Abstract
Free full text

Tetraethylammonium blockade distinguishes two inactivation mechanisms in voltage-activated K+ channels.
Abstract
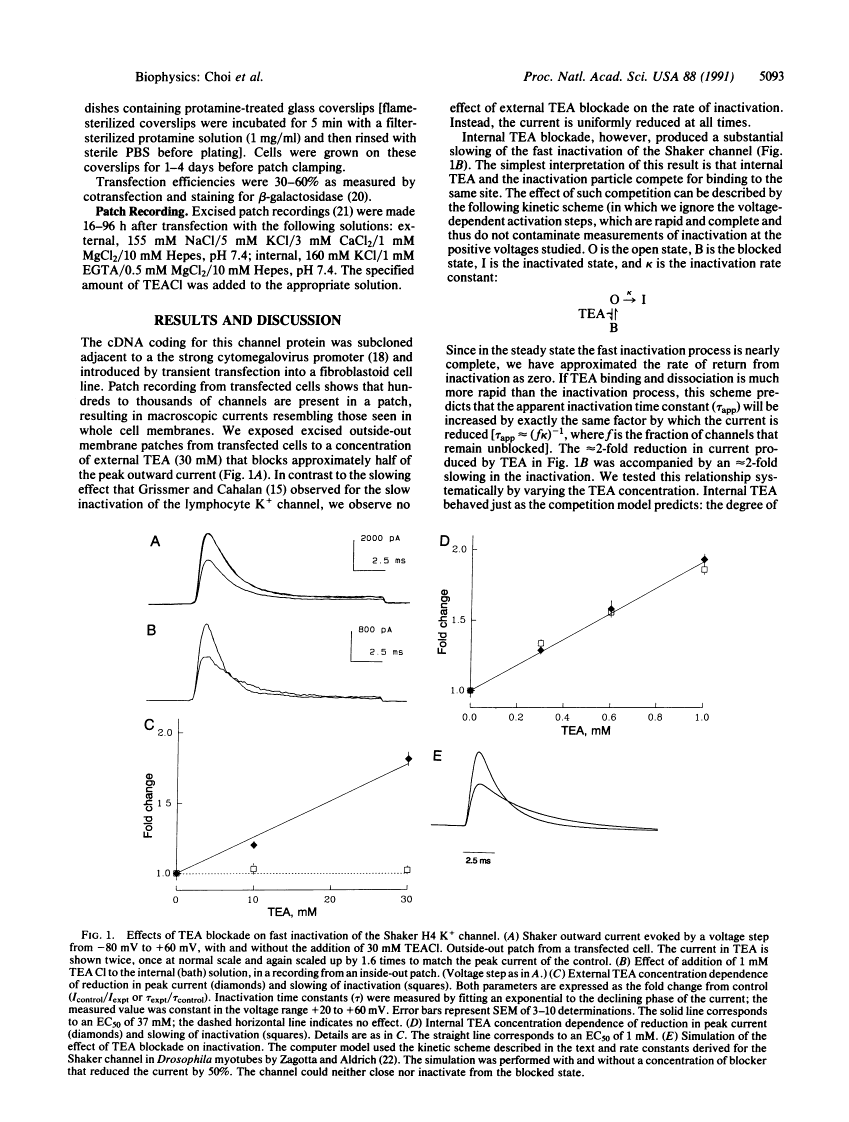
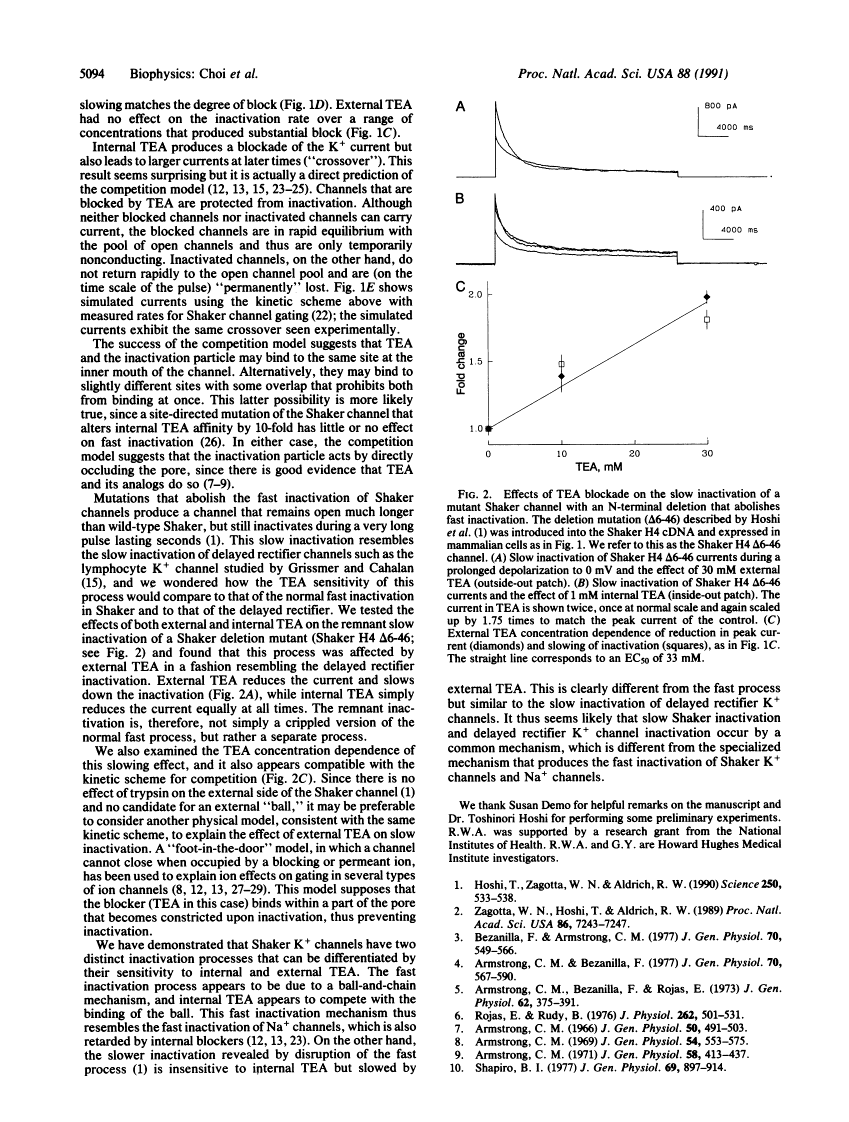
Voltage-activated K+ channels are a family of closely related membrane proteins that differ in their gating behavior, conductance, and pharmacology. A prominent and physiologically important difference among K+ channels is their rate of inactivation. Inactivation rates range from milliseconds to seconds, and K+ channels with different inactivation properties have very different effects on signal integration and repetitive firing properties of neurons. The cloned Shaker B (H4) potassium channel is an example of a K+ channel that inactivates in a few milliseconds. Recent experiments have shown that removal of an N-terminal region of the Shaker protein by site-directed deletion practically abolishes this fast inactivation, but the modified channel does still inactivate during a prolonged depolarization lasting many seconds. Here we report that this remnant inactivation must occur by a distinct mechanism from the rapid inactivation of the wild-type Shaker channel. Like the inactivation of another K+ channel [Grissmer, S. & Calahan, M. (1989) Biophys. J. 55, 203-206], this slow inactivation is retarded by the application of a channel blocker, tetraethylammonium, to the extracellular side of the channel. By contrast, the fast inactivation of the wild-type Shaker channel is sensitive only to intracellular application of tetraethylammonium. Intracellular tetraethylammonium slows down the fast inactivation process, as though it competes with the binding of the inactivation particle.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (686K), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hoshi T, Zagotta WN, Aldrich RW. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 1990 Oct 26;250(4980):533–538. [Abstract] [Google Scholar]
- Zagotta WN, Hoshi T, Aldrich RW. Gating of single Shaker potassium channels in Drosophila muscle and in Xenopus oocytes injected with Shaker mRNA. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7243–7247. [Europe PMC free article] [Abstract] [Google Scholar]
- Bezanilla F, Armstrong CM. Inactivation of the sodium channel. I. Sodium current experiments. J Gen Physiol. 1977 Nov;70(5):549–566. [Europe PMC free article] [Abstract] [Google Scholar]
- Armstrong CM, Bezanilla F. Inactivation of the sodium channel. II. Gating current experiments. J Gen Physiol. 1977 Nov;70(5):567–590. [Europe PMC free article] [Abstract] [Google Scholar]
- Armstrong CM, Bezanilla F, Rojas E. Destruction of sodium conductance inactivation in squid axons perfused with pronase. J Gen Physiol. 1973 Oct;62(4):375–391. [Europe PMC free article] [Abstract] [Google Scholar]
