Abstract
Free full text

Monomeric and trimeric forms of photosystem I reaction center of Mastigocladus laminosus: crystallization and preliminary characterization.
Abstract
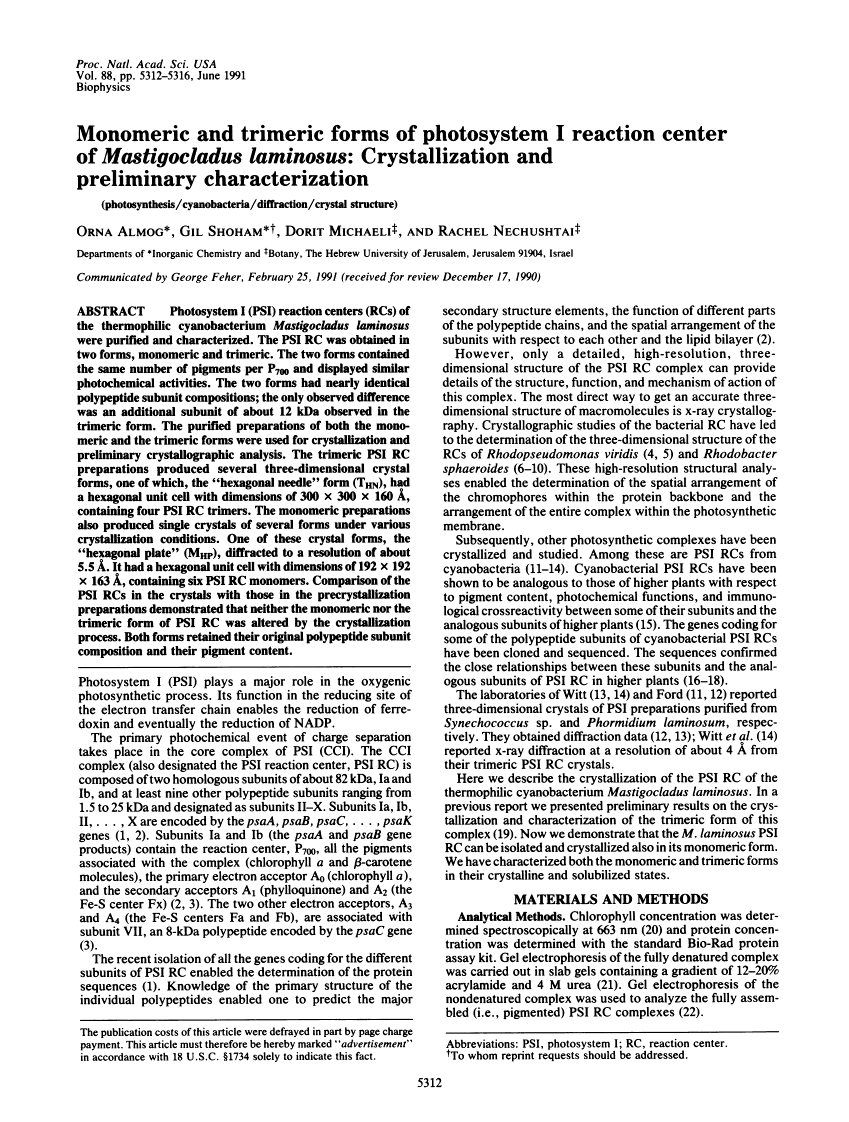
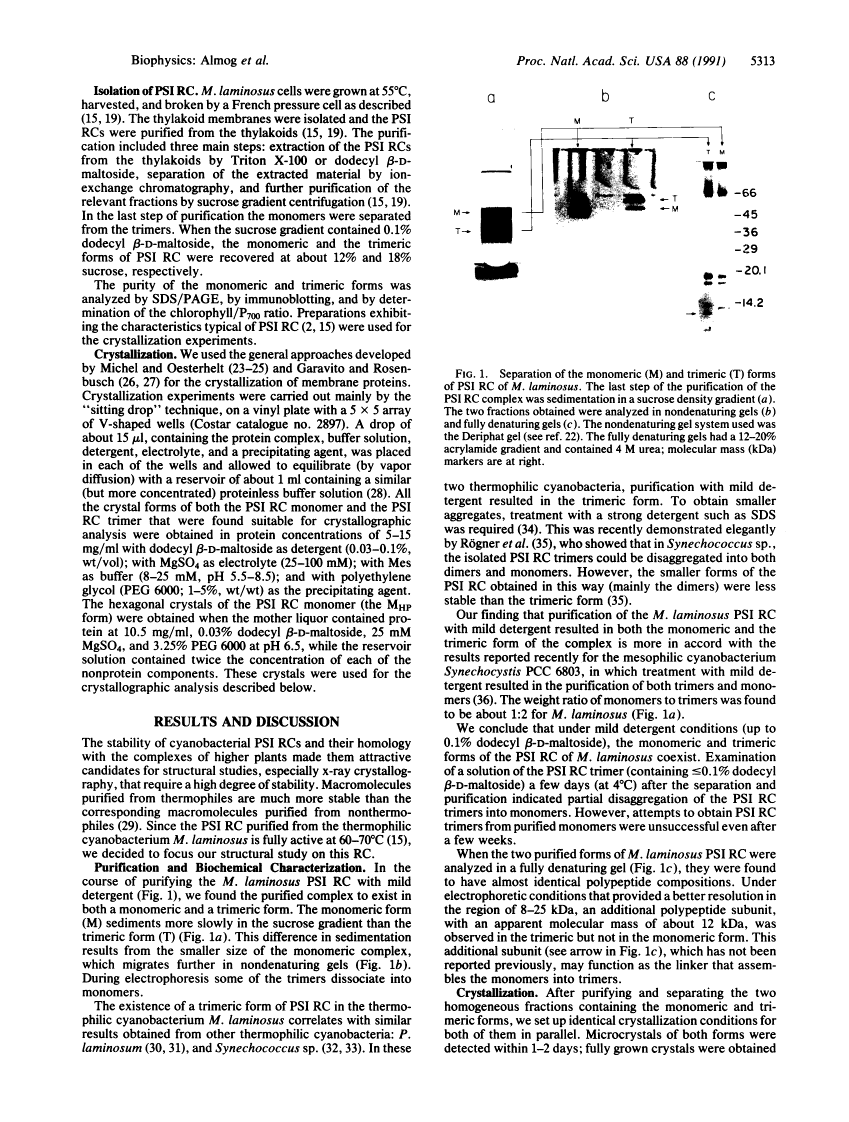
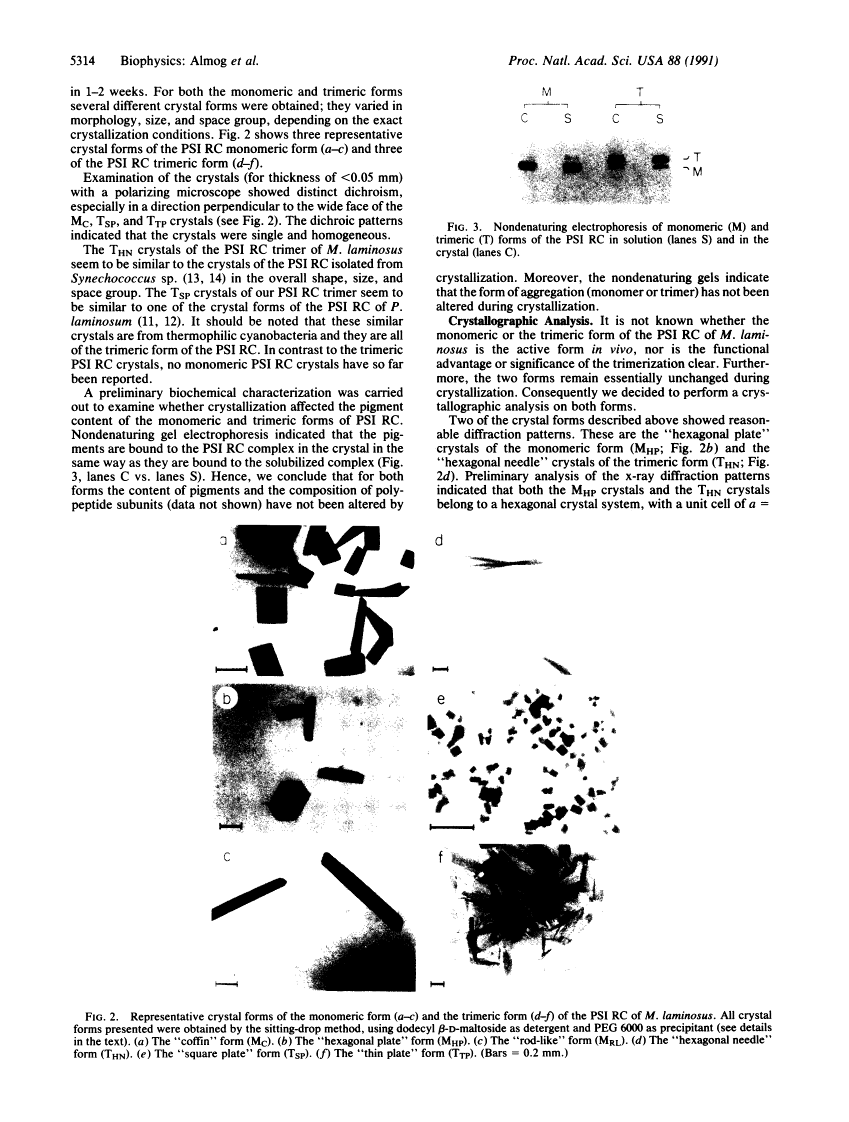
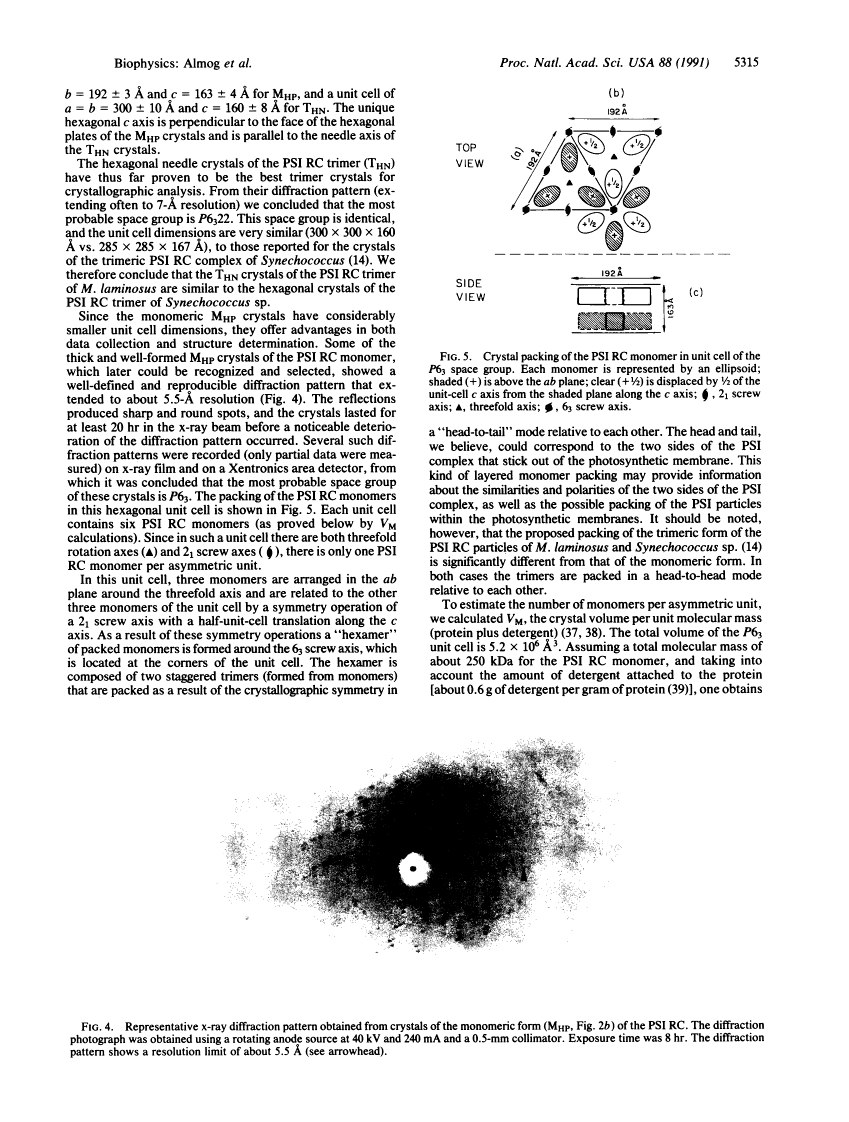
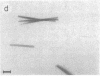
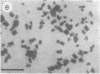
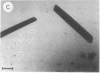
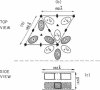
Photosystem I (PSI) reaction centers (RCs) of the thermophilic cyanobacterium Mastigocladus laminosus were purified and characterized. The PSI RC was obtained in two forms, monomeric and trimeric. The two forms contained the same number of pigments per P700 and displayed similar photochemical activities. The two forms had nearly identical polypeptide subunit compositions; the only observed difference was an additional subunit of about 12 kDa observed in the trimeric form. The purified preparations of both the monomeric and the trimeric forms were used for crystallization and preliminary crystallographic analysis. The trimeric PSI RC preparations produced several three-dimensional crystal forms, one of which, the "hexagonal needle" form (THN), had a hexagonal unit cell with dimensions of 300 x 300 x 160 A, containing four PSI RC trimers. The monomeric preparations also produced single crystals of several forms under various crystallization conditions. One of these crystal forms, the "hexagonal plate" (MHP), diffracted to a resolution of about 5.5 A. It had a hexagonal unit cell with dimensions of 192 x 192 x 163 A, containing six PSI RC monomers. Comparison of the PSI RCs in the crystals with those in the precrystallization preparations demonstrated that neither the monomeric nor the trimeric form of PSI RC was altered by the crystallization process. Both forms retained their original polypeptide subunit composition and their pigment content.