclusions) in any of these regions. We also created distinct stages of TDP-43 distribution guided by previously literature (Hu et al., 2008) and observation of distribution in our cohort: stage 0, no presence of TDP-43; stage 1, localized to amygdala; stage 2, extension to hippocampus and/or entorhinal cortex; stage 3, extension to the neocortex, as previously described (Nag et al., 2015).
Alzheimer’s disease pathology
A modified Bielschowsky silver stain was used to visualize neuritic and neurofibrillary tangles in the frontal, temporal, parietal, entorhinal, and hippocampal cortices (Schneider et al., 2004). As described previously (Schneider et al., 2009a), the neuropathological diagnosis of Alzheimer’s disease was made based on National Institute on Aging (NIA)-Reagan criteria (1997) using semi-quantitative estimates of neuritic plaque density as recommended by Consortium to Establish a Registry for Alzheimer’s Disease (CERAD; Mirra et al., 1991), modified to be implemented without adjustment for age and clinical diagnosis, and modified Braak stage for neurofibrillary tangles severity (Braak and Braak, 1991). A diagnosis of Alzheimer’s disease required an ‘intermediate likelihood Alzheimer’s disease,’ or ‘high likelihood Alzheimer’s disease’ by NIA-Reagan criteria: at least intermediate stage Braak (i.e. Braak III/IV) and at least moderate neocortical neuritic plaques (CERAD probable Alzheimer’s disease) (Bennett et al., 2006b). Pathologic absence of a diagnosis of Alzheimer’s disease included subjects with no Alzheimer’s disease and low likelihood of Alzheimer’s disease by NIA-Reagan criteria.
Cerebral infarcts
Cerebral infarcts visible to the naked eye (macroscopic infarcts) were recorded (Schneider et al., 2004). Only chronic, estimated as over 3 to 6 months in age, macroscopic infarcts were used in this analysis. Lacunar infarcts were defined as gross infarcts in the white matter or deep grey, 10 mm or less in diameter. Microscopic infarcts were recorded from haematoxylin and eosin stained 6 -µm paraffin-embedded section from at least nine regions (Arvanitakis et al., 2011). We have shown that microinfarcts are independently related to dementia in older subjects (Arvanitakis et al., 2011). We created a dichotomous summary diagnosis of infarcts (the presence of any chronic macroscopic and/or microscopic infarctions versus the absence of both types), for these analyses.
Arteriolosclerosis
Small vessel disease, here described as arteriolosclerosis, was evaluated on histological examination, on haematoxylin and eosin stained sections of the anterior basal ganglia (Buchman et al., 2011). Severity of this pathology was graded semi-quantitatively based on concentric hyaline thickening of vessel walls with emphasis placed on evaluation of smaller arterioles, less than ~50 microns (Arvanitakis et al., 2016). For these analyses, severity of arteriolosclerosis was dichotomized into moderate/severe versus not present/mild.
Lewy body pathology
Immunohistochemistry with alpha-synuclein (Zymed LB 509; 1:50; pSyn, 1:20 000; Wako Chemicals) was used to detect Lewy bodies in the substantia nigra, entorhinal, cingulate, midfrontal, middle temporal, and inferior parietal cortex (Schneider et al., 2007). Criteria for Lewy body disease used a modified version of the Consensus guidelines for a diagnosis of dementia with Lewy bodies (McKeith et al., 2005; Schneider et al., 2007). A dichotomous summary diagnosis of Lewy body disease, present or absent, was used for these analyses.
Hippocampal sclerosis
Hippocampal sclerosis was evaluated unilaterally in a coronal section of the mid-hippocampus at the level of the lateral geniculate body. Hippocampal sclerosis was graded as absent or present based on severe neuronal loss and gliosis in the CA1and/or subiculum (Nag et al., 2015).
Statistics
We first examined the frequency of each pathology type and the co-occurrence of TDP-43 pathology with the pathologic diagnosis of Alzheimer’s disease, infarcts, Lewy bodies, and hippocampal sclerosis. We examined the number of subjects who would be considered ‘pure pathological Alzheimer’s disease’ (i.e. pathological Alzheimer’s disease with no infarcts, Lewy bodies, or hippocampal sclerosis) with and without the consideration of TDP-43 pathology. To examine the relationship of pathological Alzheimer’s disease and TDP-43 severity, we cross tabulated NIA Reagan score with presence of TDP-43 and TDP-43 stage. We then examined the presence and co-occurrence of each pathology type and other characteristics by clinical Alzheimer’s-type dementia status. For all bivariate analyses, chi-square or t-tests were used to test for significance of associations.
We then used multiple logistic regression models to determine whether TDP-43 was associated with odds of clinical Alzheimer’s-type dementia, independent of and in combination with pathologic Alzheimer’s disease and other dementia-related pathologies. All models included terms for age (years, centred), sex (male), and education (years, centred). We first fit a model with a term for the presence of TDP-43. We then added a term for pathologic diagnosis of Alzheimer’s disease to test whether TDP-43 was associated with odds of clinical Alzheimer’s-type dementia above and beyond pathological Alzheimer’s disease. A third model added terms for infarcts, Lewy bodies, and hippocampal sclerosis to test whether TDP-43 was associated with odds of clinical Alzheimer’s-type dementia above and beyond the other common pathologies of dementia. We then tested for an interaction between Alzheimer’s disease pathology and TDP-43 pathology on odds of dementia by adding an interaction term for Alzheimer’s disease pathology multiplied by TDP-43. Then, to examine whether Alzheimer’s disease mixed with TDP-43 pathology was more strongly associated with odds of clinical Alzheimer’s-type dementia than Alzheimer’s disease pathology alone, we created dichotomous variables for Alzheimer’s disease mixed with TDP-43, Alzheimer’s disease without TDP-43, and TDP-43 without Alzheimer’s disease pathology, and entered these terms into a model with infarcts, Lewy bodies, and hippocampal sclerosis. We then examined whether specific stages of TDP-43 progression were associated with odds of clinical Alzheimer’s-type dementia by entering dichotomous variables for TDP-43 stage (no TDP-43 as reference) into a model with terms for pathologic Alzheimer’s disease, infarcts, Lewy bodies, and hippocampal sclerosis. Finally, we fit a model with the 6-point scale of TDP-43 severity as the term for TDP-43.
