Abstract
Free full text

Matrix metalloproteinase (MMP)-19-deficient fibroblasts display a profibrotic phenotype
Abstract
Idiopathic pulmonary fibrosis (IPF) is a progressive and usually lethal interstitial lung disease of unknown etiology characterized by aberrant activation of epithelial cells that induce the migration, proliferation and activation of fibroblasts. The resulting distinctive fibroblastic/myofibroblastic foci are responsible for the excessive extracellular matrix (ECM) production and abnormal lung remodeling. We have recently found that matrix metalloproteinase 19 (MMP-19)-deficient (Mmp19−/−) mice develop an exaggerated bleomycin-induced lung fibrosis, but the mechanisms are unclear. In this study, we explored the effect of MMP-19 deficiency on fibroblast gene expression and cell behavior. Microarray analysis of Mmp19−/− lung fibroblasts revealed the dysregulation of several profibrotic pathways, including ECM formation, migration, proliferation, and autophagy. Functional studies confirmed these findings. Compared with wild-type mice, Mmp19−/− lung fibroblasts showed increased α1 (I) collagen gene and collagen protein production at baseline and after transforming growth factor-β treatment and increased smooth muscle-α actin expression (P < 0.05). Likewise, Mmp19-deficient lung fibroblasts showed a significant increase in proliferation (P < 0.01) and in transmigration and locomotion over Boyden chambers coated with type I collagen or with Matrigel (P < 0.05). These findings suggest that, in lung fibroblasts, MMP-19 has strong regulatory effects on the synthesis of key ECM components, on fibroblast to myofibroblast differentiation, and in migration and proliferation.
idiopathic pulmonary fibrosis (IPF) is a progressive and usually lethal lung disease of unknown etiology and without current effective therapy (15). It is characterized by injury and aberrant activation of the alveolar epithelium, which induces, through the release of a variety of growth factors, cytokines, and matrix metalloproteinases (MMPs), a flow of dysregulated epithelial-fibroblast crosstalk (20). Activated epithelial cells provoke the migration, proliferation, and activation of mesenchymal cells, with the formation of fibroblast and myofibroblast foci, whereas activated myofibroblasts secrete exaggerated amounts of extracellular matrix (ECM) molecules culminating in the destruction of the lung parenchyma (5). However, the molecular mechanisms involved in IPF development and progression are uncertain.
Studies in our group and others have shown that some MMPs such as MMP-1, MMP-2, MMP-3, MMP-7, MMP-9, and MMP-13 are highly expressed in IPF, playing diverse roles in the fibrotic response; however, the exact mechanisms are not well characterized (4, 10, 11, 21, 24, 27). Recently, we identified the overexpression of MMP-19 in the hyperplastic alveolar epithelium of IPF lungs and demonstrated that, surprisingly, mice lacking this MMP developed an exacerbated bleomycin-induced lung fibrosis (25). Perturbations of MMP-19 levels in epithelial cells in vivo and in vitro were associated with changes in prostaglandin-endoperoxide synthase 2, suggesting that the protective role of MMP-19 might be in part through the induction of this enzyme in alveolar epithelial cells. A main observation in the fibrotic lungs of the MMP-19-deficient (Mmp19−/−) mice was the extensive formation of fibroblast/myofibroblast foci although the underlying mechanisms were not explored. Importantly, these foci represent areas of active fibrogenesis that play a critical role in the progressive fibrotic response that characterizes IPF.
In the present study, we evaluated the global gene expression as well as the functional behavior of Mmp19−/− compared with Mmp19+/+ lung fibroblasts. Microarray analysis of MMP-19-deficient fibroblasts revealed the upregulation of numerous profibrotic genes, including several collagens and other ECM components, as well as the dysregulation of functional pathways, such as migration, proliferation, and autophagy. Functional analysis corroborated that MMP-19-deficient fibroblasts have increased growth rate and migratory capacity, as well as increased collagen production and smooth muscle-α actin expression with decreased autophagy-related protein 4 (ATG4) expression.
MATERIALS AND METHODS
Fibroblast isolation and culture.