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.5M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Golbeck JH. Structure, function and organization of the Photosystem I reaction center complex. Biochim Biophys Acta. 1987;895(3):167–204. [Abstract] [Google Scholar]
- Deisenhofer J, Epp O, Miki K, Huber R, Michel H. X-ray structure analysis of a membrane protein complex. Electron density map at 3 A resolution and a model of the chromophores of the photosynthetic reaction center from Rhodopseudomonas viridis. J Mol Biol. 1984 Dec 5;180(2):385–398. [Abstract] [Google Scholar]
- Allen JP, Feher G, Yeates TO, Rees DC, Deisenhofer J, Michel H, Huber R. Structural homology of reaction centers from Rhodopseudomonas sphaeroides and Rhodopseudomonas viridis as determined by x-ray diffraction. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8589–8593. [Europe PMC free article] [Abstract] [Google Scholar]
- Allen JP, Feher G, Yeates TO, Komiya H, Rees DC. Structure of the reaction center from Rhodobacter sphaeroides R-26: the protein subunits. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6162–6166. [Europe PMC free article] [Abstract] [Google Scholar]
- Allen JP, Feher G, Yeates TO, Komiya H, Rees DC. Structure of the reaction center from Rhodobacter sphaeroides R-26: the cofactors. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5730–5734. [Europe PMC free article] [Abstract] [Google Scholar]
- Yeates TO, Komiya H, Rees DC, Allen JP, Feher G. Structure of the reaction center from Rhodobacter sphaeroides R-26: membrane-protein interactions. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6438–6442. [Europe PMC free article] [Abstract] [Google Scholar]