Because the reference category (no clinical Alzheimer’s-type dementia) for the analyses above included both subjects with no cognitive impairment and those with mild cognitive impairment, we conducted secondary analyses in which we used ordinal multiple logistic regression models to test whether the odds of clinical Alzheimer’s-type dementia and the odds of clinical Alzheimer’s-type dementia or mild cognitive impairment (i.e. any cognitive impairment) are associated with TDP-43. The score test for proportional odds indicated that the assumption that odds of mild cognitive impairment were always the same multiple of odds for clinical Alzheimer’s-type dementia was not violated. Data were analysed using SAS 9.3 (SAS Institute Inc., 2011).
Results
Distribution of TDP-43 pathology, Alzheimer’s disease pathology, and other neuropathologies in older subjects
As shown in Table 1, TDP-43 was present in more than half of the 946 autopsied brains. TDP-43 proteinopathy was the second most frequent pathology after a pathologic diagnosis of Alzheimer’s disease. TDP-43 positivity was present as the only pathology (e.g. no pathologic diagnosis of Alzheimer’s disease, Lewy bodies, infarcts, arteriolosclerosis, or hippocampal sclerosis) in 4.2% of the participants, but was more commonly combined with other pathologies (48.2% of participants). TDP-43 positivity most commonly co-occurred with hippocampal sclerosis (90.3% versus 47.7% in those with no hippocampal sclerosis, P < 0.001) followed by a pathologic diagnosis of Alzheimer’s disease (58.4%, versus 42.3% in those without pathologic diagnosis of Alzheimer’s disease, P < 0.001), Lewy bodies (58.4% versus 50.6% in those without Lewy bodies, P = 0.038), and arteriolosclerosis (57.8% versus 50.0% in those without arteriolosclerosis, P = 0.026). TDP was not significantly associated with cerebral infarcts (53.0% versus 51.8% in those with no infarcts, P = 0.70); when subtypes were examined, TDP-43 was not associated with gross infarcts (54.5% versus 51.3%, P = 0.36), lacunar infarcts (55.0% versus 51.7%, P = 0.40), or microinfarcts (53.3% versus 52.0%, P = 0.72).
Table 1
Characteristics and pathologies for all participants, and by clinical diagnosis
| All participants | Clinical Alzheimer’s-type dementia | No dementia | P-value | ||
|---|---|---|---|---|---|
| (n = 946) | (n = 398) | (n = 548) | |||
| Characteristic | |||||
| Age | 89.3 (6.5) | 90.8 (5.9) | 88.2 (6.7) | F(1,944) = 35.4 | <0.001 |
| Male sex (%) | 292 (32.3) | 118 (29.7) | 187 (34.1) | X2 = 2.1 | 0.15 |
| Education (years) | 16.1 (3.6) | 16.1 (3.6) | 16.0 (3.6) | F(1,944) = 0.52 | 0.47 |
| MMSE (proximate to death) | 20.7 (9.3) | 12.4 (8.5) | 26.8 (3.1) | F(1,942) = 1341.6 | <0.001 |
| Pathology | |||||
| Anya | 859 (90.8%) | 391 (98.2)% | 468 (85.4)% | X2 = 45.5 | <0.001 |
| TDP-43b | 496 (52.4%) | 257 (64.6%) | 239 (43.6%) | X2 = 40.6 | <0.001 |
| Stage 1 | 166 (17.6%) | 56 (14.1%) | 110 (20.2%) | X2 = 81.7 | <0.001 |
| Stage 2 | 194 (20.6%) | 104 (26.3%) | 90 (16.5%) | ||
| Stage 3 | 134 (14.2%) | 95 (24.0%) | 39 (7.1%) | ||
| Pathologic diagnosis of AD | 594 (62.8%) | 322 (80.9%) | 272 (49.6%) | X2 = 96.5 | <0.001 |
| TDP-43 + pathologic diagnosis of AD | 347 (36.7%) | 213 (53.5%) | 134 (24.5%) | X2 = 83.9 | <0.001 |
| Infarctsc | 460 (48.9%) | 225 (57.0%) | 235 (43.0%) | X2 = 17.8 | <0.001 |
| Gross | 325 (34.5%) | 174 (44.1%) | 151 (27.7%) | X2 = 27.2 | <0.001 |
| Microscopic | 272 (28.9%) | 126 (31.9%) | 146 (26.7%) | X2 = 2.97 | 0.085 |
| Lacunar | 321 (24.5%) | 120 (30.2%) | 102 (18.7%) | X2 = 17.0 | <0.001 |
| Arteriolosclerosis | 294 (32.2%) | 145 (36.5%) | 149 (27.3%) | X2 = 9.02 | 0.003 |
| LB | 233 (24.7%) | 135 (34.1%) | 98 (17.9%) | X2 = 32.3 | <0.001 |
| HS | 103 (10.9%) | 80 (20.2%) | 23 (4.2%) | X2 = 60.0 | <0.001 |
aIncludes pathologic diagnosis of Alzheimer’s disease (AD), TDP-43, infarcts, Lewy bodies (LB) and/or hippocampal sclerosis (HS).
bStaging not available for four individuals due to missing regional information.
cIncludes the presence of any chronic macroscopic and/or microscopic infarctions versus the absence of both types.
TDP-43 was most common in those with high likelihood of pathologic Alzheimer’s disease (68.7%) based on NIA-Reagan scoring criteria for pathologic diagnosis of Alzheimer’s disease, but was also seen in just over half of those with intermediate likelihood (54.0%). Table 2 displays the cross-tabulation of distribution of TDP-43 stage (and mean severity for each stage) by pathological Alzheimer’s disease likelihood. There was a clear relationship between TDP-43 stage and level of Alzheimer’s disease neuropathological changes (X2 = 61.81, P < 0.001), but there were individuals with the highest stage of TDP-43 and no or low Alzheimer’s disease neuropathological changes, and conversely, there were individuals with no TDP-43 with high Alzheimer’s disease neuropathological changes. Of the 142 subjects with a pathologic diagnosis of Alzheimer’s disease who would have previously been considered to have ‘pure Alzheimer’s disease’ when considering all the pathologies in this analysis aside from TDP-43 (i.e. infarcts, arteriolosclerosis, Lewy bodies, and hippocampal sclerosis), almost half (n = 64; 45.1%) had mixed Alzheimer’s disease and TDP-43 pathologies. In those without a pathologic diagnosis of Alzheimer’s disease (no/low NIA Reagan), TDP-43 pathology was also very common (42.3%), but tended to be at a lower stage (median stage = 0) compared to those with a pathologic diagnosis (median stage = 1).
