Abstract
Objective
The aim of this study was to investigate whether cortisol and oxidative stress levels and DNA damage differ between individuals who developed PTSD or not following a sexual trauma.Methods
The study included 61 children aged between 5 and 17 years who sustained sexual abuse (M/F: 18/43). The patients were divided into two groups: patients with PTSD and patients without PTSD based, based on the results of a structured psychiatric interview (K-SADS-PL and CAPS-CA). Cortisol, glutathione peroxidase (GPx), superoxide dismutase (SOD), coenzyme Q, 8-Hydroxy-2-Deoxyguanosine (8-OHdG) were all evaluated by the ELISA method.Results
Our evaluation revealed a diagnosis of PTSD in 51% (n=31) of victims. There was no significant difference between the groups with or without PTSD in terms of cortisol, GPx, SOD, coenzyme Q, and 8-OHdG levels. There was no correlation between CAPS scores and GPx, SOD, coenzyme Q, and 8-OHdG levels between patients with or without PTSD. In patients with PTSD, both cortisol and 8-OHdG levels decreased with increasing time after trauma, and there was no significant correlation with cortisol and 8-OHdG levels in patients without PTSD.Conclusion
Although the present study did not find any difference between the groups in terms of 8-OHdG concentrations, the decreases in both cortisol and 8-OHdG levels with increasing time after trauma is considered to indicate a relationship between cortisol and DNA damage.Free full text

The Levels of Cortisol and Oxidative Stress and DNA Damage in Child and Adolescent Victims of Sexual Abuse with or without Post-Traumatic Stress Disorder
Abstract
Objective
The aim of this study was to investigate whether cortisol and oxidative stress levels and DNA damage differ between individuals who developed PTSD or not following a sexual trauma.
Methods
The study included 61 children aged between 5 and 17 years who sustained sexual abuse (M/F: 18/43). The patients were divided into two groups: patients with PTSD and patients without PTSD based, based on the results of a structured psychiatric interview (K-SADS-PL and CAPS-CA). Cortisol, glutathione peroxidase (GPx), superoxide dismutase (SOD), coenzyme Q, 8-Hydroxy-2-Deoxyguanosine (8-OHdG) were all evaluated by the ELISA method.
Results
Our evaluation revealed a diagnosis of PTSD in 51% (n=31) of victims. There was no significant difference between the groups with or without PTSD in terms of cortisol, GPx, SOD, coenzyme Q, and 8-OHdG levels. There was no correlation between CAPS scores and GPx, SOD, coenzyme Q, and 8-OHdG levels between patients with or without PTSD. In patients with PTSD, both cortisol and 8-OHdG levels decreased with increasing time after trauma, and there was no significant correlation with cortisol and 8-OHdG levels in patients without PTSD.
Conclusion
Although the present study did not find any difference between the groups in terms of 8-OHdG concentrations, the decreases in both cortisol and 8-OHdG levels with increasing time after trauma is considered to indicate a relationship between cortisol and DNA damage.
