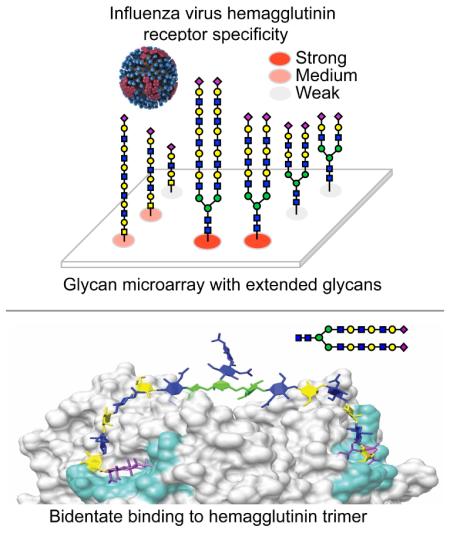
Abstract
Free full text

Recent H3N2 Viruses Have Evolved Specificity for Extended Branched Human-type Receptors Conferring Potential for Increased Avidity
SUMMARY
Human and avian influenza viruses recognize different sialic acid-containing receptors, referred to as human-type (NeuAcα2-6Gal) and avian-type (NeuAcα2-3Gal) respectively. This presents a species barrier for aerosol droplet transmission of avian viruses in humans and ferrets. Recent reports have suggested that current human H3N2 viruses no longer have strict specificity towards human-type receptors. Using an influenza receptor glycan microarray with extended airway glycans we find that H3N2 viruses have in fact maintained human-type specificity, but have evolved preference for a subset of receptors comprising branched glycans with extended poly-N-acetyl-lactosamine (poly-LacNAc) chains, a specificity shared with the 2009 pandemic H1N1 (Cal/04) hemagglutinin. Lipid-linked versions of extended sialoside receptors can restore susceptibility of sialidase-treated MDCK cells to infection by both recent (A/Victoria/361/11) and historical (A/Hong Kong/8/1968) H3N2 viruses. Remarkably, these human-type receptors with elongated branches have the potential to increase avidity by simultaneously binding to two subunits of a single hemagglutinin trimer.
INTRODUCTION
Human and animal influenza viruses are classified by the antigenic serotypes of the two major surface glycoproteins, hemagglutinin (HA), which binds to sialic acid containing receptors on the host cell, and neuraminidase (NA), which destroys receptors and releases virus progeny from infected cells. Although 18 HA and 11 NA serotypes are found in influenza A viruses that circulate in avian and mammalian species, only three combinations have successfully adapted to humans (H1N1, H2N2 and H3N2) (Yoon et al., 2014). Currently, only two viral subtypes, circulate within humans, H3N2, that emerged in the population in 1968, and the novel H1N1 (pandemic H1N1) established in 2009 that replaced the seasonal H1N1 strains.
Human viruses and their avian virus progenitors differ in their receptor specificity, which is believed to represent a major barrier for avian virus transmission in humans (de Graaf and Fouchier, 2014; Imai and Kawaoka, 2012; Paulson and de Vries, 2013; Raman et al., 2014; Shi et al., 2014). Human influenza viruses prefer receptors that are sialic acid α2-6-linked to galactose (human-type), which are dominant on epithelial cells of the human airway. In contrast, avian influenza viruses exhibit preferred recognition of receptors with sialic acid α2-3-linked to galactose (avian-type) and bind poorly to human airway epithelium. In previous human pandemics (H1, H2, H3) that originated from avian viruses, only two amino-acid mutations in the receptor-binding pocket of the HA were sufficient to produce a switch from avian-type to human-type specificity, providing a molecular basis for this simple receptor paradigm (Connor et al., 1994; Matrosovich et al., 2000; Skehel and Wiley, 2000; Stevens et al., 2006).
Evolution of H3N2 influenza viruses through antigenic drift has produced changes in receptor binding that have begun to blur the definition of human-type receptor specificity (Li et al., 2013; Lin et al., 2012). Changes in receptor binding properties were first noticed by lack of agglutination of red blood cells in hemagglutination assays, and difficulty in recovery of virus from patient samples through propagation in laboratory hosts (e.g. eggs, MDCK cells). Moreover, recent H3 isolates showed increasingly reduced avidity and inconsistent specificity for human-type receptors in receptor binding assays (Gulati et al., 2013; Lin et al., 2012; Medeiros et al., 2001; Nobusawa et al., 2000; Stevens et al., 2010; Yang et al., 2015). These observations have suggested that human H3N2 viruses have not maintained consistent receptor specificity during evolution in the human population.
While the terms ‘human-type’ and ‘avian-type’ receptor specificity refer to the sequences NeuAcα2-6Gal versus NeuAcα2-3Gal, respectively, these are the terminal sequences fragments found on a diverse array of glycans that decorate glycoproteins on the surface of a cell. Thus, these terms belie the true complexity of the glycome and the potential for other aspects of glycan structure to be an important factor in receptor recognition. Glycan profiling of a human airway epithelial cell line (Chandrasekaran et al., 2008), human and ferret respiratory tract tissues (Jia et al., 2014; Walther et al., 2013), and porcine airway epithelial cells (Bateman et al., 2010) has revealed the presence of Asn-linked glycans (N-glycans) with extended branches as a unique characteristic of the airway glycome. While the branches of N-glycans on most cell types extend from the mannose core (Man3GlcNAc2Asn) by a single LacNAc (Galβ1-4GlcNAc) sequence, N-glycans in airway tissues exhibit extensions with multiple LacNAc repeats. Although reports have observed that LacNAc extensions enhance recognition of human-type receptors by some human influenza HAs, a consistent picture has not yet emerged (Chandrasekaran et al., 2008; Gulati et al., 2013; Nycholat et al., 2012; Yang et al., 2015).
To investigate the importance of this unique feature of glycan structure and to determine its contribution to receptor preference during antigenic drift, we chemo-enzymatically synthesized a series of N- and O-linked glycans extended with one to five LacNAc repeats and evaluated the specificity of HAs from H3N2 influenza viruses isolated between 1968-2011 using glycan microarrays. We find that recombinant HAs and whole H3N2 viruses exhibit preferential recognition of extended N- and O-glycans terminating in α2-6 linked sialic acids. After 2003, however, a pronounced preference was observed for branched glycans that carry two α2-6 sialic acids and at least three LacNAc repeats. Since little or no binding is observed to the same glycans that terminate with α2-3 linked sialic acids, the basic H3N2 preference for human receptors is maintained. Initially it was thought that selectivity for extended glycan chains exhibited by late H3 isolates might have arisen through steric hindrance by N-glycans added proximal to the HA receptor-binding pocket during antigenic drift, thus limiting the ability of shorter receptors to access the binding site. However, comparison with endoglycosidase-treated H3s, and with the 2009 H1N1 pandemic strain (Cal/04/09), revealed a similar restricted preference for extended, branched, N-glycans. We propose that preference for branched glycans may result from increased avidity attained by simultaneous bidentate binding to two HA protomers within an HA trimer.
RESULTS
Preparation of an Influenza Receptor Glycan Microarray with Extended Airway Glycans
Glycan microarray technology has become a widely used tool to rapidly assess the receptor specificity of both HAs and whole viruses (Blixt et al., 2004; Childs et al., 2009; Gulati et al., 2013; Stevens et al., 2006). However, among the glycan arrays available to date, none have systematically covered N-glycans with extended poly-N-acetyl-lactosamine (poly-LacNAc) sequences found in human, swine and ferret airway tissues. To develop a glycan array library including these structures, we employed an efficient chemo-enzymatic approach to extend poly-LacNAc arms on N- and O-glycan core structures using H. pylori β1-3 N-acetylglucosaminyltransferase (HP β1-3GnT) and mammalian β1-4-galactosyltransferase (GalT-1), followed by capping the terminal galactose residues with sialic acids in either α2-3 or α2-6 linkage to create a equivalent set of avian-type and human-type receptor analogs (Figure 1A).