Table 2
Cross-tabulation of TDP-43 stage and Alzheimer’s disease severity by NIA-Reagan score
| Pathologic Alzheimer’s disease by NIA-Reagan criteria | |||
|---|---|---|---|
| TDP-43 pathology stage | No/low | Intermediate | High |
| (Mean severity score)* | |||
| 0: No TDP-43 | 202 (57.7%) | 190 (46.0%) | 56 (31.3%) |
| 1: Amygdala | 65 (18.6%) | 77 (18.6%) | 24 (13.4%) |
| (0.36, SD = 0.21) | |||
| 2: Hippocampus/ entorhinal cortex | 55 (15.7%) | 88 (21.3%) | 51 (28.5%) |
| (1.19, SD = 0.72) | |||
| 3: Neocortex | 28 (8.0%) | 58 (14.0%) | 48 (26.8%) |
| (2.46, SD = 1.03) | |||
Percentages refer to proportion of each stage of TDP-43 progression within each level of Alzheimer’s disease pathology.
Mean severity score and standard deviation based on a 6-point scale based on the number of inclusions in a 0.25-mm2 area of greatest density within that region [none, sparse (1–2 inclusions), sparse to moderate (3–5 inclusions), moderate (6–12 inclusions), moderate to severe (13–19 inclusions), and severe (20 or more inclusions)].
TDP-43 with and without pathologic Alzheimer's diagnosis, by clinical Alzheimer's dementia status
About 42% of the cohort had clinical Alzheimer’s-type dementia at the time of death (Table 1). The clinical diagnosis of Alzheimer’s-type dementia was pathologically confirmed as Alzheimer’s disease (NIA-Reagan intermediate or high) in over 80% of these 398 subjects. Interestingly, though Alzheimer’s disease was the most common pathologic diagnosis in subjects with clinical Alzheimer’s-type dementia, this was followed by TDP-43 pathology, which was present in the brains of almost two-thirds of those with clinical Alzheimer’s-type dementia.
In the 322 subjects with clinical Alzheimer’s-type dementia who had pathologically confirmed Alzheimer’s disease, mixed pathologies were much more common than single or ‘pure’ pathologies, and mixed Alzheimer’s disease and TDP-43 was the most common category of mixed pathologies. Figure 1 displays the proportion of mixed Alzheimer’s disease and TDP-43 pathologies in the brains of older subjects both with and without clinical Alzheimer’s-type dementia. Figure 1 shows that more than half of subjects with clinical Alzheimer’s-type dementia have mixed Alzheimer’s disease and TDP-43 pathologies, and that more than half of subjects in this group with Alzheimer’s disease pathologies also had TDP-43 pathology in their brain. In subjects with clinical Alzheimer’s-type dementia, a pathologic diagnosis of Alzheimer’s disease was more likely to be mixed with TDP-43 pathology (66.2%) than with infarcts (54.4%), arteriolosclerosis (34.8%), Lewy bodies (34.0%), or hippocampal sclerosis (19.9%). It was also much more common than ‘pure’ Alzheimer’s disease, defined as those with a pathologic diagnosis of Alzheimer’s disease without TDP-43, infarcts, arteriolosclerosis, Lewy bodies, or hippocampal sclerosis. Overall, the proportion of subjects with clinical Alzheimer’s-type dementia formerly labelled ‘pure pathologic Alzheimer’s disease’ was reduced by 60% from 16.1% to 6.5% when including TDP-43 as a mixed pathology. Conversely, the proportion of mixed pathologies in those with pathologically confirmed clinical Alzheimer’s-type dementia increased from 83.5% to 93.5% when considering TDP-43.
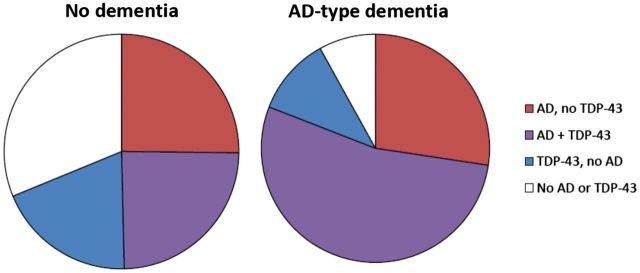
Mixed Alzheimer’s disease and TDP-43 pathologies by clinical Alzheimer’s-type dementia status proximate to death. Red = presence of pathologic diagnosis of Alzheimer’s disease (AD) without TDP-43. Blue = presence of pathologic transactive response DNA-binding protein 43 (TDP-43) without AD. Purple = presence of both AD and TDP-43. White = absence of AD and TDP-43.
TDP-43 was also present in those without clinical Alzheimer’s-type dementia (43.6%) and was generally at a lower stage; it extended out of the amygdala in just over half of those who had TDP-43 (54.0%), compared to 78.0% of those who had TDP-43 and had clinical Alzheimer’s-type dementia. In subjects without clinical Alzheimer’s-type dementia but with a pathologic diagnosis of Alzheimer’s disease, 49.4% had Alzheimer’s disease mixed with TDP-43 and again the TDP-43 tended to be at a lower stage. When separating those without clinical Alzheimer’s-type dementia into those with mild cognitive impairment versus those with no cognitive impairment, TDP-43 was slightly more prevalent in the brains of those with mild cognitive impairment (49.2% versus 39.1% in those with no impairment, P = 0.018).