C57BL/6/129O1 Mmp19−/− mice kindly donated by Carlos Lopez Otin were generated as previously described (13). Primary mouse lung fibroblasts were obtained by trypsin dispersion. Briefly, lungs from Mmp19−/− and wild-type (WT) Mmp19+/+ mice were harvested, minced, and incubated with trypsin-EDTA solution 0.5 g/l (Sigma-Aldrich, St. Louis, MO) for 20 min. Cells were grown at 37°C in 5% CO2-95% air in 25-cm2 Falcon flasks containing Dulbecco's Modified Eagle Medium (DMEM) (Life Technologies, Gaithersburg, MD) supplemented with 10% FBS, 100 U/ml of penicillin, 100 μg/ml of streptomycin, and 2.5 mg/ml of amphotericin B. Passages 4–6 were used for all experiments. For some experiments, cells were stimulated with transforming growth factor (TGF)-β (5 ng/ml) for 48 h.
The Bioethics Committee at the Instituto Nacional de Enfermedades Respiratorias in Mexico DF approved the protocol.
Growth rate assay.
Mice fibroblasts (n = 5 from each genotype) were seeded in a 96-well plate at a cell density of 4 × 103 cells/well and incubated at 37°C in 5% CO2 in DMEM medium supplemented with 10% FBS. Cell growth was determined using the water-soluble tetrazolium salt (WST-1) reagent (Roche Applied Science, Mannheim, Germany) according to manufacturer's instructions. Absorbance (450–620 nm) was measured on a plate reader, and the results are shown as growth rate increase relative to basal values (day 0). Cell growth was also analyzed by cell proliferation assay (CyQUANT; Life Technologies, Eugene, OR). Mmp19+/+ and Mmp19−/− fibroblasts were incubated in DMEM with 2% FBS alone or in the presence of 100 ng/ml of recombinant human MMP-19 (Creative BioMart, New York, NY). The fluorescence was determined in a microplate reader with filters set to 480 nm excitation/520 nm emission, and the results are shown as cell proliferation increases relative to basal values (day 0).
Transmigration assay (chemotaxis and chemokinesis).
Migration of Mmp19−/− and Mmp19+/+ mice fibroblasts (n = 6 from each genotype) was assayed as previously described using collagen-coated chambers (QCM Haptotaxis Cell Migration Assay - Collagen 1; EMD Millipore, Billerica, MA) or BD BioCoat Matrigel Invasion Chambers with an 8-mm pore size (16). Fibroblasts (1.5 × 105 cells) were added to the upper chamber, and the lower chamber contained 0.3 ml of medium with PDGF-BB (20 ng/ml; R&D Systems, Minneapolis, MN). The cells that migrated were analyzed 12 h after.
For determination of chemokinesis of lung fibroblasts, a checkerboard analysis was performed (7). PDGF (10, 20, and 50 ng/ml) were placed in the upper or/and lower compartments of collagen-coated Boyden chambers. Fibroblasts (1.5 × 105 cells) were added to the upper chamber and assayed as above.
Quantitative real-time RT-PCR.
Total RNA was extracted from lung fibroblasts using TRIzol reagent (Invitrogen Life Technologies, Grand Island, NY). Total RNA (1 μg) was reverse transcribed with cDNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions.
TaqMan probes were for α1 type I collagen expression (Mm00801666_g1), for Mmp19 (Mm01159954_g1), for nidogen 2 (Nid2; Mm00456212_m1), for integrin α11 (Itga11; Mm00723741_m1), for Col14a1 (Mm00805269_m1), for fibronectin type 3 domain 1 (Fndc1; Mm01297904_m1), for Mmp14 (Mm00485054_m1), and 4352930E for eukaryotic 18S rRNA that was used as endogenous control (Applied Biosystems). Time PCR amplification was performed using an i-Cycler iQ Detection System (Bio-Rad Laboratories, Hercules, CA).
Results from three different Mmp19−/− and Mmp19+/+ mice fibroblasts are expressed as means ± SD of a relative quantification of the target gene normalized to 18S rRNA. Each cDNA was quantified in triplicate using a Mastermix solution (Applied Biosystems).
Collagen synthesis.
Collagen was quantified in cell-conditioned medium using the Sircol collagen assay (Biocolor, Belfast, UK) according to the manufacturer's protocol. Briefly, fibroblasts (n = 3 from each genotype) were seeded in a six-well plate at a density of 3 × 105 cells/well and incubated overnight at 37°C in 5% CO2 in DMEM medium plus 10% FBS. Afterward, cells were incubated in serum-free medium for 48 h or with TGF-β (3 ng/ml). The media were collected and dialyzed against deionized water in the presence of protease inhibitors (2 M N-ethylmaleimide, 100 mM phenylmethylsulfonyl fluoride, and 1 M EDTA) and 1% sodium azide. All samples were lyophilized and resuspended in 50 μl of deionized water, and 30 μg of protein concentration per sample was used in the assay.