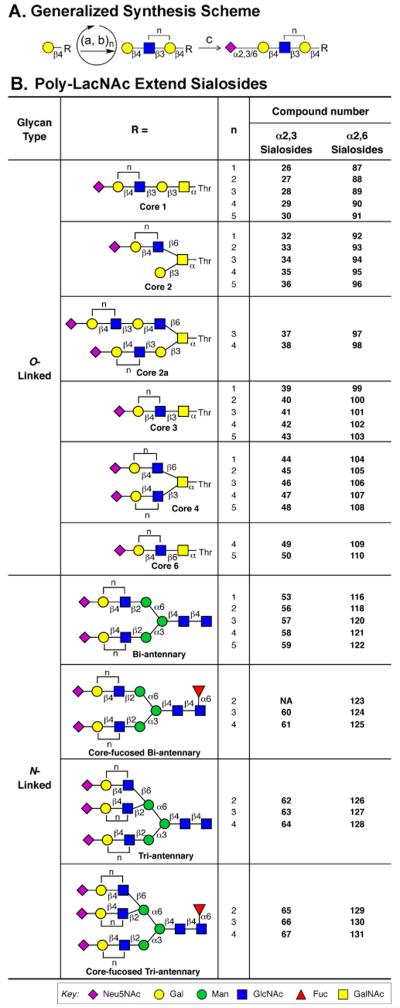
(A) Scheme for addition of poly-LacNAc structures on N-linked and O-linked glycan core structures. Terminal Gal residues on core structures (R) are iteratively elongated to contain a poly-LacNAc chain using H. pylori β1-3 N-acetylglucosaminyltransferase (a) and mammalian β1-4-galactosyltransferase (b, GalT-1). Each compound with terminal galactose is sialylated by either rat ST3Gal-III or human ST6Gal-I sialyltransferases (c) to elaborate the final sialylated structures with α2-3 or α2-6 linked sialic acid, respectively. Synthesis of O-linked and N-glycan core structures are summarized in Supplemental Figure S1. An example of the poly-LacNAc extension for a biantennary N-glycan is shown in Supplemental Figure S2. (B) List of sialosides with poly-LacNAc extended O-linked and N-glycan cores prepared for the glycan array. Compound numbers (bold) are used in glycan array figures and throughout the manuscript. See also Figure S3 and Table S1.
The strategy was applied to six O-glycan cores prepared as previously described (Peng et al., 2012), and with four N-glycan cores prepared from the sialyl biantennary-N-glyco-hexapeptide (SGP) extracted from egg yolk (Seko et al., 1997) as illustrated in Figures S1A and S1B. The hexapeptide was retained as a linker since we found that trimming to single β-Asn reduces subsequent printing efficiency on NHS-activated slides (Nycholat et al., 2012). Treatment of SGP with neuraminidase and β-galactosidase yielded 53c, which could further be elaborated to an N-linked core structure containing Fucα1-6GlcNAc (60c) by reaction with fucosyltransferase FUT8 and GDP-Fuc (Ihara et al., 2006). An N-linked core with a third branch (62c) is produced by reaction with N-acetylglucosaminyltransferase GlcNAcT-5 and UDP-GlcNAc (Alvarez-Manilla et al., 2010), and a core with both a third branch and core fucose by reaction with both enzymes (65c) (Figure S1B).
An example of the poly-LacNAc extension is illustrated for the biantennary N-linked Core 53a in Figure S2. Each intermediate with terminal galactose was further sialylated by either rat ST3Gal-III or human ST6Gal-I sialyltransferases to elaborate the sialylated structures with α2-3 or α2-6 sialic acid, respectively. For each step, prior to purification, the reaction mixture was subjected to analysis by mass spectrometry to ensure that no starting material remained. The homogeneous product was obtained by size exclusion chromatography and characterized by NMR and/or MS analysis. In total, over 70 new sialylated O- and N-glycans were prepared with extended chains comprising one to five LacNAc repeats as summarized in Figure 1B. Details of the synthesis and characterization of these compounds are provided in supplementary materials.
A sialoside glycan array was prepared for analysis of influenza receptor specificity by combining a library of the newly synthesized sialosides with a library of sialylated glycans reported previously (Nycholat et al., 2012), and printing directly onto NHS-activated glass slides. The resulting sialoside microarray contained 135 glycans, including 10 non-sialylated glycans controls (1-10), 69 avian-type sialosides (11-79) and 56 human-type sialosides (80-135) (Table S1). Arrays were subjected to rigid quality control analysis using plant lectins, such as SNA, AAL and RCA (Figure S3).
Receptor Specificity of HAs from Human H3N2 Viruses 1968 to 2011
Several reports document loss of H3 avidity to human-type receptors during antigenic drift, which has been attributed to the accumulation of N-glycosylation that impacts receptor binding, protein folding, and shielding of immunogenic sites (Gulati et al., 2013; Lin et al., 2012; Medeiros et al., 2001; Nobusawa et al., 2000; Skehel et al., 1984; Vigerust et al., 2007) (Table S2). The potential for glycans added near the receptor binding pocket to impact receptor interactions is illustrated in Figure 2A with a bi-antennary N-glycan modeled at each confirmed glycosylation site on the HA trimer. To compare the impact of antigenic drift of H3 viruses on receptor avidity and on receptor specificity, we produced representative recombinant HAs spanning 1968-2011 in mammalian cells (293F) (Figure S4).
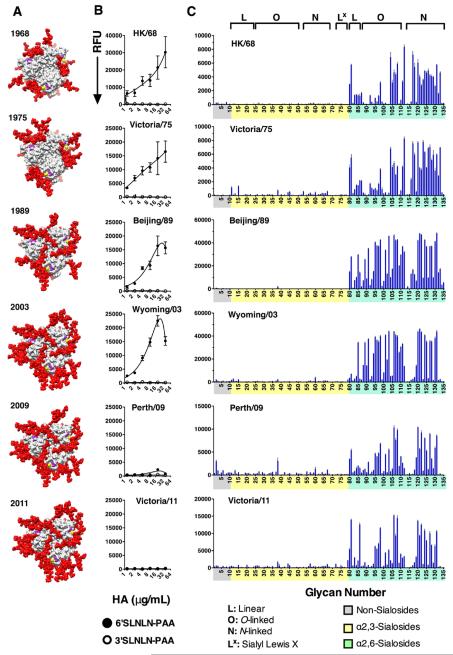
(A) Visualization of the increase in the number of N-glycans that have accrued during H3N2 antigenic drift. A top view of HA is shown with the HA protein in gray with sialic acid (magenta) α2-6 linked to a galactose (yellow) in the receptor-binding site of each protomer of the trimer. Asialo-LacNAc-bi-antennary-N-glycans (red spheres) are modeled at each known glycosylation site on the HA surface (see also Figures S4, S5, Table S2). Strain-specific glycosylation sites modelled onto the structure of Vic/11 H3 in each case. (B) H3 isolates isolated after 2009 lose avidity to polyacrylamide (PAA) conjugates bearing NeuAcα2-3diLacNAc (3SLN2 – open circles) or NeuAcα2-6diLacNAc (6SLN2 – closed circles) glycans. These PAA-glycans were imprinted in a multi-well microarray and overlayed with serially diluted HA (50 to 0.4 μg/ml). After washing, bound HA was detected with mouse anti-HIS and AlexaFluor488 labeled anti-mouse-IgG as described in Experimental Procedures. (C) Recombinant H3 HA proteins exhibit specificity for a subset of human-type receptors. Glycans on the array comprise non-sialoside controls (1-10; Grey), α2-3 sialosides (11-79; Yellow) and α2-6 sialosides (80-135; Green). Glycans are grouped by structure type (see top): L, linear; O, O-linked; N, N-linked and Lx, sialyl Lex (see also Table S1).
The avidity of the HAs for simple sialosides was assessed using an ELISA-like assay that was adapted to microarray format by immobilization of linear NeuAcα2-3diLacNAc (3SLN2-L) or NeuAcα2-6diLacNAc (6SLN2-L) sialosides conjugated to polyacrylamide (PAA) in micro wells of a glass slide (Figure 2B) (McBride et al., 2016; Zhang et al., 2015). All H3 proteins from 1968 to 2003 showed high avidity and specificity to 6SLN2, with no apparent binding to 3SLN2. By contrast, later H3 proteins, Perth/09 and Vic/11, lost all binding avidity to 6SLN2. These results are in agreement with previous reports .