TDP-43 with and without a pathologic diagnosis of Alzheimer’s disease in the clinical expression of Alzheimer’s-type dementia
We used a series of multiple logistic regression models adjusted for age, sex, and education to determine how TDP-43 pathology contributes to the clinical expression of clinical Alzheimer’s-type dementia, both independently and in combination with Alzheimer’s disease pathology. We first fit a model with a term for pathologic diagnosis of Alzheimer’s disease alone and as expected, a pathologic diagnosis of Alzheimer’s disease was associated with an increase in odds of clinical Alzheimer’s-type dementia (Table 3, Model 1). We then fit a model with TDP-43 pathology alone; TDP-43 was associated with more than double the odds of clinical Alzheimer’s-type dementia compared to no TDP-43 (Table 3, Model 2). In a model with both pathologic diagnosis of Alzheimer’s disease and TDP-43 pathology, TDP-43 pathology was associated with double the odds of clinical Alzheimer’s-type dementia independent of Alzheimer’s disease pathology (Table 3, Model 3). We then added terms for Lewy bodies, infarcts, arteriolosclerosis, and hippocampal sclerosis pathologies; the association of TDP-43 with clinical Alzheimer’s-type dementia was attenuated to a 51% increase in odds but still statistically significant independent of all other dementia-related pathologies (Table 3, Model 4). In a model adding an interaction term for TDP-43 and pathologic diagnosis of Alzheimer’s disease, the interaction was not significant (P = 0.69) suggesting there is an additive but not multiplicative effect of Alzheimer’s disease pathology and TDP-43 on the expression of clinical Alzheimer’s-type dementia. To illustrate the additive effect of Alzheimer’s disease with TDP-43 pathology, we fit a model with terms for (i) Alzheimer’s disease and TDP-43 mixed pathologies; (ii) Alzheimer’s disease without TDP-43 pathology; and (iii) TDP-43 without Alzheimer’s disease pathology, as well as terms for infarcts, arteriolosclerosis, Lewy bodies, and hippocampal sclerosis. As shown in Fig. 2, pathologic Alzheimer’s disease mixed with TDP-43 pathologies was associated with a much higher odds of clinical Alzheimer’s-type dementia [odds ratio (OR) = 6.73, 95% confidence interval (CI) = 4.18, 10.85] than pathologic Alzheimer’s disease without TDP-43 (OR = 4.62, 95% CI = 2.84, 7.52) or TDP-43 alone (OR = 1.67, 95% CI = 0.94, 2.94). We also fit an ordinal logistic regression model to test the association of these terms with any cognitive impairment (i.e. three-level ordinal outcome: clinical Alzheimer’s-type dementia, and mild cognitive impairment or clinical Alzheimer’s-type dementia compared to no cognitive impairment as the reference category) and the results were similar: pathologic Alzheimer’s disease mixed with TDP-43 pathologies was associated with a much higher odds of cognitive impairment (OR = 5.01, 95% CI = 3.45, 7.28) than pathological Alzheimer’s disease without TDP-43 (OR = 3.14, 95% CI = 2.16, 4.55) or TDP-43 alone (OR = 1.24, 95% CI = 0.81, 1.90).
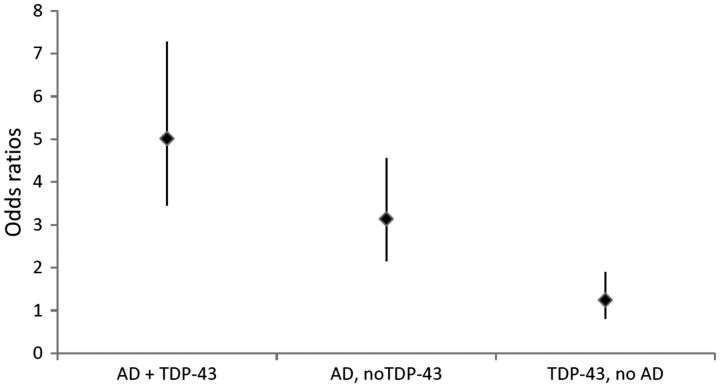
Odds ratios for clinical Alzheimer’s-type dementia. Odds ratios provided for pathologic diagnosis of Alzheimer’s disease (AD) mixed with TDP-43 pathology, for pathologic diagnosis of AD without TDP-43 pathology, and for TDP-43 pathology without pathologic AD compared to not having pathologic diagnosis of AD or TDP-43 pathology, from a model adjusted for age, sex, education, cerebral infarcts, arteriolosclerosis, Lewy bodies, and hippocampal sclerosis.
Table 3
Odds ratios and 95% confidence intervals for clinical Alzheimer’s-type dementia (adjusted for age, sex, and education; n = 946)
| Pathology type | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| TDP-43 | 2.07 (1.58, 2.72) | 1.91 (1.44, 2.54) | 1.51 (1.11, 2.05) | |
| Pathologic diagnosis of Alzheimer’s disease | 3.88 (2.86, 5.27) | 3.71 (2.73, 5.06) | 4.30 (3.08, 6.01) | |
| Cerebral infarcts | 1.69 (1.25, 2.29) | |||
| Arteriolosclerosis | 1.51 (1.10, 2.09) | |||
| Lewy bodies | 2.54 (1.81, 3.58) | |||
| Hippocampal sclerosis | 4.37 (2.55, 7.48) |
Values are OR (95% CI).
Finally, we examined the odds of clinical Alzheimer’s-type dementia by stage of TDP-43 pathology progression. In a model with terms for specific TDP-43 stage, as well as terms for Alzheimer’s disease, infarcts, arteriolosclerosis, Lewy bodies, and hippocampal sclerosis pathologies, a dose-response relationship was observed for odds of clinical Alzheimer’s-type dementia by increasing progression of TDP-43. As shown in Table 4, a significant association with odds of clinical Alzheimer’s-type dementia was only observed starting at stage 2 (extension beyond the amygdala to hippocampus and/or entorhinal cortex). Finally, in a model with the 6-point scale of TDP-43 severity as the term for TDP-43, each additional point of TDP-43 severity was associated with increased odds of clinical Alzheimer’s-type dementia (OR = 1.58, 95% CI = 1.31, 1.89).
Table 4
Odds ratio for clinical Alzheimer’s-type dementia comparing each stage of TDP-43 progression to no TDP-43, adjusted for age, sex, education, pathologic Alzheimer’s disease, infarcts, arteriolosclerosis, Lewy bodies, and hippocampal sclerosis pathologies
| TDP-43 stage | OR (95% CI) |
|---|---|
| 1: Amygdala | 0.89 (0.58, 1.35) |
| 2: Hippocampus/ entorhinal cortex | 1.84 (1.25, 2.71) |
| 3: Neocortex | 2.44 (1.48, 4.02) |
Discussion
In a cohort of almost 1000 older community-dwelling subjects we performed a comprehensive study of TDP-43 pathology, TDP-43 pathology mixed with a pathologic diagnosis of Alzheimer’s disease and the roles of these pathologies in the clinical expression of Alzheimer’s-type dementia. TDP-43 pathology was very common in the brains of older subjects, present in over half of all brains, and much more common in conjunction with other neurodegenerative pathologies, including not only Alzheimer’s disease but also hippocampal sclerosis (where it is extraordinarily common), arteriolosclerosis, and Lewy body disease. TDP-43 pathology was present in almost two-thirds of those with clinical Alzheimer’s-type dementia, being the second most common pathology after a pathologic diagnosis of Alzheimer’s disease. A pathologic diagnosis of Alzheimer’s disease mixed with TDP-43 was also the most common mixed pathology in subjects with a pathologic diagnosis of Alzheimer’s disease (compared to pathological Alzheimer’s disease mixed with infarcts, arteriolosclerosis, Lewy bodies or hippocampal sclerosis). We found no evidence of a multiplicative effect between TDP-43 pathology and a pathologic diagnosis of Alzheimer’s disease on the odds of clinical Alzheimer’s-type dementia; rather, TDP-43 acted as an additional additive pathology similar to other age-related pathologies. A pathologic diagnosis of Alzheimer’s disease mixed with TDP-43 pathology was associated with much higher odds of dementia than a pathologic diagnosis of Alzheimer’s disease without TDP-43, independent of other age-related pathologies. Finally, we found a dose-response relationship between TDP-43 pathologic stage and clinical Alzheimer’s-type dementia such that TDP-43 pathology needed to progress from the amygdala to the hippocampus/entorhinal cortex to have an association with clinical Alzheimer’s-type dementia.