INTRODUCTION
Trauma is defined as a direct exposure of an individual to a severely distressing event such as experiencing or witnessing a real death or death threat, severe injury or any threat to the physical integrity of an individual.1 Sexual abuse is one of the most severe forms of trauma, considering its future consequences. Brain tissue continues its development in childhood and during adolescence.2 Traumas experienced during these periods have been reported to have more serious consequences.3
The individuals who have experienced trauma often exhibit one or more psychiatric disorders, including post-traumatic stress disorder (PTSD), depression, dissociative disorder, alcohol and substance abuse, personality disorders, and suicide attempts.4 Although PTSD is the most commonly encountered psychiatric condition after trauma, not all patients who experience trauma develop PTSD. Some individual and environmental risk factors are associated with the development of PTSD. The type and severity of trauma are the most important predictors of PTSD.5
Oxidative stress implies overproduction of reactive oxygen species (ROS) and/or failure of antioxidant defense mechanisms.6 Brain tissue is vulnerable to oxidative stress due to its higher demand for oxygen.7,8,9 Oxidative stress also impairs normal brain functions through the inhibition of neurogenesis, altering neuronal transmission, and inducing mitochondrial dysfunction.10
The anti-oxidant defense system involves enzymatic and non-enzymatic components. Superoxide dismutase (SOD) and glutathione peroxidase (GPx) are anti-oxidant enzymes.11 Coenzyme Q is a non-enzymatic anti-oxidant.12 Coenzyme Q is a lipid-soluble vitamin-like substance, and it is an important cofactor in mitochondrial electron transport from complex I to complex III.13 It is found at high concentrations in the brain and heart.14 8-Hydroxy-2-Deoxyguanosine (8-OHdG) is a biochemical marker of ROS-mediated DNA damage.15
The human and animals studies demonstrated that oxidative stress is related to anxiety.16,17,18,19,20,21 In animal model of PTSD, inflammation and oxidative stress were reported to play a critical role in the development and exacerbation of PTSD.22 There are also studies that did not report a significant difference between patients with PTSD and the control group in terms of oxidative stress.23,24
It was reported that oxidative stress could be a critical molecular linkage between the hypothalamic–pituitary–adrenal (HPA) axis dysfunction and mental disorders.25 It was also reported that the stress-induced increase in cortisol levels accelerates glucose metabolism and production of reactive oxygen species.26
The present study considered the fact that children and adolescents are more susceptible to the effects of trauma, and there are no studies demonstrating the relationship between oxidative stress and PTSD in this particular age group; the studies on adults have reported controversial data. The aim of this study was to investigate whether cortisol and oxidative stress levels and DNA damage differ between individuals who developed PTSD or not following a sexual trauma.
METHODS
Study sample
The study was conducted in the Department of Child Psychiatry at Dicle University Training and Research Hospital. The study data were collected between July 2013 and February 2014.
A total of 61 children, aged between 5 and 17 years (18 males and 43 females), participated in the study. CSA included intercourse, attempted intercourse, oral-genital contact, fondling of genitals directly or through clothing, exhibitionism or exposing children to adult sexual activity or pornography. The cases were selected from among the children who were subjected to the psychiatric examinations required by the legal authorities. A total of 160 CSA cases were admitted within the data collection period. Of these 105 cases agreed to be involved in the study, and 44 cases were excluded from the study based on the exclusion criteria. The patients were divided into two groups, patients with PTSD and patients without PTSD, based on the results of a structured psychiatric interview. Children who had mental retardation, history of head trauma, and those who received oral contraceptives and psychotropics, previous or current cortisol therapy, and vitamins, and patients who had ≥30 body mass index, chronic systemic disorders, and clinically active infection were excluded in order to prevent interference with biochemical parameters. Furthermore, cases with a psychopathology different from PTSD and depression were excluded from the study. They weren't paid for participation in the study. The patients were evaluated by two experienced psychiatric doctors who had been working with these patient groups for four years. Parents provided informed consent for their children to participate in the study. Inter-rater agreement was 0.70. Ethical approval of the study protocol was obtained from the Non-Interventional Clinical Research Ethics Committee at Dicle University Faculty of Medicine.
Study procedures
Sociodemographic features and clinical dates of the participants were recorded by the psychiatrists. This was followed by a structured psychiatric interview (K-SADS-PL and CAPS-CA) and administration of the self-reported Children's Depression Inventory (CDI). Finally, a 2 mL venous blood sample was obtained for biochemical tests.
Forms and scales
Sociodemographic data and clinical data form
This form included questions about age, gender, age of the parents, education level, number of siblings, age at menarche and menstrual cycle, type of abuse, duration and frequency of abuse, relationship with the abuser, and abuse history.
Affective disorders and schizophrenia for school age children-present and lifetime version
The schedule (K-SADS-PL) was originally developed by Kaufman et al.27 It was adapted to the Turkish language by Gökler et al. in 2004.28 K-SADS-PL is administered during an interview with the parents and children, and the final evaluation is performed using input from all data sources. The presence of common psychopathologies in children and adolescents may be investigated with this tool.