In contrast to the altered binding in the ELISA assay, HAs from 1968-2011 all exhibited remarkably similar receptor specificity on the expanded sialoside array, with exclusive binding to α2-6 sialosides (Figure 2C). On further inspection, the earliest HAs bind a broader range of glycans than the later strains. Binding of HAs to linear fragments of larger glycans (83-86) and linear O-glycans (87-91, 99-103, 109, 110) was most variable, with weakest binding to glycans with only one or two LacNAc (83, 84, 86, 87, 88, 92, 93, 99 & 100). Branched O-glycans with at least three LacNAc repeats were strongly bound by all the HAs (95-98, 106-108), while glycans with one or two repeats were bound more weakly (92, 93, 104, 105). Strikingly, the region of the array displaying N-glycans (116-131) exhibits a comb-like appearance for HAs after 1975. Strongest binding is observed to extended glycans with three to five LacNAc repeats in each core series, including biantennary glycans without (120-122) and with (124,125) core fucose and triantennary chains both without (127,128) or with (130-131) core fucose (see Figure 1B). Conversely, in each series, there is relatively poor binding to glycans with two LacNAc repeats (118, 123, 126, 129), resulting in the gaps, and a comb-like appearance. Although one N-glycan with three LacNAc repeats (119) showed less avid binding, this glycan has a single β−Asn linker that has reduced coupling to the NHS-activated slides compared to the hexapeptide linker of the other N-glycans (118, 120-131). In summary, despite the apparent loss of avidity of H3 after 2003, these HAs maintain binding to extended, branched, N- and O-glycans on the array, providing evidence of conserved receptor specificity during almost 50 years of antigenic drift.
Comparison with Previous Specificity Results of 2006-2007 H3N2 Viruses
Previous glycan array analysis of influenza viruses isolated in 2006 and 2007 reported widely varying receptor specificity for different isolates, and inconsistent preference for ‘human-type’ α2-6 sialosides (Gulati et al., 2013; Stevens et al., 2010). Using aliquots of eight of the same viruses that were isolated from respiratory secretions and produced in MDCK cells, we evaluated receptor specificities on the expanded array (Figure 3). It is apparent that these viruses have a very similar binding pattern compared to one another, and to recombinant H3 HAs (Figure 2). All H3N2 viruses consistently bound to branched α2-6 sialosides with three to five LacNAc repeat unit extensions including, for most viruses, the comb-like pattern resulting from reduced binding to di-LacNAc extended glycans for each N-glycan core (116-131).
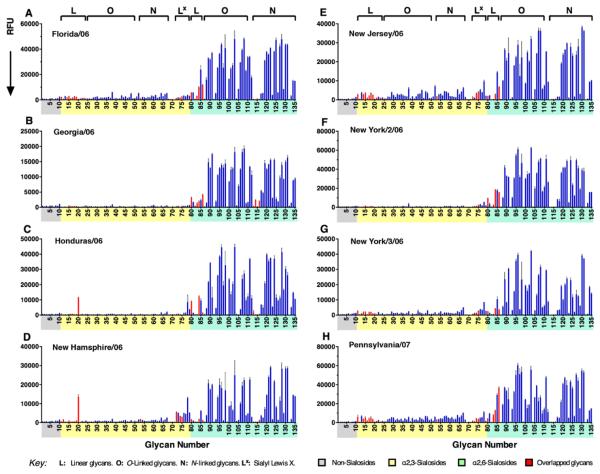
Intact H3N2 viruses exhibit consistent human-type receptor specificity on the expanded sialoside array. Viruses tested were from frozen aliquots of human influenza isolates grown on MDCK cells and were identical to those previously found to have varied specificity on a glycan array without poly-LacNAc extended N-linked and O-linked glycans (Hatakeyama et al., 2005; Oh et al., 2008). The signals highlighted in red correspond to the glycan structures on the microarray used in the previous study. See also Table S1.
These results are in contrast to glycan array analyses reported previously for the same viruses (Stevens et al., 2010), where it was concluded that there was no consistent receptor specificity and that some viruses bound preferentially or equally to α2-3 sialosides. In light of data presented here, this discrepancy now clearly results from a lack of extended receptors on previously available glycans arrays (Figure 1B). Our expanded array library also features glycans employed in these earlier studies and are shown highlighted in red in Figure 3. Looking at the red highlighted signals, it is apparent that binding to these receptors is weak relative to the robust binding to extended α2-6 sialylated glycans. Moreover, if the highlighted red signals in Figure 3 are normalized to full scale (as normally done in array experiments), and only these signals are considered in assigning receptor specificity, specificity of the various viruses do indeed appear varied as reported previously (Stevens et al., 2010). However, on this expanded array that includes glycans with extended glycan chains, the receptor specificities of the viruses are remarkably similar.
Specificity of H5N1, Avian H3N8, Human H3N2, and Pandemic H1N1 HAs
To place the preference of H3 HAs for extended α2-6 sialosides into broader context, we compared receptor specificities from an H5N1 virus (A/VN/1203/04, isolated from human but with avian-type receptor specificity), the avian H3 pandemic progenitor virus (A/Duck/UKR/63 (H3N8)), a human 2010 H3N2 virus sequenced directly from the clinical isolate without prior growth in laboratory hosts (A/HK/6934/10), and the H1N1 2009 pandemic virus (A/Cal/04/09) (Figure 4). The H5 and avian H3 progenitor HAs recognized only α2-3 sialosides, with preference for branched N- and O-glycans and little selectivity for length, thus confirming the specificity of these avian viruses is maintained across a diverse range of glycans (Figure 4A, B). In contrast, the 2010 H3N2 clinical isolate exhibited the most restricted specificity, binding exclusively to branched N- and O-linked α2-6 sialosides (Figure 4C).
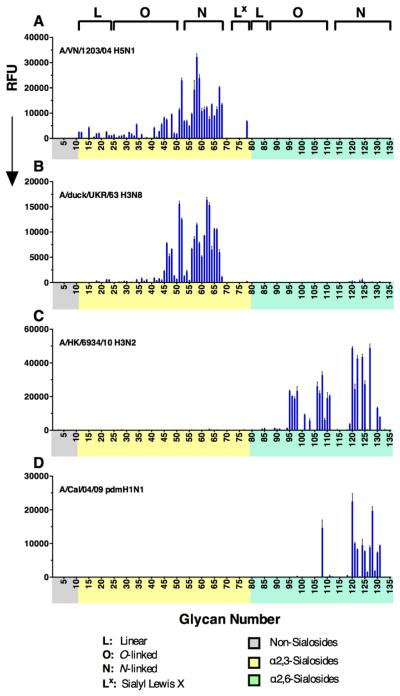
Glycan microarray analysis of (A) recombinant H5 HA from A/Vietnam/1203/04 H5N1, and (B) an avian H3 precursor to the 1968 human pandemic strain (A/Duck/Ukraine/1/1963 (H3N8)), show typical avian-type specificity for α2-3 sialosides. (C) Recombinant H3 from an A/HK/6934/2010 (H3N2) patient isolate, and (D) H1 Cal/04/09 HA show binding to almost exclusively to N-glycans with three or more LacNAc repeats.
The 2009 H1N1 pandemic virus Cal/04/09 is well documented to exhibit low avidity in receptor binding assays, precluding analysis of receptor specificity on glycan microarrays due to inefficient binding (Stevens et al., 2010). Remarkably, on this expanded array, Cal/04/09 HA exhibited robust and restricted binding to extended branched α2-6 sialosides with preference for N-glycans with three or more LacNAc repeats (120-122, 124, 125, 127, 128, 130, 131), and a single branched O-glycan with five LacNAc repeats (108). Thus, as exemplified by the H3 HAs (Figures 2,,3)3) and this low-avidity 2009 pandemic virus (Figure 4D), human influenza HAs consistently bind branched N- and O-glycans with three or more LacNAc repeats with higher avidity than linear glycans and branched glycans with shorter chains.
Extended Glycan Receptors Increase Avidity and Support H3N2 Infection of MDCK Cells
To directly assess the biological impact of glycan length, we coupled linear and N-glycans with one to four LacNAc repeats to biotin for ELISA-type assays, and to lipids to assess their ability to serve as receptors for virus infection in MDCK cells (Figure 5A). The avidity of H3 HAs from HK/68 and Vic/11, representative of early and late strains, were assessed by overlaying purified HA onto biotinylated-glycans adsorbed to streptavidin-coated plates. For HK/68 H3, binding to linear glycans (6SLN1-4-L) was weak and had little dependence on glycan length. Binding to shorter branched N-glycans (6SLN1-2-N) showed similarly low avidity to linear receptors, while binding to longer N-glycans (6SLN3-4-N) was dramatically stronger (Figure 5B, left). In contrast, for Vic/11 HA, binding was strongly dependent on glycan length for both linear and branched N-glycans. Indeed, there was no detectable binding to shorter receptors (6SLN1-2-L and 6SLN1-2-N) over the avian virus receptor control (3SLN2-L), and strong binding to longer glycans (6SLN3-4-L and 6SLN3-4-N) amounting to > 100-fold higher avidity relative to the non-binding shorter glycans (Figure 5B, right).