TDP-43 pathology has a short but significant historical footprint in the field of Alzheimer’s disease and dementia (Wilson et al., 2011; Youmans and Wolozin, 2012; Josephs et al., 2014b; Josephs and Nelson, 2015; Chang et al., 2016). It was first recognized as a pathology that accumulated in the brains of patients with FTLD/ALS (Arai et al., 2006; Neumann et al., 2006), but has since been described as common in the brains of patients with Alzheimer’s disease (Amador-Ortiz et al., 2007; Hu et al., 2008; Josephs et al., 2014b), as well as hippocampal sclerosis (Amador-Ortiz et al., 2007; Nag et al., 2015; Cykowski et al., 2016), and possibly other neurodegenerative diseases (Higashi et al., 2007; Arai et al., 2009). For the most part, previous ageing and Alzheimer’s disease research has focused on TDP-43 pathology in those with clinical or pathological Alzheimer’s disease, with research participants identified from clinic samples or patient registries. The proportion of pathologically confirmed Alzheimer’s disease cases that had TDP-43 proteinopathy has been shown to range from between 19 and 57% in studies of 50 or more subjects (Amador-Ortiz et al., 2007; Uryu et al., 2008; Arai et al., 2009; Kadokura et al., 2009; Bigio et al., 2010; Davidson et al., 2011; Rauramaa et al., 2011; Josephs et al., 2014a), including 27% in a population-based sample (Keage et al., 2014). Our study, using community-based cohort of both subjects with and without clinical Alzheimer’s-type dementia, showed 58.5% of subjects with a pathologic diagnosis of Alzheimer’s disease had TDP-43 proteinopathy. In studies involving only subjects clinically diagnosed with clinical Alzheimer’s-type dementia or any dementia, the proportion with TDP-43 was lower (29 to 41%) (Davidson et al., 2011; Robinson et al., 2011) than the proportion that we observed. Our study may have a larger proportion of TDP-43 positivity than some other studies because we investigated the amygdala which has been noted as the most commonly involved region in some studies including the current one (Higashi et al., 2007; Hu et al., 2008).
Previous work has shown that TDP-43 pathology is important in dementia, lowers cognition and accelerates cognitive decline. A study using a subset of an autopsy cohort found an association between TDP-43 and cognitive impairment, independent of Alzheimer’s disease pathology or hippocampal sclerosis (Nelson et al., 2010). In the Religious Orders Study, investigators previously demonstrated that TDP-43 positivity is higher in clinical Alzheimer’s-type dementia than in normal cognition, with mild cognitive impairment at an intermediate level (Tremblay et al., 2011) and that TDP-43 was related to more rapid cognitive decline and a higher likelihood of dementia (but not mild cognitive impairment, though the sample size was much smaller than the current study) and accounted for nearly as much variability in cognitive decline as did tau tangles (Wilson et al., 2013). Another prospective population-based study in the oldest-old also found an association with dementia (Robinson et al., 2011). Studies in pathologically confirmed Alzheimer’s dementia have shown TDP-43 related to lower cognition (Josephs et al., 2014b). Importantly, the current study extends these findings to a large, community-based cohort with and without clinical Alzheimer’s-type dementia, and shows that a pathologic diagnosis of Alzheimer’s disease mixed with TDP-43 pathology was associated with much higher odds for clinical Alzheimer’s-type dementia than Alzheimer’s disease pathology without TDP-43, after accounting for other age-related pathologies. It has been long recognized that subjects with a pathologic diagnosis of Alzheimer’s disease may not exhibit dementia. The current study extends the data on mixed Alzheimer’s disease pathologies, and indicates that TDP-43 is another important mixed pathology linked to crossing the threshold to dementia in subjects with a pathologic diagnosis of Alzheimer’s disease (Schneider et al., 2007). TDP-43 may therefore help to explain some of the unexplained variance accounted for by age-related pathologies in the expression of dementia and cognitive impairment in later life (Boyle et al., 2013).
There is increasing recognition of the role of mixed or comorbid pathologies in the expression of clinical Alzheimer’s-type dementia (Schneider et al., 2007). Almost all of the subjects with clinical Alzheimer’s-type dementia in this cohort had two or more pathologies when considering TDP-43 in addition to the other most commonly studied age-related pathologies. Overall, <10% of subjects with clinical Alzheimer’s-type dementia were left with ‘pure Alzheimer’s disease’ when TDP-43 was taken into account, highlighting that the clinical syndrome often assumed to be the sole manifestation of Alzheimer’s disease pathology is influenced by numerous other age-related pathologies (Jellinger and Attems, 2015). This has public health implications for treatment and research on dementia, as innovations on interventions for alternate pathologies such as TDP-43 could help to alleviate the societal burden of Alzheimer’s dementia even in the absence of prevention or treatment of the amyloid plaques and PHF-tau tangles that are the hallmarks of Alzheimer’s disease pathology (Chang et al., 2016). Moreover, the dose-response relationship between TDP-43 stage progression and Alzheimer’s disease-dementia, along with the findings that TDP-43 is present in lower stages in the brains of people with no Alzheimer’s disease pathology and in those without clinical Alzheimer’s-type dementia, suggest there may be a critical window for intervention prior to the point where the progression of Alzheimer’s disease and TDP-43 pathology manifest as dementia. From a research perspective, it remains unclear why some subjects with amyloid-β and tau tangles exhibit TDP-43 while others do not. Genetic factors, e.g. variants in TMEM106B, are likely to be factors (Yu et al., 2015). Whether genetic interactions or pathologic triggers (e.g. amyloid-β deposition) also play a role is not clear (Youmans and Wolozin, 2012; Chang et al., 2016). Clearly, mixed neurodegenerative pathologies and the relationship between specific comorbid pathologies and dementia deserve further study.