Clinician-administered post-traumatic stress disorder scale for children and adolescents
CAPS-CA is a semi-structured interview developed to evaluate the frequency and severity of present and past PTSD in children and adolescents according to DSM-III and DSM-IV diagnostic criteria. It was adapted from the Clinician-Administered Post-Traumatic Stress Disorder Scale (CAPS) by Nader et al. in 1996.29 There are totally 36 items in the scale. While seventeen evaluate PTSD symptoms, eight examine other symptoms related to PTSD, such as feelings of guilt, shame, dissociation, change in attachment behavior and trauma related fears. In the current study, other symptoms related to PTSD were not examined, and only PTSD symptoms occurring within the last one month were examined. The total score of the scale ranges between 0 and 68. A diagnosis of PTSD could be made based on the presence of at least one sign of re-experiencing, three symptoms of avoidance or numbing, and two symptoms of a hyper-arousal state. If the patient did not meet these criteria, they were diagnosed as subthreshold PTSD. Each item is scored based both on the children's answers and on a clinical judgment made during the interview. It was adapted to the Turkish language by Karakaya et al.30
The children's depression inventory
The Children's Depression Inventory (CDI) developed by Kovasc based on the Beck Depression scale was used in the study.31 However, questions specific to the childhood period such as school success and relationship with the friends were added. The scale was adapted to the Turkish language by Öy32 and the scale contains 27 items. Each item is scored as “0”, “1”, or “2” points depending on the severity of the symptom. The highest score is 54 points. High scores indicate the level or severity of depression. The cutoff point for the scale is 19 points.
Biochemical analysis
The blood samples were obtained in the morning between 1000 h and 1200 h. The samples were collected into gel tubes. After collection, blood samples were allowed to set for 15 minutes for clotting. Then, blood samples were centrifuged at 5000 rpm for 6 minutes. The sera were transferred to 1.5 mL polypropylene tubes and stored at −80 degrees centigrade until the analysis. Serum levels of cortisol, GPx, coenzyme Q, 8-OHdG, and SOD were determined with an enzyme linked immunosorbent assay (ELISA) method (Hangzhou Eastbiopharm CO. LTD China), according to the manufacturer's instructions. Briefly, samples were added to monoclonal antibody enzyme well which is pre-coated with cortisol monoclonal antibody, incubated; then, cortisol antibodies labeled with biotin were added, and combined with Streptavidin-HRP to form immune complex; then incubation and washing were carried out again to remove the uncombined enzyme. Then Chromogen Solution A, B were added, the color of the liquid changed into the blue, And at the effect of acid, the color was finally become yellow. The absorbance was measured at 450 nm.
Statistical analysis
The statistical analysis was performed using SPSS 18.0 software package. The student's t-test was used to compare normally distributed variables in independent groups, and the Mann-Whitney test was used to compare not-normally distributed categorical variables. The significance of the difference between the groups in terms of gender, history of psychiatric disorders, or substance abuse in the family members or relatives, type of abuse, frequency and duration of abuse, relationship with the abuser was evaluated using the chi-square test or Fisher's exact test, where appropriate. Pearson's test was used to evaluate correlation coefficients and statistically significance of normally distributed variables, and Spearman's test was used to evaluate not normally distributed variables. A p value below 0.05 was considered statistically significant.
RESULTS
The mean age was 13.3±2.4 years (range: 5-17 years) among the victims of sexual abuse. Our evaluation revealed a diagnosis of PTSD in 51% (n=31) of victims. There was no significant difference between patients with or without PTSD in terms of gender, smoking status, and menstrual cycle, the latter being assessed for adolescent patients. There was also no significant difference between the groups in terms of age, age of the mother/father. Data related to some sociodemographic variables and scale scores are presented in Table 1.