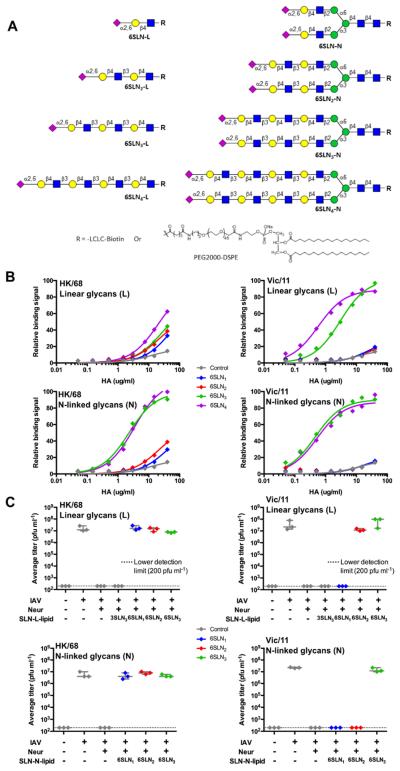
(A) α2-6 Sialosides with 1-4 LacNAc extensions used in ELISA analysis of HA binding specificity (biotinylated glycans) or virus infection study (lipidated glycans). L, Linear glycans; N, N-glycans. (B) ELISA analysis of H3N2 HK/68 (left) and Vic/11 (right). HK/68 HA shows weak binding to all linear α2-6-sialosides regardless of length, and binds preferentially to branched N-glycans with tri- and tetra-LacNAc extensions. Apparent Kd for each glycan was calculated using the Prism 6 software package: 6SLN3-N = 1.83 ± 0.16 μg/ml, 6SLN4-N = 2.09 ± 0.22 μg/ml. Vic/11 HA shows specificity toward α2-6 receptors with tri- and tetra-LacNAc extensions both in Linear and N-glycan structures. Kd for respective glycans were: 6SLN3-L = 2.95 ± 0.21 μg/ml, 6SLN4-L = 0.57 ± 0.04 μg/ml, 6SLN3-N = 0.46 ± 0.05 μg/ml, 6SLN4-N = 0.53 ± 0.07 μg/ml. Linear, biotinylated NeuAcα2-3diLacNAc (3SLN2-L) was used as a negative control (gray diamonds) in all experiments. (C) Infection of glycolipid-engineered MDCK cells (linear & N-linked) with both HK/68 (left) and Vic/11 (right) H3N2 viruses. Plots are average virus titers, measured by plaque assay, at 48 HPI. From left to right in each panel, MDCK monolayers were treated with PBS only (mock infected), 50-80 pfu virus (positive control), CPN then 50-80 pfu virus (background), or treated with CPN, overlaid with SLN-glycolipids, then virus. Linear NeuAcα2-3triLacNAc (3SLN3-L) glycolipid was used as a negative control (gray diamonds). See also Figure S7.
To assess the length dependence of sialosides as receptors for infection, we reasoned that MDCK cells could be engineered to carry synthetic sialoside receptors coupled to lipid anchors that would spontaneously insert into the cell membrane, as shown for glycolipid receptors of Sendai virus (Markwell and Paulson, 1980; Markwell et al., 1981). To this end, MDCK cells were treated with C. perfringens neuraminidase (CPN) to destroy endogenous receptors, overlaid with lipid-conjugated glycans and incubated briefly with low-titer virus samples. After washing, bound viruses were allowed to replicate for 48 hours, and final titers assessed by plaque assay. For both HK/68 and Vic/11, CPN pre-treatment of cells effectively abolished susceptibility to infection, while cells treated with the lipid-linked avian type receptor (3SLN3) produced no virus. However, for HK/68, susceptibility to infection was fully restored by lipid-linked human-type receptor glycans (6SLN1-3-L and 6SLN1-3-N) regardless of length (1-3 LacNAc repeats) or branching (Figure 5C, left). In contrast, for Vic/11, susceptibility to infection was restored more selectively, with strong preference for longer glycans. For the linear series 6SLN3-L restored replication equivalent to native MDCK cells, with 6SLN2-L somewhat less, and virus did not replicate with 6SLN1-L (Figure 5C, right). For the bi-antennary N-glycans, only the longest human-type receptor (6SLN3-N) restored replication, recapitulating the binding activity results in ELISA assays and glycan arrays.
Preference for Extended Branched N-linked Receptors is Not Due to the HA Glycan Shield
One possible explanation for the striking preference of the exemplary Vic/11 HA for extended glycan receptors is that they are of sufficient length to allow the sialic acid to reach the receptor binding site without being sterically restricted by N-glycans added around the HA receptor binding site. To directly assess this possibility, we expressed the A/Vic/11 and A/HK/68 HAs in HEK 293S cells that produce only high mannose glycans, allowing them to be removed by treatment with endoglycosidase H (Endo H). As shown in Figure 6A, Endo H removed the glycans efficiently, yielding an apparent molecular weight (63.2 kDa), equivalent to that of Endo H treated HK/68 HA (Figure S7A). Remarkably, glycan arrays showed no change in specificity as a result of deglycosylation (Figures 6B, S7B). In particular, Vic/11 maintains its extreme preference for human-type branched N-glycans with at least three LacNAc repeats, resulting in the familiar ‘comb-like’ appearance for binding to N-glycans 115-131
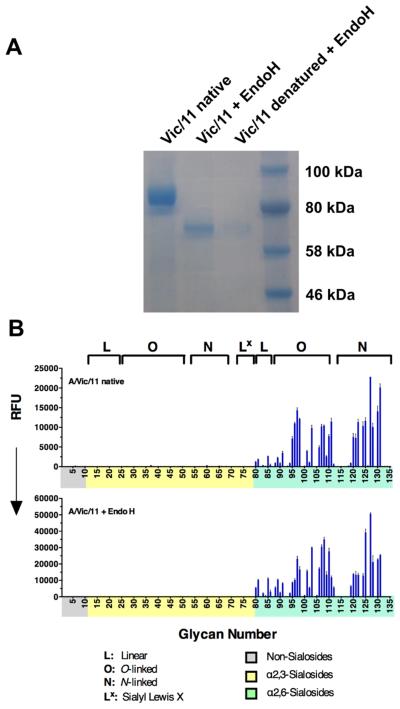
(A) Victoria/11 HA was expressed in 293S cells (left) and natively deglycosylated using Endo H (center). As a control, a small HA sample was denatured prior to Endo H treatment (right) to ensure complete deglycosylation of the folded protein. (B) Receptor specificities of glycosylated (upper) and Endo H-treated (lower) Vic/11 HA were analyzed by glycan microarray. See also Figure S7.
Molecular Dynamics Simulation of Bidentate Binding of an N-glycan to an HA Trimer
It is striking that H3 HAs from 1989-2011 and the Cal/04/09 H1 HA bind with higher avidity to branched, extended N-glycans with more than two LacNAc repeats. Since this specificity does not appear to be sensitive to the presence of HA N-glycans, we considered that the extended, branched glycans could adopt a unique geometry that permits simultaneous bidentate binding to receptor sites on two protomers within the same HA trimer, which are ~45 Å apart (Figure 7A & B). To assess this possibility, 3D structures for biantennary glycans were generated and grafted (Nivedha et al., 2014) onto the bound receptor oligosaccharide in a crystal structure of HA (CA/04/09; PDB code 3UBE) such that the NeuAcα2-6Gal receptor fragment was constrained to bind in the same conformation in the reported structure (Grant et al., 2016; Xu et al., 2012). NeuAcα2-6Gal on the other arm of the glycan was then brought towards the binding site of an adjacent protomer by varying the intervening glycosidic linkages within normal angular bounds (Nivedha et al., 2014). Structures that were closest to displaying bidentate binding were subsequently refined using molecular dynamics simulation. Modeling data shows that SLN2 glycans are too short to form bidendate complexes (Figure 7C), whereas longer glycans with SLN3, SLN4, and SLN5 extensions, are capable of adopting low energy shapes that permit simultaneous binding of the two arms, as illustrated for SLN3 in Figure 7D (see also Movies S1-S4). It is notable that attempts to simultaneously dock both branches of a bi-antennary SLN5 glycan terminating with the NeuAcα2-3Gal linkage failed to generate bidentate complexes.