This study extends previous research seeking to develop a staging scheme for TDP-43 in Alzheimer’s disease. Josephs et al. (2014a) originally developed a scheme identifying five stages of progression of TDP-43 deposition in Alzheimer’s disease and demonstrated a relationship between higher TDP-43 stage and lower cognitive ability. Recently, the investigators extended this staging scheme to include limbic regions of the brain, identifying six stages of TDP-43 deposition, progressing from the amygdala (stage 1), to entorhinal cortex and subiculum (stage 2), to the dentate gyrus of the hippocampus and occipitotemporal cortex (stage 3) to insular cortex, ventral striatum, basal forebrain and inferior temporal cortex (stage 4), to substantia nigra, inferior olive and midbrain tectum (stage 5), and to basal ganglia and middle frontal cortex (stage 6) (Josephs et al., 2016). Our staging scheme recognized only four stages (including no TDP-43), but the progression from amygdala to hippocampus and entorhinal cortex to neocortex followed the same order as the Josephs et al. (2014a) staging. Uniquely, our staging scheme was rendered in participants without regard to pathological or clinical Alzheimer’s disease status. Our study thus extends previous research by showing a relationship between TDP-43 stage and pathological likelihood of Alzheimer’s disease, as well as between TDP-43 stage and likelihood of clinical Alzheimer’s-type dementia. A novel finding was that TDP-43 had to progress to stage 2 (hippocampus and entorhinal cortex) before a relationship with clinical Alzheimer’s-type dementia was observed.
Strengths of this study include the use of autopsy data from a large, community-based cohort of older subjects, some of whom developed clinical Alzheimer’s-type dementia prior to death over the course of follow-up and some of whom did not. Findings are therefore more generalizable to the general population of older adults than a memory clinic-based sample, or a brain bank of brains with confirmed Alzheimer’s disease. Further, annual follow-up and autopsy rates were high, minimizing the risk for bias due to selective attrition before autopsy, and the time between last clinical evaluation and autopsy was relatively short. Uniform clinical evaluation and established criteria were used to clinically classify participants. Neuropathological evaluations were conducted blinded to clinical evaluations and diagnoses. Limitations include a relatively healthy and racially homogeneous volunteer cohort that agreed to autopsy, potentially limiting generalizability. For this analysis, pathologies like TDP-43 without clearly defined pathologic diagnoses were dichotomized to facilitate comparisons with pathologic diagnosis of Alzheimer’s disease and across pathologies; this dichotomizaton was data-driven, but using different thresholds for presence of pathology could affect our results. Autopsy procedures were carried out on only one hemisphere of the brain, raising the possibility that certain pathologies that present unilaterally were not observed. We did not include data on TDP-43 neurites, which may lead us to underestimate the per cent of mixed Alzheimer’s disease; further study of TDP-43 neurites in relation to Alzheimer’s disease and other pathologies is needed. At the time of analyses, the caudate had not been assessed for TDP-43 in the full cohort, limiting our ability to compare to other TDP-43 staging schemes that involve the caudate (Josephs et al., 2014a, 2016). More research is needed to understand how TDP-43 contributes to dementia in the context of Alzheimer’s disease and other known neuropathologies.
Acknowledgements
We thank the nuns, priests, and brothers from across the country participating in the Religious Orders Study, the men and women across Northeastern Illinois participating in the Memory and Aging Project, and the staff of the Rush Alzheimer’s Disease Center.
Funding
This research was supported by National Institute on Aging Grants P30AG10161, RF1AG15819, R01AG17917, R01AG042210, P30AG10124, and K01AG050823. The funding agencies played no part in the design or conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. B.D.J. is a consultant for Partners Healthcare and the Alzheimer’s Association. J.A.S. is also a consultant for AVID Radiopharmaceuticals Inc., and Navidea Biopharmaceuticals and has been on the Advisory Boards for GE Healthcare and Eli Lilly and Company. None of the authors report any conflicts of interests.
References
- Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer's disease. Ann Neurol 2007; 61: 435–45. [Europe PMC free article] [Abstract] [Google Scholar]
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 2006; 351: 602–11. [Abstract] [Google Scholar]
- Arai T, Mackenzie IR, Hasegawa M, Nonoka T, Niizato K, Tsuchiya K, et al. Phosphorylated TDP-43 in Alzheimer's disease and dementia with Lewy bodies. Acta Neuropathol 2009; 117: 125–36. [Abstract] [Google Scholar]
- Arnold SJ, Dugger BN, Beach TG. TDP-43 deposition in prospectively followed, cognitively normal elderly individuals: correlation with argyrophilic grains but not other concomitant pathologies. Acta Neuropathol 2013; 126: 51–7. [Europe PMC free article] [Abstract] [Google Scholar]
- Arvanitakis Z, Capuano AW, Leurgans SE, Buchman AS, Bennett DA, Schneider JA. The relationship of cerebral vessel pathology to brain microinfarcts. Brain Pathol 2016. 10.1111/bpa.12365. [Epub ahead of print] [Europe PMC free article] [Abstract] [Google Scholar]
- Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA. Microinfarct pathology, dementia, and cognitive systems. Stroke 2011; 42: 722–7. [Europe PMC free article] [Abstract] [Google Scholar]
- Attems J, Jellinger KA. Hippocampal sclerosis in Alzheimer disease and other dementias. Neurology 2006; 66: 775. [Abstract] [Google Scholar]