- Chang CH, Tiede D, Tang J, Smith U, Norris J, Schiffer M. Structure of Rhodopseudomonas sphaeroides R-26 reaction center. FEBS Lett. 1986 Sep 1;205(1):82–86. [Abstract] [Google Scholar]
- Ford RC, Picot D, Garavito RM. Crystallization of the photosystem I reaction centre. EMBO J. 1987 Jun;6(6):1581–1586. [Europe PMC free article] [Abstract] [Google Scholar]
- Nechushtai R, Muster P, Binder A, Liveanu V, Nelson N. Photosystem I reaction center from the thermophilic cyanobacterium Mastigocladus laminosus. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1179–1183. [Europe PMC free article] [Abstract] [Google Scholar]
- Reilly P, Hulmes JD, Pan YC, Nelson N. Molecular cloning and sequencing of the psaD gene encoding subunit II of photosystem I from the cyanobacterium, Synechocystis sp. PCC 6803. J Biol Chem. 1988 Nov 25;263(33):17658–17662. [Abstract] [Google Scholar]
- Chitnis PR, Reilly PA, Miedel MC, Nelson N. Structure and targeted mutagenesis of the gene encoding 8-kDa subunit of photosystem I from the cyanobacterium Synechocystis sp. PCC 6803. J Biol Chem. 1989 Nov 5;264(31):18374–18380. [Abstract] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. [Abstract] [Google Scholar]
- Michel H, Oesterhelt D. Three-dimensional crystals of membrane proteins: bacteriorhodopsin. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1283–1285. [Europe PMC free article] [Abstract] [Google Scholar]
- Michel H. Three-dimensional crystals of a membrane protein complex. The photosynthetic reaction centre from Rhodopseudomonas viridis. J Mol Biol. 1982 Jul 5;158(3):567–572. [Abstract] [Google Scholar]
- Garavito RM, Rosenbusch JP. Three-dimensional crystals of an integral membrane protein: an initial x-ray analysis. J Cell Biol. 1980 Jul;86(1):327–329. [Europe PMC free article] [Abstract] [Google Scholar]