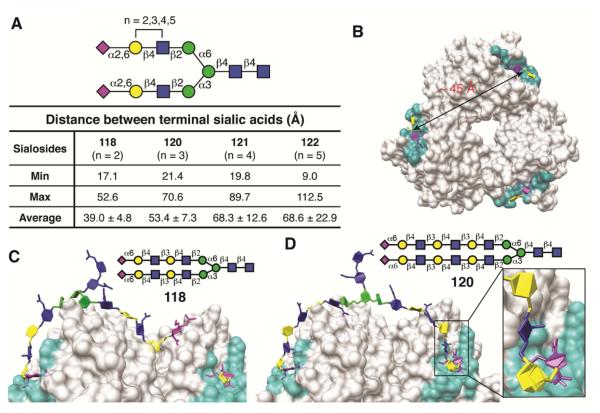
(A) Summary of the distances between the C2 atoms in the sialic acids in each arm of each biantennary N-glycan (see Figure S6 for triantennary sialosides). (B) The distance between the C2 atoms of the sialic acid in two binding sites (cyan) of the HA1 head group trimer; bound disaccharide ligands (NeuAcα2-6Gal) are shown in sticks (magenta/yellow). (C) The di-LacNAc sialoside 118 is incapable of bivalent binding to two receptor-binding sites within a Cal/04 HA trimer. (D) The tri-LacNAc sialoside 120 is capable of spanning the distance between two receptor-binding sites within a H1 Cal/04/09 HA trimer; inset, the sialic acid of the biantennary N-glycan adopts the same conformation as the receptor glycan (NeuAcα2-6Gal) in the crystal structure (see also Movies S1-S4; Figure S5 for top view of H3 HA trimer in complex with sialoside 120).
Modeling also confirmed the potential for bidentate binding to the HA of glycosylated H3N2 viruses. To generate an ensemble of conformations, MD simulations for a glycosylated model of an H3 (Vic/11; pdb code 4O5N) trimer with asialo-LacNAc-bi-antennary-N-glycans at positions N165 and N246. Although some conformations of glycans at N165 and N246 lead to clashes with a bound bidentate receptor glycan, orientations of glycans at N165 and N246 were identified that tolerated bidentate binding (see Figure S5).
The results confirm the ability of extended (SLN3 or longer) branched glycans to form bidentate complexes with either glycosylated or non-glycosylated H3 HAs. Bidentate binding would increase the avidity of the glycan-HA binding, consistent with the comb-like binding pattern in the glycan array data (Figures 2--44).
DISCUSSION
Despite reports that evolution of the human H3N2 virus has been accompanied by diminished recognition of human-type receptors (Gulati et al., 2013; Yang et al., 2015), we find that the virus has maintained consistent specificity for a subset of branched human-type receptors that have sialic acid α2-6 linked to poly-LacNAc extended glycans, with little or no binding to the same glycans with α2-3 sialic acids. Although the preference for longer glycans has become exaggerated over time and coincides with acquisition of additional glycans around the receptor binding pocket during antigenic drift, we show here that restricted specificity of the recent H3 isolates is unrelated to the steric clash of glycans added to the HA (Figure 6). We suggest that the strong preference for branched longer-chain glycans of the receptor can result from the potential to increase avidity by binding to two protomers of the same HA trimer. It appears that the enhanced specificity of late H3N2 isolates is largely due to a process of “avidity maturation” where H3 HAs are evolving a binding mode favoring multivalent receptor interactions and simultaneously losing specificity for shorter, linear receptors incapable of supporting these contacts.
N-glycans used in our array here are observed in respiratory tissue of different influenza-susceptible species including humans. Although the requirement for N-glycans in cell-based experiments is still unclear (Chu and Whittaker, 2004; de Vries et al., 2012), it is remarkable that the extended bi-antennary N-glycans linked to lipids could by themselves restore infection to receptor-deficient MDCK cells. Clearly, they are sufficient to mediate binding of the virus to the surface of the cell. However, we know that sialic acids are replaced on the surface of neuraminidase-treated cells within hours of treatment. Thus, it remains to be seen if the lipid-linked glycans mediate binding only, or both binding and endocytosis of the virus.
Here we also provide evidence that branched N-glycans with at least three LacNAc repeats are capable of forming bidentate binding with their sialic acid termini, where two branches can bridge the binding sites of two protomers on the HA same trimer. This binding mode effectively increases avidity, and is particularly relevant to the human 2009 pandemic Cal/04/09 H1N1 virus, which has the strictest specificity for branched N-glycans (Figure 4D). In this context, it is notable that the NeuAcα2-6Gal linkage has a preferred low energy solution conformation of the terminal tri-saccharide that allows NeuAcα2-6Galβ1-4GlcNAc to project the glycan chain up over the 190 helix towards the top of HA (Chandrasekaran et al., 2008), as a consequence, positions the rest of the glycan is located at the apex of the trimer, allowing the other branch to bridge to the receptor binding site of an adjacent HA subunit (Figure 7, movies S1-S4).
In this report, observations of H3 HA binding to a series of structurally related glycans in different assays and using an expanded glycan array provides relevant insights to ambiguous receptor specificity analyses from previous studies. For recent H3 isolates, it is evident that shorter glycans cannot reliably be used to assess receptor specificity since the binding mode evolved over the evolution of H3N2 from 1968 to present day favors receptors with extended glycans reduce without any change in specificity for human-type receptors. We and others have shown that historic H3N2 strains bind to linear α2-6 sialylated glycans (Gulati et al., 2013; Lin et al., 2012; Yang et al., 2015). However, after 2003, H3 HAs appear to exhibit relatively weak and inconsistent binding to linear glycans, even when featuring up to 5 LacNAc repeats (e.g. glycans 83-85, 87-91). However, N-glycans with 3 or more LacNAc units exhibit robust and reproducible binding to all H3s tested (Figures 2--4).4). While these observations fully support our proposed model of bidentate binding, factors other than glycan structure can influence binding avidity. For instance, in the glycan array, the linear glycans are coupled to the array surface with a short linker (ethanolamine), while the N-glycans are further extended from the array surface by the Man3GlcNA2 core and a hexapeptide linker. In the ELISA assay, the same glycans are uniformly coupled to longer biotinylated linkers (LCLC-biotin) bound to streptavidin. Here, linear glycans featuring four LacNAc repeats exhibit comparable avidities to N-linked counterparts when binding to Vic/11 HA. In infectivity assays, glycans are attached to lipid via a 2000 MW PEG linker, dramatically extending distance from the surface again. In this format, linear glycans now with only two or three LacNAc repeats are able to restore infectivity to receptor-deficient MDCK cells. Converse to this apparent linear variability, in all three assays, length dependence for biantennary N-glycans is far more strongly conserved, with three or more LacNAc repeats required for binding. This suggests that for receptors featuring multiple antennae, the length of the glycan arm relative to its attachment to the branching core is what is important, and extending the linker has no additional impact.
Although glycomics profiling of an airway epithelial cell line (Chandrasekaran et al., 2008), human and ferret respiratory lung tissues (Jia et al., 2014; Walther et al., 2013), and swine respiratory epithelium (Bateman et al., 2010), suggest the presence of extended branched N-glycans similar to those studied here, definitive analysis of human airway epithelium and, in particular, different sections of the airway, still remain to be carried out. A recent report suggesting that the soft palate of ferrets plays a prominent role in selection of HA mutations that promote a switch from avian-type to human-type receptor specificity (Lakdawala et al., 2015), emphasizes how potential variation in the expression of receptor glycans in different regions of the upper airway could account for selection of viruses with specificity for extended, human-type receptors. Clearly, additional information on the types of glycans expressed along the human airway epithelium will help refine our understanding of selective pressures that maintain human-type receptor specificity in human influenza viruses, and the role of receptor binding as a barrier for the emergence of new pandemic viruses from avian and other animal influenza viruses.