- Bennett DA, Schneider JA, Aggarwal NT, Arvanitakis Z, Shah RC, Kelly JF, et al. Decision rules guiding the clinical diagnosis of Alzheimer's disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology 2006a; 27: 169–76. [Abstract] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 2006b; 66: 1837–44. [Abstract] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the religious orders study. Curr Alzheimer Res 2011; 9: 628–45. [Europe PMC free article] [Abstract] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the rush memory and aging project. Curr Alzheimer Res 2012; 9: 646–63. [Europe PMC free article] [Abstract] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush memory and aging project: study design and baseline characteristics of the study cohort. Neuroepidemiology 2005; 25: 163–75. [Abstract] [Google Scholar]
- Bennett DA, Wilson RS, Arvanitakis Z, Boyle PA, de Toledo-Morrell L, Schneider JA. Selected findings from the religious orders study and rush memory and aging project. J Alzheimers Dis 2013; 33 (Suppl 1): S397–403. [Europe PMC free article] [Abstract] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, et al. Natural history of mild cognitive impairment in older persons. Neurology 2002; 59: 198–205. [Abstract] [Google Scholar]
- Bigio EH, Mishra M, Hatanpaa KJ, White CL, 3rd, Johnson N, Rademaker A, et al. TDP-43 pathology in primary progressive aphasia and frontotemporal dementia with pathologic Alzheimer disease. Acta Neuropathol 2010; 120: 43–54. [Europe PMC free article] [Abstract] [Google Scholar]
- Boyle PA, Wilson RS, Yu L, Barr AM, Honer WG, Schneider JA, et al. Much of late life cognitive decline is not due to common neurodegenerative pathologies. Ann Neurol 2013; 74: 478–89. [Europe PMC free article] [Abstract] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991; 82: 239–59. [Abstract] [Google Scholar]
- Buchman AS, Leurgans SE, Nag S, Bennett DA, Schneider JA. Cerebrovascular disease pathology and parkinsonian signs in old age. Stroke 2011; 42: 3183–9. [Europe PMC free article] [Abstract] [Google Scholar]
- Chang XL, Tan MS, Tan L, Yu JT. The Role of TDP-43 in Alzheimer's disease. Mol Neurobiol 2016; 53: 3349–59. [Abstract] [Google Scholar]
- Cykowski MD, Takei H, Van Eldik LJ, Schmitt FA, Jicha GA, Powell SZ, et al. Hippocampal sclerosis but not normal aging or Alzheimer disease is associated with TDP-43 pathology in the basal forebrain of aged persons. J Neuropathol Exp Neurol 2016; 75: 397–407. [Europe PMC free article] [Abstract] [Google Scholar]
- Davidson YS, Raby S, Foulds PG, Robinson A, Thompson JC, Sikkink S, et al. TDP-43 pathological changes in early onset familial and sporadic Alzheimer's disease, late onset Alzheimer's disease and Down's syndrome: association with age, hippocampal sclerosis and clinical phenotype. Acta Neuropathol 2011; 122: 703–13. [Abstract] [Google Scholar]
- Geser F, Prvulovic D, O'Dwyer L, Hardiman O, Bede P, Bokde AL, et al. On the development of markers for pathological TDP-43 in amyotrophic lateral sclerosis with and without dementia. Prog Neurobiol 2011; 95: 649–62. [Europe PMC free article] [Abstract] [Google Scholar]
- Geser F, Robinson JL, Malunda JA, Xie SX, Clark CM, Kwong LK, et al. Pathological 43-kDa transactivation response DNA-binding protein in older adults with and without severe mental illness. Arch Neurol 2010; 67: 1238–50. [Europe PMC free article] [Abstract] [Google Scholar]
- Higashi S, Iseki E, Yamamoto R, Minegishi M, Hino H, Fujisawa K, et al. Concurrence of TDP-43, tau and alpha-synuclein pathology in brains of Alzheimer's disease and dementia with Lewy bodies. Brain Res 2007; 1184: 284–94. [Abstract] [Google Scholar]
- Hu WT, Josephs KA, Knopman DS, Boeve BF, Dickson DW, Petersen RC, et al. Temporal lobar predominance of TDP-43 neuronal cytoplasmic inclusions in Alzheimer disease. Acta Neuropathol 2008; 116: 215–20. [Europe PMC free article] [Abstract] [Google Scholar]
- Jellinger KA, Attems J. Challenges of multimorbidity of the aging brain: a critical update. J Neural Trans 2015; 122: 505–21. [Abstract] [Google Scholar]
- Josephs KA, Murray ME, Whitwell JL, Parisi JE, Petrucelli L, Jack CR, et al. Staging TDP-43 pathology in Alzheimer's disease. Acta Neuropathol 2014a; 127: 441–50. [Europe PMC free article] [Abstract] [Google Scholar]
- Josephs KA, Murray ME, Whitwell JL, Tosakulwong N, Weigand SD, Petrucelli L, et al. Updated TDP-43 in Alzheimer's disease staging scheme. Acta Neuropathol 2016; 131: 571–85. [Abstract] [Google Scholar]
- Josephs KA, Nelson PT. Unlocking the mysteries of TDP-43. Neurology 2015; 84: 870–1. [Abstract] [Google Scholar]
- Josephs KA, Whitwell JL, Knopman DS, Hu WT, Stroh DA, Baker M, et al. Abnormal TDP-43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology 2008; 70 (19 Pt 2): 1850–7. [Europe PMC free article] [Abstract] [Google Scholar]
- Josephs KA, Whitwell JL, Weigand SD, Murray ME, Tosakulwong N, Liesinger AM, et al. TDP-43 is a key player in the clinical features associated with Alzheimer's disease. Acta Neuropathol 2014b; 127: 811–24. [Europe PMC free article] [Abstract] [Google Scholar]
- Kadokura A, Yamazaki T, Lemere CA, Takatama M, Okamoto K. Regional distribution of TDP-43 inclusions in Alzheimer disease (AD) brains: their relation to AD common pathology. Neuropathology 2009; 29: 566–73. [Abstract] [Google Scholar]
- Keage HA, Hunter S, Matthews FE, Ince PG, Hodges J, Hokkanen SR, et al. TDP-43 pathology in the population: prevalence and associations with dementia and age. J Alzheimers Dis 2014; 42: 641–50. [Abstract] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB consortium. Neurology 2005; 65: 1863–72. [Abstract] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of department of health and human services task force on Alzheimer's disease. Neurology 1984; 34: 939–44. [Abstract] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 1991; 41: 479–86. [Abstract] [Google Scholar]
- Nag S, Yu L, Capuano AW, Wilson RS, Leurgans SE, Bennett DA, et al. Hippocampal sclerosis and TDP-43 pathology in aging and Alzheimer disease. Ann Neurol 2015; 77: 942–52. [Europe PMC free article] [Abstract] [Google Scholar]
- Nelson PT, Abner EL, Schmitt FA, Kryscio RJ, Jicha GA, Smith CD, et al. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol 2010; 20: 66–79. [Europe PMC free article] [Abstract] [Google Scholar]