Table 1
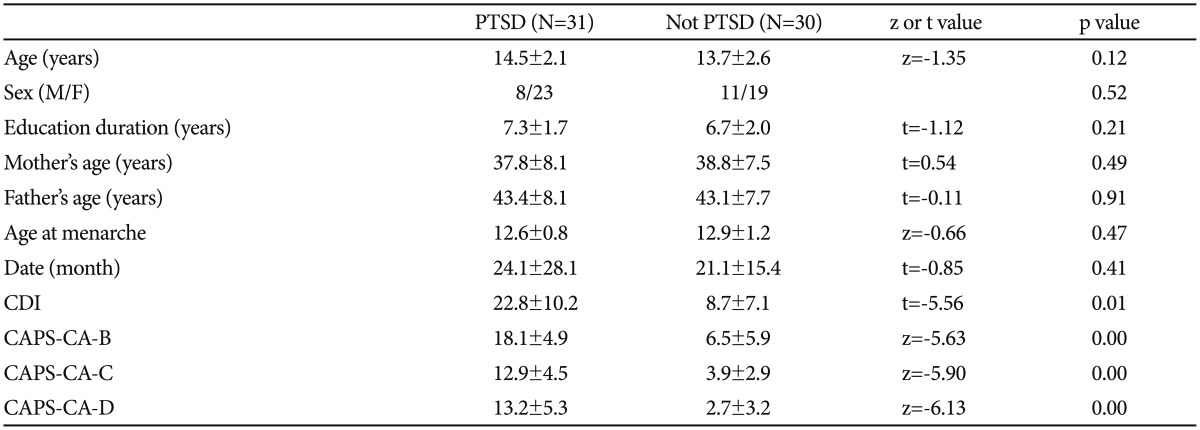
PTSD: post-traumatic stress disorder, Date (month): time between the first date of trauma and examination date, CDI: Child Depression Inventory, CAPS-CA: Clinician-Administered Post-Traumatic Stress Disorder Scale for Children and Adolescents
Regarding the parameters related to sexual abuse, 48% (n=29) of the victims experienced sexual abuse involving penetration. Of the victims, 46% (n=28) experienced single assault and 54% (n=33) experienced multiple assaults. 21% (n=13) of victims experienced sexual abuse within the family (incestuous), and 79% (n=48) experienced sexual abuse committed by non-related persons. There was no significant difference between patients with or without PTSD in terms of relationship with the abuse and presence of penetration (p=0.34 and p=0.68, respectively). However, the rate of PTSD was higher in patients who experienced multiple sexual assaults compared to the victims of a single assault (p=0.01). The rate of depression was significantly higher in patients with PTSD (p=0.01).
There was no significant difference between the groups with or without PTSD in terms of cortisol, GPx, SOD, coenzyme Q, and 8-OHdG levels (Table 2). Likewise, there was no significant difference between the groups with or without depression in terms of cortisol, GPx, SOD, coenzyme Q, and 8-OHdG levels (p=0.43, p=0.46, p=0.38, p=0.53, and p=0.48, respectively). There was no correlation between CAPS scores and GPx, SOD, coenzyme Q, and 8-OHdG levels between patients with or without PTSD.