The practical implications of these findings extend to the difficulty of isolating and propagating recent H3N2 viruses for production of vaccines (Asaoka et al., 2006). It is well documented that propagation of primary isolates in MDCK cells and eggs is difficult, and often results in mutations in the HA and neuraminidase (NA) that compensate for weak binding of the virus to receptor glycans found in those hosts (Hardy et al., 1995; Lee et al., 2013; Nobusawa et al., 2000). When the problem for isolation of virus in MDCK cells was first recognized, MDCK cells engineered to contain a higher density of human-type receptors were found to improve recovery of virus from human samples (Oh et al., 2008). Knowledge that recent H3N2 viruses prefer extended branched glycan receptors provides a further rational basis to identify or engineer laboratory hosts that contain receptors favorable for binding of human influenza viruses.
EXPERIMENTAL PROCEDURES
Synthesis of Glycans
Glycan core structures were prepared as previous described (Peng et al., 2012). The poly-LacNAc chains were obtained by chemoenzymatic extension using H. pylori β1,3-GlcNAcT and mammalian GalT-1, subsequently followed with sialylation by either hST6Gal-I or rST3Gal-III (Figure 1A). Full details of all synthesis steps are included in the supplemental experimental procedures.
Glycan Array Printing and Quality Control
Glycan arrays were custom printed on a MicroGridII (Digilab) using a contact microarray robot equipped with StealthSMP4B microarray pins (Telechem) as previously described (Xu et al., 2012). Briefly, samples of each glycan were diluted to 100 μM in 150 mM Na3PO4 buffer, pH 8.4. Aliquots of 10 μl were loaded in 384-well plates and imprinted on NHS-activated glass slides (SlideH, Schott/Nexterion), each containing 6 replicates of each glycan. Remaining NHS-ester residues were quenched by immersing slides in 50 mM ethanolamine in 50 mM borate buffer, pH 9.2, for 1 hr. Blocked slides were washed with water, centrifuged dry, and stored at room temperature until use.
Expression and Purification of Recombinant HA
Recombinant H3 subtype HAs from 1968 to 2011 viral isolates, A/HK/1/68 (HK/68), A/Victoria/3/77, A/Beijing/353/89, A/Wyoming/3/03, A/Perth/16/09 and A/Victoria/361/11 (Vic/11) were expressed in 293F freestyle expression system as described previously (Lee et al., 2014). H1 A/Cal/04/09 (Cal/04/09), H3 A/HK/6934/10, H3 A/Duck/UKR/63 and H5 A/VN/1203/04, and for some experiments Vic/11 HAs were expressed in HEK293S (GnTI−/−) cells and purified from the cell culture supernatants as described previously (de Vries et al., 2013).
Preparation of H3N2 Viruses
The H3N2 viruses used in this study, A/Florida/2/06, A/Georgia/4/06, A/Honduras/3112/06, A/New Hampshire/3/06, A/New Jersey/2/06, A/New York/2/06, A/New York/3/06, A/Pennsylvania/4/07 and Vic/11 were obtained from the Centers for Disease Control and Prevention (CDC) and the CDC-Influenza Reagent Resource (CDC-IRR). They were grown in MDCK cells, purified and frozen in aliquots for glycan array analysis as previously described (Stevens et al., 2010).
Glycan Array Analysis Experiments
Purified, soluble trimeric HA (50 μg/ml) was pre-complexed with either an anti-HIS or anti-Strep mouse antibody and an Alexa647-linked anti-mouse IgG was added (4:2:1 molar ratio) for 15 min on ice in 100 μl PBS-T, and incubated on the array surface in a humidified chamber for 90 min. Array analysis of inactivated virus stocks was performed as described previously (Stevens et al., 2010).
ELISA with Biotinylated Glycans
For glycan ELISA, purified HA trimers were concentrated to 500 μg/ml, precomplexed with anti-HIS mouse antibody and HRP-conjugated anti-mouse IgG, then diluted in series to appropriate assay concentrations (typically 40 – 0.05 μg/ml final). Preparation of streptavidin-coated plates with biotinylated glycans, incubation and washing of pre-complexed HA dilutions was exactly as previously described (Chandrasekaran et al., 2008).
Virus Infection Study
MDCK cells were grown to confluency in 6-well tissue culture trays and digested with 50 units (per well) of commercial CPN (New England BioLabs). Cells were then incubated with 3 μM (final) glycolipid (6SLN1-3-L/N-lipid) for 20 min, followed by 50-80 pfu virus for 10 min. Bound virus was allowed to grow for 48 hours at 37oC, with final solution titers assessed by plaque assay. Full virus infection protocols are described in supplementary experimental procedures.
Molecular Modeling
For 3D-structure generation, initial conformations for bi-antennary α2-6 di-, tri-, tetra- and penta-LacNAc glycans were built and energy minimized using GLYCAM-Web (www.glycam.org). The trimeric HA1 domains of H1 Cal/04/09 and H3 Victoria/11 were generated from PDB codes 3UBE and 4O5N, respectively. For complexes, each N-glycan receptor was grafted into an HA binding site by superimposing the NeuAcα2-6Gal binding motif of one branch onto the crystal structure of the ligand bound to the HA. Structures were then refined and energy minimized to assess the ability of the sialic acid on the other branch to reach the binding site of another protomer. Details are provided in Supplementary Experimental Procedures.
Supplementary Material
1
2
3
4
5
6
ACKNOWLEDGEMENTS
This work was funded in part by grants from National Institutes of Health, AI099274 and AI114730 (to J.C.P.), CA207824 and GM103390 (to R.J.W.) and R56 AI117675 (to I.A.W.), and the Kuang Hua Educational Foundation (to J.C.P.). R.P.d.V. is a recipient of Rubicon and VENI grants from the Netherlands Organization for Scientific Research (NWO). A.J.T. is the recipient of a Long-term Fellowship from the European Molecular Biology Organization (EMBO ALTF 963-2014). We thank Li-Mei Chen and Ruben Donis of the Centers for Disease Control (Atlanta, GA) for samples of 2006 H3N2 viruses, Jennifer Pranskevich for preparation of enzymes used in synthesis of glycans, and Anna Crie for help in preparation of figures and tables. The Consortium for Functional Glycomics (http://www.functionalglycomics.org/) funded by NIGMS grant GM062116 (J.C.P.) provided several glycans used in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Figures S1-S7, Tables S1 and S2, Movies S1-S4, and Supplementary Experimental Procedures for synthesis and characterization of synthetic compounds, and for energy minimization and molecular dynamics.
AUTHOR CONTRIBUTIONS
W.P., R.P.d.V., I.A.W., R.J.W. and J.C.P. designed the project; W.P. synthesized glycans; W.P. synthesized biotinylated and lipidated sialosides. B.T. assisted in synthesis of glycans. R.P.d.V., A.J.T. and P.S.L. expressed recombinant proteins; R.M. produced glycan arrays; W.P., R.P.d.V., A.J.T. R.M. and J.C.P. analyzed glycan array data; O.C.G. performed molecular modeling; A.J.T. performed glycan ELISA and virus infection study; W.P., R.P.d.V. and J.C.P wrote the manuscript, and all authors reviewed and edited the manuscript.
In Brief
To clarify H3N2 human influenza virus receptor specificity, Peng et al. developed a glycan array that included extended glycans. Recent H3N2 and 2009 pandemic H1N1 viruses share specificity for human-type receptors with extended glycan chains, conferring potential for increase avidity by simultaneously binding two subunits of a single hemagglutinin trimer.