- Nelson PT, Schmitt FA, Lin Y, Abner EL, Jicha GA, Patel E, et al. Hippocampal sclerosis in advanced age: clinical and pathological features. Brain 2011; 134 (Pt 5): 1506–18. [Europe PMC free article] [Abstract] [Google Scholar]
- Neumann M, Kwong LK, Lee EB, Kremmer E, Flatley A, Xu Y, et al. Phosphorylation of S409/410 of TDP-43 is a consistent feature in all sporadic and familial forms of TDP-43 proteinopathies. Acta Neuropathol 2009; 117: 137–49. [Europe PMC free article] [Abstract] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006; 314: 130–3. [Abstract] [Google Scholar]
- Rauramaa T, Pikkarainen M, Englund E, Ince PG, Jellinger K, Paetau A, et al. TAR-DNA binding protein-43 and alterations in the hippocampus. J Neural Transm 2011; 118: 683–9. [Abstract] [Google Scholar]
- Robinson JL, Geser F, Corrada MM, Berlau DJ, Arnold SE, Lee VM, et al. Neocortical and hippocampal amyloid-beta and tau measures associate with dementia in the oldest-old. Brain 2011; 134 (Pt 12): 3708–15. [Europe PMC free article] [Abstract] [Google Scholar]
- SAS Institute Inc. SAS 9.3 Help and documentation. Cary, NC: SAS Institute Inc.; 2011. [Google Scholar]
- Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA. The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis 2009a; 18: 691–701. [Europe PMC free article] [Abstract] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007; 69: 2197–204. [Abstract] [Google Scholar]
- Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol 2009b; 66: 200–8. [Europe PMC free article] [Abstract] [Google Scholar]
- Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology 2004; 62: 1148–55. [Abstract] [Google Scholar]
- The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer s Disease. Consensus recommendations for the postmortem diagnosis of Alzheimer s disease. Neurobiol Aging 1997; 18 (4 Suppl): S1–2. [Abstract] [Google Scholar]
- Tremblay C, St-Amour I, Schneider J, Bennett DA, Calon F. Accumulation of transactive response DNA binding protein 43 in mild cognitive impairment and Alzheimer disease. J Neuropathol Exp Neurol 2011; 70: 788–98. [Europe PMC free article] [Abstract] [Google Scholar]
- Uryu K, Nakashima-Yasuda H, Forman MS, Kwong LK, Clark CM, Grossman M, et al. Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol 2008; 67: 555–64. [Europe PMC free article] [Abstract] [Google Scholar]
- Wilson AC, Dugger BN, Dickson DW, Wang DS. TDP-43 in aging and Alzheimer's disease—a review. Int J Clin Exp Pathol 2011; 4: 147–55. [Europe PMC free article] [Abstract] [Google Scholar]
- Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. J Int Neuropsychol Soc 2005; 11: 400–7. [Abstract] [Google Scholar]
- Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging 2002; 17: 179–93. [Abstract] [Google Scholar]
- Wilson RS, Yu L, Trojanowski JQ, Chen EY, Boyle PA, Bennett DA, et al. TDP-43 pathology, cognitive decline, and dementia in old age. JAMA Neurol 2013; 70: 1418–24. [Europe PMC free article] [Abstract] [Google Scholar]
- Youmans KL, Wolozin B. TDP-43: a new player on the AD field? Exp Neurol 2012; 237: 90–5. [Europe PMC free article] [Abstract] [Google Scholar]
- Yu L, De Jager PL, Yang J, Trojanowski JQ, Bennett DA, Schneider JA. The TMEM106B locus and TDP-43 pathology in older persons without FTLD. Neurology 2015; 84: 927–34. [Europe PMC free article] [Abstract] [Google Scholar]
- Zarow C, Weiner MW, Ellis WG, Chui HC. Prevalence, laterality, and comorbidity of hippocampal sclerosis in an autopsy sample. Brain Behav 2012; 2: 435–42. [Europe PMC free article] [Abstract] [Google Scholar]
Articles from Brain are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/brain/aww224
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/brain/article-pdf/139/11/2983/24173003/aww224.pdf
Citations & impact
Impact metrics
Article citations
Amyloid deposition and its association with depressive symptoms and cognitive functions in late-life depression: a longitudinal study using amyloid-β PET images and neuropsychological measurements.
Alzheimers Res Ther, 16(1):232, 19 Oct 2024
Cited by: 0 articles | PMID: 39427221 | PMCID: PMC11490031
HDGFL2 cryptic protein: a portal to detection and diagnosis in neurodegenerative disease.
Mol Neurodegener, 19(1):79, 25 Oct 2024
Cited by: 0 articles | PMID: 39456026 | PMCID: PMC11515212
Grey matter ageing-related tau astrogliopathy: associations with brain pathologies and cognitive decline.
Brain, 147(10):3501-3512, 01 Oct 2024
Cited by: 0 articles | PMID: 39045644
Alzheimer's disease CSF biomarkers correlate with early pathology and alterations in neuronal and glial gene expression.
Alzheimers Dement, 20(10):7090-7103, 27 Aug 2024
Cited by: 1 article | PMID: 39192661 | PMCID: PMC11485399
Association of Alzheimer's Disease and Other Neuropathologies With Functional Disability in Persons With and Without Dementia.
J Gerontol A Biol Sci Med Sci, 79(9):glae118, 01 Sep 2024
Cited by: 0 articles | PMID: 38757945
Go to all (218) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Evaluation of TDP-43 proteinopathy and hippocampal sclerosis in relation to APOE ε4 haplotype status: a community-based cohort study.
Lancet Neurol, 17(9):773-781, 06 Aug 2018
Cited by: 84 articles | PMID: 30093249 | PMCID: PMC6154505
Dichotomous scoring of TDP-43 proteinopathy from specific brain regions in 27 academic research centers: associations with Alzheimer's disease and cerebrovascular disease pathologies.
Acta Neuropathol Commun, 6(1):142, 19 Dec 2018
Cited by: 41 articles | PMID: 30567576 | PMCID: PMC6299605
Comprehensive assessment of TDP-43 neuropathology data in the National Alzheimer's Coordinating Center database.
Acta Neuropathol, 147(1):103, 19 Jun 2024
Cited by: 0 articles | PMID: 38896163 | PMCID: PMC11186885
TDP-43 in aging and Alzheimer's disease - a review.
Int J Clin Exp Pathol, 4(2):147-155, 30 Jan 2011
Cited by: 127 articles | PMID: 21326809 | PMCID: PMC3037200
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIA NIH HHS (9)
Grant ID: RF1 AG015819
Grant ID: R01 AG015819
Grant ID: R01 AG042210
Grant ID: P30 AG010161
Grant ID: P01 AG017586
Grant ID: K01 AG050823
Grant ID: R01 AG034374
Grant ID: P30 AG010124
Grant ID: R01 AG017917