Table 2
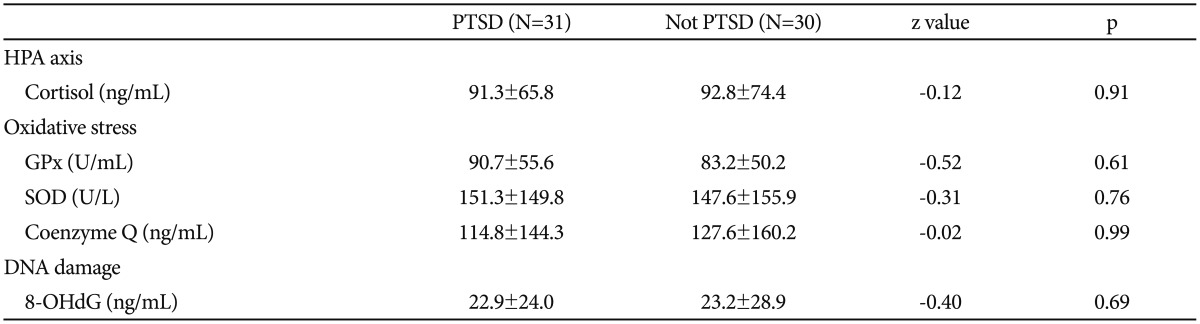
PTSD: post-traumatic stress disorder, GPx: Glutathione peroxidase, SOD: Superoxide dismutase, 8-OHdG: 8-Hydroxy-2-Deoxyguanosine
The mean time that elapsed since the first sexual abuse until the date of examination was 23.9±24.1 months (range: 1-115 months). There is no difference between PTSD and non-PTSD group according to their mean time that elapsed since the first sexual abuse until the date of examination (p=0.41). In the PTSD group, cortisol levels decreased with increasing time after trauma, and there was no significant correlation with the cortisol levels in patients without PTSD (r=-0.46, p=0.01 and r=-0.07, p=0.73, respectively). Similarly, 8-OHdG levels in the PTSD group decreased with increasing time after trauma, and there was no significant correlation with 8-OHdG levels in patients without PTSD (r=-0.42, p= 0.03 and r=-0.04, p=0.85, respectively) (Figure 1).
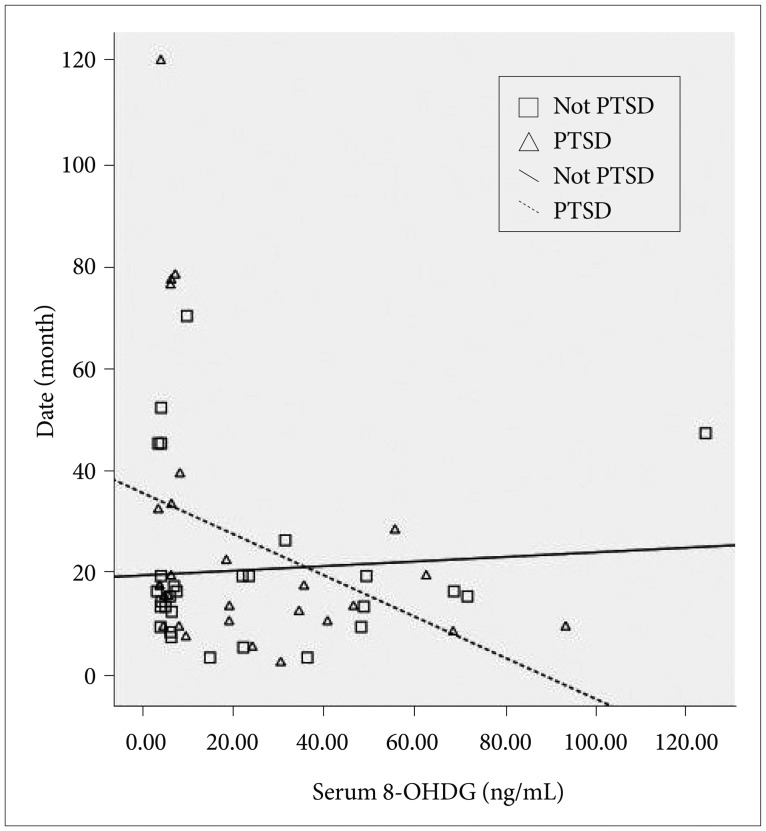
DISCUSSION
In the present study, there was no significant difference between patients with or without PTSD in terms of oxidative stress and DNA damage. Furthermore, no relationship was found between the severity of the symptoms of PTSD and oxidative stress and DNA damage. In their study, Tezcan et al.24 and Čeprnja et al.23 did not report any association between PTSD and oxidative stress. However, healthy volunteers having no past history of trauma were selected as the control group in their study. In addition, the type of trauma in their study was different compared to the present study. In contrast to our findings, human and animal studies showed an association between oxidative stress and anxiety.16,17,18,19,20,21 In an animal model of PTSD, inflammation and oxidative stress were reported to play a critical role in the development and exacerbation of PTSD.22
In the present study, cortisol and 8-OHdG levels decreased with increasing time after trauma in the PTSD group. Although we did not find any difference between the groups in terms of 8-OHdG concentrations, this finding was considered to be a reflection of the relationship between cortisol and DNA damage. In addition, the decrease in cortisol levels in time was suggested to be a reflection of adaptive process preventing harmful effects of prolonged exposure to high cortisol levels on brain structures such as the hippocampus and frontal cortex.33
The present study had several limitations. Biochemical parameters were analyzed in serum samples. The sources other than brain may have effects on the consequences. The present study performed single measurements to determine cortisol levels. This study did not include a healthy control group that did not have a history of trauma.