REFERENCES
- Alvarez-Manilla G, Troupe K, Fleming M, Martinez-Uribe E, Pierce M. Comparison of the substrate specificities and catalytic properties of the sister N-acetylglucosaminyltransferases, GnT-V and GnT-Vb (IX) Glycobiology. 2010;20:166–174. [Europe PMC free article] [Abstract] [Google Scholar]
- Asaoka N, Tanaka Y, Sakai T, Fujii Y, Ohuchi R, Ohuchi M. Low growth ability of recent influenza clinical isolates in MDCK cells is due to their low receptor binding affinities. Microbes Infect. 2006;8:511–519. [Abstract] [Google Scholar]
- Bateman AC, Karamanska R, Busch MG, Dell A, Olsen CW, Haslam SM. Glycan analysis and influenza A virus infection of primary swine respiratory epithelial cells: the importance of NeuAcα2-6 glycans. J Biol Chem. 2010;285:34016–34026. [Europe PMC free article] [Abstract] [Google Scholar]
- Blixt O, Head S, Mondala T, Scanlan C, Huflejt ME, Alvarez R, Bryan MC, Fazio F, Calarese D, Stevens J, et al. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc Natl Acad Sci U S A. 2004;101:17033–17038. [Europe PMC free article] [Abstract] [Google Scholar]
- Chandrasekaran A, Srinivasan A, Raman R, Viswanathan K, Raguram S, Tumpey TM, Sasisekharan V, Sasisekharan R. Glycan topology determines human adaptation of avian H5N1 virus hemagglutinin. Nat Biotech. 2008;26:107–113. [Abstract] [Google Scholar]
- Childs RA, Palma AS, Wharton S, Matrosovich T, Liu Y, Chai W, Campanero-Rhodes MA, Zhang Y, Eickmann M, Kiso M, et al. Receptor-binding specificity of pandemic influenza A (H1N1) 2009 virus determined by carbohydrate microarray. Nat Biotech. 2009;27:797–799. [Europe PMC free article] [Abstract] [Google Scholar]
- Chu VC, Whittaker GR. Influenza virus entry and infection require host cell N-linked glycoprotein. Proc Natl Acad Sci U S A. 2004;101:18153–18158. [Europe PMC free article] [Abstract] [Google Scholar]
- Connor RJ, Kawaoka Y, Webster RG, Paulson JC. Receptor specificity in human, avian, and equine H2 and H3 influenza virus isolates. Virology. 1994;205:17–23. [Abstract] [Google Scholar]
- de Graaf M, Fouchier RA. Role of receptor binding specificity in influenza A virus transmission and pathogenesis. EMBO J. 2014;33:823–841. [Europe PMC free article] [Abstract] [Google Scholar]
- de Vries E, de Vries RP, Wienholts MJ, Floris CE, Jacobs MS, van den Heuvel A, Rottier PJ, de Haan CA. Influenza A virus entry into cells lacking sialylated N-glycans. Proc Natl Acad Sci U S A. 2012;109:7457–7462. [Europe PMC free article] [Abstract] [Google Scholar]
- de Vries RP, de Vries E, Martinez-Romero C, McBride R, van Kuppeveld FJ, Rottier PJ, Garcia-Sastre A, Paulson JC, de Haan CA. Evolution of the hemagglutinin protein of the new pandemic H1N1 influenza virus: maintaining optimal receptor binding by compensatory substitutions. J Virol. 2013;87:13868–13877. [Europe PMC free article] [Abstract] [Google Scholar]
- Grant OC, Tessier MB, Meche L, Mahal LK, Foley BL, Woods RJ. Combining 3D structure with glycan array data provides insight into the origin of glycan specificity. Glycobiology. 2016;26:772–783. [Europe PMC free article] [Abstract] [Google Scholar]
- Gulati S, Smith DF, Cummings RD, Couch RB, Griesemer SB, George K, Webster RG, Air GM. Human H3N2 influenza iiruses isolated from 1968 to 2012 show varying preference for receptor substructures with no apparent consequences for disease or spread. PLoS One. 2013;8:e66325. [Europe PMC free article] [Abstract] [Google Scholar]
- Hardy CT, Young SA, Webster RG, Naeve CW, Owens RJ. Egg fluids and cells of the chorioallantoic membrane of embryonated chicken eggs can select different variants of influenza A (H3N2) viruses. Virology. 1995;211:302–306. [Abstract] [Google Scholar]
- Hatakeyama S, Sakai-Tagawa Y, Kiso M, Goto H, Kawakami C, Mitamura K, Sugaya N, Suzuki Y, Kawaoka Y. Enhanced expression of an α2,6-linked sialic acid on MDCK cells improves isolation of human influenza viruses and evaluation of their sensitivity to a neuraminidase inhibitor. J Clin Microbiol. 2005;43:4139–4146. [Europe PMC free article] [Abstract] [Google Scholar]
- Ihara H, Ikeda Y, Taniguchi N. Reaction mechanism and substrate specificity for nucleotide sugar of mammalian α1,6-fucosyltransferase--a large-scale preparation and characterization of recombinant human FUT8. Glycobiology. 2006;16:333–342. [Abstract] [Google Scholar]
- Imai M, Kawaoka Y. The role of receptor binding specificity in interspecies transmission of influenza viruses. Curr Opin Virol. 2012;2:160–167. [Europe PMC free article] [Abstract] [Google Scholar]
- Jia N, Barclay WS, Roberts K, Yen HL, Chan RW, Lam AK, Air G, Peiris JS, Dell A, Nicholls JM, et al. Glycomic characterization of respiratory tract Tissues of ferrets: Implications for its use in influenza virus infection studies. J Biol Chem. 2014;289:28489–28504. [Europe PMC free article] [Abstract] [Google Scholar]
- Lakdawala SS, Jayaraman A, Halpin RA, Lamirande EW, Shih AR, Stockwell TB, Lin X, Simenauer A, Hanson CT, Vogel L, et al. The soft palate is an important site of adaptation for transmissible influenza viruses. Nature. 2015;526:122–125. [Europe PMC free article] [Abstract] [Google Scholar]
- Lee HK, Tang JW, Kong DH, Loh TP, Chiang DK, Lam TT, Koay ES. Comparison of mutation patterns in full-genome A/H3N2 influenza sequences obtained directly from clinical samples and the same samples after a single MDCK passage. PLoS One. 2013;8:e79252. [Europe PMC free article] [Abstract] [Google Scholar]
- Lee PS, Ohshima N, Stanfield RL, Yu W, Iba Y, Okuno Y, Kurosawa Y, Wilson IA. Receptor mimicry by antibody F045-092 facilitates universal binding to the H3 subtype of influenza virus. Nat Commun. 2014;5:3614. [Europe PMC free article] [Abstract] [Google Scholar]
- Li Y, Bostick DL, Sullivan CB, Myers JL, Griesemer SB, Stgeorge K, Plotkin JB, Hensley SE. Single hemagglutinin mutations that alter both antigenicity and receptor binding avidity influence influenza virus antigenic clustering. J Virol. 2013;87:9904–9910. [Europe PMC free article] [Abstract] [Google Scholar]
- Lin YP, Xiong X, Wharton SA, Martin SR, Coombs PJ, Vachieri SG, Christodoulou E, Walker PA, Liu J, Skehel JJ, et al. Evolution of the receptor binding properties of the influenza A(H3N2) hemagglutinin. Proc Natl Acad Sci U S A. 2012;109:21474–21479. [Europe PMC free article] [Abstract] [Google Scholar]
- Markwell MA, Paulson JC. Sendai virus utilizes specific sialyloligosaccharides as host cell receptor determinants. Proc Natl Acad Sci U S A. 1980;77:5693–5697. [Europe PMC free article] [Abstract] [Google Scholar]
- Markwell MA, Svennerholm L, Paulson JC. Specific gangliosides function as host cell receptors for Sendai virus. Proc Natl Acad Sci U S A. 1981;78:5406–5410. [Europe PMC free article] [Abstract] [Google Scholar]
- Matrosovich M, Tuzikov A, Bovin N, Gambaryan A, Klimov A, Castrucci MR, Donatelli I, Kawaoka Y. Early alterations of the receptor-binding properties of H1, H2, and H3 avian influenza virus hemagglutinins after their introduction into mammals. J Virol. 2000;74:8502–8512. [Europe PMC free article] [Abstract] [Google Scholar]
- McBride R, Paulson JC, de Vries RP. A miniaturized glycan microarray assay for assessing avidity and specificity of influenza A virus hemagglutinins. J Vis Exp. 2016 May 29;:111. [Europe PMC free article] [Abstract] [Google Scholar]