- Rojas E, Rudy B. Destruction of the sodium conductance inactivation by a specific protease in perfused nerve fibres from Loligo. J Physiol. 1976 Nov;262(2):501–531. [Abstract] [Google Scholar]
- Armstrong CM. Time course of TEA(+)-induced anomalous rectification in squid giant axons. J Gen Physiol. 1966 Nov;50(2):491–503. [Europe PMC free article] [Abstract] [Google Scholar]
- Armstrong CM. Inactivation of the potassium conductance and related phenomena caused by quaternary ammonium ion injection in squid axons. J Gen Physiol. 1969 Nov;54(5):553–575. [Europe PMC free article] [Abstract] [Google Scholar]
- Armstrong CM. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J Gen Physiol. 1971 Oct;58(4):413–437. [Europe PMC free article] [Abstract] [Google Scholar]
- Shapiro BI. Effects of strychnine on the potassium conductance of the frog node of Ranvier. J Gen Physiol. 1977 Jun;69(6):897–914. [Europe PMC free article] [Abstract] [Google Scholar]
- Shapiro BI. Effects of strychnine on the sodium conductance of the frog node of Ranvier. J Gen Physiol. 1977 Jun;69(6):915–926. [Europe PMC free article] [Abstract] [Google Scholar]
- Yeh JZ, Narahashi T. Kinetic analysis of pancuronium interaction with sodium channels in squid axon membranes. J Gen Physiol. 1977 Mar;69(3):293–323. [Europe PMC free article] [Abstract] [Google Scholar]
- Cahalan MD. Local anesthetic block of sodium channels in normal and pronase-treated squid giant axons. Biophys J. 1978 Aug;23(2):285–311. [Europe PMC free article] [Abstract] [Google Scholar]
- Zagotta WN, Hoshi T, Aldrich RW. Restoration of inactivation in mutants of Shaker potassium channels by a peptide derived from ShB. Science. 1990 Oct 26;250(4980):568–571. [Abstract] [Google Scholar]
- Grissmer S, Cahalan M. TEA prevents inactivation while blocking open K+ channels in human T lymphocytes. Biophys J. 1989 Jan;55(1):203–206. [Europe PMC free article] [Abstract] [Google Scholar]
- Tempel BL, Papazian DM, Schwarz TL, Jan YN, Jan LY. Sequence of a probable potassium channel component encoded at Shaker locus of Drosophila. Science. 1987 Aug 14;237(4816):770–775. [Abstract] [Google Scholar]
- Kamb A, Tseng-Crank J, Tanouye MA. Multiple products of the Drosophila Shaker gene may contribute to potassium channel diversity. Neuron. 1988 Jul;1(5):421–430. [Abstract] [Google Scholar]
- Boshart M, Weber F, Jahn G, Dorsch-Häsler K, Fleckenstein B, Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985 Jun;41(2):521–530. [Abstract] [Google Scholar]
- Hall CV, Jacob PE, Ringold GM, Lee F. Expression and regulation of Escherichia coli lacZ gene fusions in mammalian cells. J Mol Appl Genet. 1983;2(1):101–109. [Abstract] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. [Abstract] [Google Scholar]
- Zagotta WN, Aldrich RW. Voltage-dependent gating of Shaker A-type potassium channels in Drosophila muscle. J Gen Physiol. 1990 Jan;95(1):29–60. [Europe PMC free article] [Abstract] [Google Scholar]