- Garavito RM, Rosenbusch JP. Isolation and crystallization of bacterial porin. Methods Enzymol. 1986;125:309–328. [Abstract] [Google Scholar]
- Kühlbrandt W. Three-dimensional crystallization of membrane proteins. Q Rev Biophys. 1988 Nov;21(4):429–477. [Abstract] [Google Scholar]
- Ford RC, Holzenburg A. Investigation of the structure of trimeric and monomeric photosystem I reaction centre complexes. EMBO J. 1988 Aug;7(8):2287–2293. [Europe PMC free article] [Abstract] [Google Scholar]
- Rögner M, Nixon PJ, Diner BA. Purification and characterization of photosystem I and photosystem II core complexes from wild-type and phycocyanin-deficient strains of the cyanobacterium Synechocystis PCC 6803. J Biol Chem. 1990 Apr 15;265(11):6189–6196. [Abstract] [Google Scholar]
- Matthews BW. Solvent content of protein crystals. J Mol Biol. 1968 Apr 28;33(2):491–497. [Abstract] [Google Scholar]
- Allen JP, Feher G. Crystallization of reaction center from Rhodopseudomonas sphaeroides: preliminary characterization. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4795–4799. [Europe PMC free article] [Abstract] [Google Scholar]
- Garavito RM, Jenkins J, Jansonius JN, Karlsson R, Rosenbusch JP. X-ray diffraction analysis of matrix porin, an integral membrane protein from Escherichia coli outer membranes. J Mol Biol. 1983 Feb 25;164(2):313–327. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.88.12.5312
Read article for free, from open access legal sources, via Unpaywall:
https://www.pnas.org/content/pnas/88/12/5312.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Article citations
Compensatory Transcriptional Response of Fischerella thermalis to Thermal Damage of the Photosynthetic Electron Transfer Chain.
Molecules, 27(23):8515, 03 Dec 2022
Cited by: 0 articles | PMID: 36500606 | PMCID: PMC9740203