- Medeiros R, Escriou N, Naffakh N, Manuguerra JC, van der Werf S. Hemagglutinin residues of recent human A(H3N2) influenza viruses that contribute to the inability to agglutinate chicken erythrocytes. Virology. 2001;289:74–85. [Abstract] [Google Scholar]
- Nivedha AK, Makeneni S, Foley BL, Tessier MB, Woods RJ. Importance of ligand conformational energies in carbohydrate docking: Sorting the wheat from the chaff. J Comput Chem. 2014;35:526–539. [Europe PMC free article] [Abstract] [Google Scholar]
- Nobusawa E, Ishihara H, Morishita T, Sato K, Nakajima K. Change in receptor-binding specificity of recent human influenza A viruses (H3N2): a single amino acid change in hemagglutinin altered its recognition of sialyloligosaccharides. Virology. 2000;278:587–596. [Abstract] [Google Scholar]
- Nycholat CM, McBride R, Ekiert DC, Xu R, Rangarajan J, Peng W, Razi N, Gilbert M, Wakarchuk W, Wilson IA, et al. Recognition of sialylated poly-N-acetyllactosamine chains on N- and O-linked glycans by human and avian influenza A virus hemagglutinins. Angew Chem Int Ed Engl. 2012;51:4860–4863. [Europe PMC free article] [Abstract] [Google Scholar]
- Oh DY, Barr IG, Mosse JA, Laurie KL. MDCK-SIAT1 cells show improved isolation rates for recent human influenza viruses compared to conventional MDCK cells. J Clin Microbiol. 2008;46:2189–2194. [Europe PMC free article] [Abstract] [Google Scholar]
- Paulson JC, de Vries RP. H5N1 receptor specificity as a factor in pandemic risk. Virus Res. 2013;178:99–113. [Europe PMC free article] [Abstract] [Google Scholar]
- Peng W, Pranskevich J, Nycholat C, Gilbert M, Wakarchuk W, Paulson JC, Razi N. Helicobacter pylori β1,3-N-acetylglucosaminyltransferase for versatile synthesis of type 1 and type 2 poly-LacNAcs on N-linked, O-linked and I-antigen glycans. Glycobiology. 2012;22:1453–1464. [Europe PMC free article] [Abstract] [Google Scholar]
- Raman R, Tharakaraman K, Shriver Z, Jayaraman A, Sasisekharan V, Sasisekharan R. Glycan receptor specificity as a useful tool for characterization and surveillance of influenza A virus. Trends Microbiol. 2014;22:632–641. [Europe PMC free article] [Abstract] [Google Scholar]
- Seko A, Koketsu M, Nishizono M, Enoki Y, Ibrahim HR, Juneja LR, Kim M, Yamamoto T. Occurence of a sialylglycopeptide and free sialylglycans in hen's egg yolk. Biochim Biophys Acta. 1997;1335:23–32. [Abstract] [Google Scholar]
- Shi Y, Wu Y, Zhang W, Qi J, Gao GF. Enabling the 'host jump': structural determinants of receptor-binding specificity in influenza A viruses. Nat Rev Microbiol. 2014;12:822–831. [Abstract] [Google Scholar]
- Skehel JJ, Stevens DJ, Daniels RS, Douglas AR, Knossow M, Wilson IA, Wiley DC. A carbohydrate side chain on hemagglutinins of Hong Kong influenza viruses inhibits recognition by a monoclonal antibody. Proc Natl Acad Sci U S A. 1984;81:1779–1783. [Europe PMC free article] [Abstract] [Google Scholar]
- Skehel JJ, Wiley DC. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu Rev Biochem. 2000;69:531–569. [Abstract] [Google Scholar]
- Stevens J, Blixt O, Paulson JC, Wilson IA. Glycan microarray technologies: tools to survey host specificity of influenza viruses. Nat Rev Microbiol. 2006;4:857–864. [Abstract] [Google Scholar]
- Stevens J, Chen LM, Carney PJ, Garten R, Foust A, Le J, Pokorny BA, Manojkumar R, Silverman J, Devis R, et al. Receptor specificity of influenza A H3N2 viruses isolated in mammalian cells and embryonated chicken eggs. J Virol. 2010;84:8287–8299. [Europe PMC free article] [Abstract] [Google Scholar]
- Vigerust DJ, Ulett KB, Boyd KL, Madsen J, Hawgood S, McCullers JA. N-linked glycosylation attenuates H3N2 influenza viruses. J Virol. 2007;81:8593–8600. [Europe PMC free article] [Abstract] [Google Scholar]
- Walther T, Karamanska R, Chan RW, Chan MC, Jia N, Air G, Hopton C, Wong MP, Dell A, Malik Peiris JS, et al. Glycomic analysis of human respiratory tract tissues and correlation with influenza virus infection. PLoS Pathog. 2013;9:e1003223. [Europe PMC free article] [Abstract] [Google Scholar]
- Xu R, McBride R, Nycholat CM, Paulson JC, Wilson IA. Structural characterization of the hemagglutinin receptor specificity from the 2009 H1N1 influenza pandemic. J Virol. 2012;86:982–990. [Europe PMC free article] [Abstract] [Google Scholar]
- Yang H, Carney PJ, Chang JC, Guo Z, Villanueva JM, Stevens J. Structure and receptor binding preferences of recombinant human A(H3N2) virus hemagglutinins. Virology. 2015;477:18–31. [Europe PMC free article] [Abstract] [Google Scholar]
- Yoon SW, Webby RJ, Webster RG. Evolution and ecology of influenza A viruses. Curr Top Microbiol Immunol. 2014;385:359–375. [Abstract] [Google Scholar]
- Zhang H, de Vries RP, Tzarum N, Zhu X, Yu W, McBride R, Paulson JC, Wilson IA. A human-infecting H10N8 influenza virus retains a strong preference for avian-type receptors. Cell Host Microbe. 2015;17:377–384. [Europe PMC free article] [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.chom.2016.11.004
Read article for free, from open access legal sources, via Unpaywall:
http://www.cell.com/article/S1931312816304796/pdf
Citations & impact
Impact metrics
Article citations
Divergent synthesis of amino acid-linked O-GalNAc glycan core structures.
Nat Protoc, 26 Sep 2024
Cited by: 0 articles | PMID: 39327537
Review
Chemoenzymatic Synthesis of Sulfated N-Glycans Recognized by Siglecs and Other Glycan-Binding Proteins.
JACS Au, 4(8):2966-2978, 26 Jul 2024
Cited by: 0 articles | PMID: 39211606 | PMCID: PMC11350573
Epistasis mediates the evolution of the receptor binding mode in recent human H3N2 hemagglutinin.
Nat Commun, 15(1):5175, 18 Jun 2024
Cited by: 1 article | PMID: 38890325 | PMCID: PMC11189414
Potential pandemic risk of circulating swine H1N2 influenza viruses.
Nat Commun, 15(1):5025, 13 Jun 2024
Cited by: 3 articles | PMID: 38871701
Cross-species spill-over potential of the H9N2 bat influenza A virus.
Nat Commun, 15(1):3449, 25 Apr 2024
Cited by: 0 articles | PMID: 38664384 | PMCID: PMC11045754
Go to all (122) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Protein structures in PDBe (5)
-
(4 citations)
PDBe - 6SLNView structure
-
(2 citations)
PDBe - 4O5NView structure
-
(2 citations)
PDBe - 3UBEView structure
-
(1 citation)
PDBe - 3GnTView structure
-
(1 citation)
PDBe - 6GalView structure
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Enhanced Human-Type Receptor Binding by Ferret-Transmissible H5N1 with a K193T Mutation.
J Virol, 92(10):e02016-17, 27 Apr 2018
Cited by: 19 articles | PMID: 29491160 | PMCID: PMC5923085
Receptor binding specificity of recent human H3N2 influenza viruses.
Virol J, 4:42, 09 May 2007
Cited by: 113 articles | PMID: 17490484 | PMCID: PMC1876801
Evolution of the receptor binding properties of the influenza A(H3N2) hemagglutinin.
Proc Natl Acad Sci U S A, 109(52):21474-21479, 10 Dec 2012
Cited by: 194 articles | PMID: 23236176 | PMCID: PMC3535595
Adaptation of influenza viruses to human airway receptors.
J Biol Chem, 296:100017, 22 Nov 2020
Cited by: 51 articles | PMID: 33144323 | PMCID: PMC7948470
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Dutch Research Council (NWO)
European Molecular Biology Organization (1)
Grant ID: EMBO ALTF 963-2014
Kuang Hua Educational Foundation
NCI NIH HHS (1)
Grant ID: U01 CA207824
NIAID NIH HHS (3)
Grant ID: R01 AI114730
Grant ID: R56 AI117675
Grant ID: T32 AI007244
NIGMS (1)
Grant ID: GM062116
NIGMS NIH HHS (2)
Grant ID: U54 GM062116
Grant ID: P41 GM103390
NIH (4)
Grant ID: CA207824
Grant ID: R56 AI117675
Grant ID: GM103390
Grant ID: AI114730