In conclusion, there was no significant difference between children and adolescents with or without PTSD after sexual abuse in terms of the level of oxidative stress and DNA damage. However, cortisol and 8-OHdG levels decreased with increasing time after trauma in the PTSD group. Although we did not find any difference between the groups in terms of 8-OHdG concentrations, this finding was considered to be a reflection of the relationship between cortisol and DNA damage. This is the first study conducted in this age group. On the other hand, longitudinal studies with larger sample size are required to confirm the findings of the current study.
Acknowledgments
We would like to thank Dr. Rümeysa Alaca, and our patients and their parents for taking part in this study.
References
Articles from Psychiatry Investigation are provided here courtesy of Korean Neuropsychiatric Association
Full text links
Read article at publisher's site: https://doi.org/10.4306/pi.2016.13.6.616
Read article for free, from open access legal sources, via Unpaywall:
http://psychiatryinvestigation.org/upload/pdf/pi-13-616.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.4306/pi.2016.13.6.616
Article citations
Psychological and biological mechanisms linking trauma with cardiovascular disease risk.
Transl Psychiatry, 13(1):25, 27 Jan 2023
Cited by: 15 articles | PMID: 36707505 | PMCID: PMC9883529
Review Free full text in Europe PMC
PTSD, Immune System, and Inflammation.
Adv Exp Med Biol, 1411:225-262, 01 Jan 2023
Cited by: 3 articles | PMID: 36949313
Mapping structural covariance networks in children and adolescents with post-traumatic stress disorder after earthquake.
Front Psychiatry, 13:923572, 15 Sep 2022
Cited by: 2 articles | PMID: 36186852 | PMCID: PMC9520616
The Evaluation of Thiol/Disulfide Homeostasis and Oxidative DNA Damage in Patients with Obsessive Compulsive Disorder.
Clin Psychopharmacol Neurosci, 20(2):240-247, 01 May 2022
Cited by: 4 articles | PMID: 35466095 | PMCID: PMC9048000
Cell Model of Depression: Reduction of Cell Stress with Mirtazapine.
Int J Mol Sci, 23(9):4942, 29 Apr 2022
Cited by: 8 articles | PMID: 35563333 | PMCID: PMC9099543
Go to all (15) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
BDNF and cortisol levels in children with or without post-traumatic stress disorder after sustaining sexual abuse.
Psychoneuroendocrinology, 56:45-51, 10 Mar 2015
Cited by: 19 articles | PMID: 25800148
The Levels of Cortisol, Oxidative Stress, and DNA Damage in the Victims of Childhood Sexual Abuse: A Preliminary Study.
J Child Sex Abus, 25(2):175-184, 01 Feb 2016
Cited by: 15 articles | PMID: 26934543
Oxidative Stress and DNA Damage in Untreated First-Episode Psychosis in Adolescents.
Neuropsychobiology, 73(2):92-97, 23 Mar 2016
Cited by: 19 articles | PMID: 27003298
Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis.
Br J Psychiatry, 191:387-392, 01 Nov 2007
Cited by: 345 articles | PMID: 17978317
Review

 1
1 