Changes in supramolecular organization of cyanobacterial thylakoid membrane complexes in response to far-red light photoacclimation.
Sci Adv, 8(6):eabj4437, 09 Feb 2022
Cited by: 9 articles | PMID: 35138895 | PMCID: PMC8827656
Cryo-EM structure of a tetrameric photosystem I from Chroococcidiopsis TS-821, a thermophilic, unicellular, non-heterocyst-forming cyanobacterium.
Plant Commun, 3(1):100248, 13 Oct 2021
Cited by: 10 articles | PMID: 35059628 | PMCID: PMC8760143
Physiological and evolutionary implications of tetrameric photosystem I in cyanobacteria.
Nat Plants, 5(12):1309-1319, 09 Dec 2019
Cited by: 19 articles | PMID: 31819227
Fischerella thermalis: a model organism to study thermophilic diazotrophy, photosynthesis and multicellularity in cyanobacteria.
Extremophiles, 23(6):635-647, 11 Sep 2019
Cited by: 14 articles | PMID: 31512055
Review
Go to all (25) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Fluorescence spectroscopy of the longwave chlorophylls in trimeric and monomeric photosystem I core complexes from the cyanobacterium Spirulina platensis.
Biochemistry, 36(45):13830-13837, 01 Nov 1997
Cited by: 33 articles | PMID: 9374860
An insight into the assembly and organization of photosystem I complex in the thylakoid membranes of the thermophilic cyanobacterium, Mastigocladus laminosus.
Indian J Biochem Biophys, 37(6):405-417, 01 Dec 2000
Cited by: 2 articles | PMID: 11355627
Trimeric forms of the photosystem I reaction center complex pre-exist in the membranes of the cyanobacterium Spirulina platensis.
FEBS Lett, 334(1):79-82, 01 Nov 1993
Cited by: 30 articles | PMID: 8224233
Organization and role of the long-wave chlorophylls in the photosystem I of the Cyanobacterium spirulina.
Membr Cell Biol, 12(5):571-584, 01 Jan 1998
Cited by: 7 articles | PMID: 10379641
Review