- Cahalan MD, Almers W. Block of sodium conductance and gating current in squid giant axons poisoned with quaternary strychnine. Biophys J. 1979 Jul;27(1):57–73. [Europe PMC free article] [Abstract] [Google Scholar]
- Beam KG. A quantitative description of end-plate currents in the presence of two lidocaine derivatives. J Physiol. 1976 Jun;258(2):301–322. [Abstract] [Google Scholar]
- Neher E, Steinbach JH. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. [Abstract] [Google Scholar]
- Yellen G, Jurman ME, Abramson T, MacKinnon R. Mutations affecting internal TEA blockade identify the probable pore-forming region of a K+ channel. Science. 1991 Feb 22;251(4996):939–942. [Abstract] [Google Scholar]
- Swenson RP, Jr, Armstrong CM. K+ channels close more slowly in the presence of external K+ and Rb+. Nature. 1981 Jun 4;291(5814):427–429. [Abstract] [Google Scholar]
- Miller C. Trapping single ions inside single ion channels. Biophys J. 1987 Jul;52(1):123–126. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.88.12.5092
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc51817?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Stability of N-type inactivation and the coupling between N-type and C-type inactivation in the Aplysia Kv1 channel.
Pflugers Arch, 476(10):1493-1516, 15 Jul 2024
Cited by: 0 articles | PMID: 39008084
Dilation of ion selectivity filters in cation channels.
Trends Biochem Sci, 49(5):417-430, 20 Mar 2024
Cited by: 1 article | PMID: 38514273
Review
Resurgent current in context: Insights from the structure and function of Na and K channels.
Biophys J, 123(14):1924-1941, 21 Dec 2023
Cited by: 1 article | PMID: 38130058 | PMCID: PMC11309984
Review Free full text in Europe PMC
Eukaryotic Kv channel Shaker inactivates through selectivity filter dilation rather than collapse.
Sci Adv, 9(49):eadj5539, 08 Dec 2023
Cited by: 3 articles | PMID: 38064553 | PMCID: PMC10708196
Calcium-gated potassium channel blockade via membrane-facing fenestrations.
Nat Chem Biol, 20(1):52-61, 31 Aug 2023
Cited by: 2 articles | PMID: 37653172 | PMCID: PMC10847966
Go to all (309) article citations
Other citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Slow inactivation in Shaker K channels is delayed by intracellular tetraethylammonium.
J Gen Physiol, 132(6):633-650, 01 Dec 2008
Cited by: 15 articles | PMID: 19029372 | PMCID: PMC2585862
Pore mutations in Shaker K+ channels distinguish between the sites of tetraethylammonium blockade and C-type inactivation.
J Physiol, 499 ( Pt 2):361-367, 01 Mar 1997
Cited by: 53 articles | PMID: 9080366
Two types of A-channels in Lymnaea neurons.
J Membr Biol, 146(3):327-341, 01 Aug 1995
Cited by: 3 articles | PMID: 8568847
Ball-and-Chain Inactivation in Potassium Channels.
Annu Rev Biophys, 52:91-111, 10 Jan 2023
Cited by: 4 articles | PMID: 36626766
Review