Western blot.
Fibroblasts from Mmp19+/+ and Mmp19−/− mice (n = 4–5 from each genotype) were plated on T-25 culture dishes and grown at 80% confluence. Cells were then lysated with RIPA buffer (Sigma-Aldrich) following the manufacturer's instructions. Proteins (30 μg) were separated on 10% SDS-polyacrylamide gels and transferred to a PVDF membrane (0.45 μm; Millipore), blocked with 5% (wt/vol) nonfat dried milk in PBS, and incubated overnight with the corresponding antibody at 4°C, including anti-smooth muscle actin (SMA) (1:500; Dako, Glostrup, Denmark), anti-ATG4C (a9482; Sigma-Aldrich), and anti-β-tubulin (1:500; Santa Cruz Biotechnology, Santa Cruz, CA) that was used to test equal loading. After being washed with PBS-Tween 20 0.05%, membranes were incubated with the corresponding secondary horseradish peroxidase-conjugated antibody (1:5,000; Sigma-Aldrich) for 1 h at room temperature. Bands were detected using a chemiluminescence detection system (Millipore). Images were acquired and analyzed with ChemiDoc XRS system (Bio-Rad).
Microarray of fibroblasts.
Mmp19−/− and Mmp19+/+ fibroblasts obtained from three different WT and MMP-19-deficient mice were lysed in QIAzol (Qiagen, Valencia, CA), and total RNA was extracted and used as a template for double-stranded cDNA synthesis. RNA quantity was determined by NanoDrop and integrity by Bioanalyzer (Agilent Technologies, Santa Clara, CA). Labeling was performed using the Agilent Low-RNA Input Linear Amplification Kit PLUS, One Color (5184-3523). After purification and fragmentation, aliquots of each sample were hybridized to Agilent Whole Human Genome 4 × 44 arrays (G4112F; Agilent Technologies). Each array was then sequentially washed and scanned by an Agilent microarray scanner. Array readout was extracted by Agilent Feature Extraction 9.5.3 software. Probes with annotations for Entrez Gene ID were extracted, and cyclic LOESS was applied to normalize the gene expression signals. Transcriptional network analysis was performed using the Ingenuity Pathway Analysis (IPA) software. The microarray data were submitted to the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/), access number: GSE49643.
Statistical methods.
The results are expressed as means ± SD. Differences were analyzed by Student's t-test or Tukey's test. Values of P < 0.05 were considered statistically significant.
RESULTS
We first evaluated by real-time PCR whether lung fibroblasts from WT and MMP-19-deficient mice express the gene under basal conditions. As shown in Fig. 1, Mmp19 was expressed in WT fibroblasts, whereas, as expected, in MMP-19-deficient fibroblasts, the gene was absent.
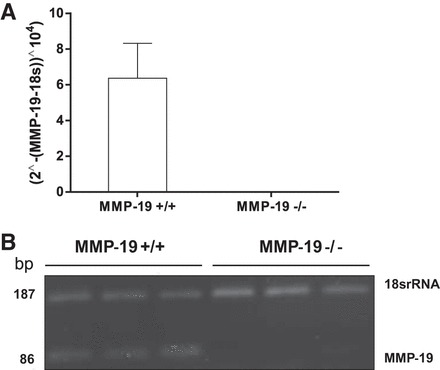
Mmp19 gene expression in Mmp19−/− and Mmp19+/+ fibroblasts. The expression of matrix metalloproteinase 19 (MMP-19) was evaluated by quantitative RT-PCR (A) and RT-PCR (B) in 3 different fibroblast cell lines from each genotype. Each cDNA was quantified in triplicate.
Microarray analysis reveals several upregulated fibrogenic pathways in MMP-19-deficient mice.
Using an Agilent Whole Human Genome array platform (G4112F), we examined the global gene expression levels in mouse lung fibroblasts derived from three Mmp19−/− mice and three Mmp19+/+ mice. Under basal conditions, 840 genes were found differentially expressed by at least twofold, 456 upregulated and 384 downregulated. The most upregulated genes (fold change ≥7) and some meaningful upregulated genes are shown in Table 1, and the most downregulated genes (fold change ≥8) are in in Table 2.
Table 1.
Most upregulated genes in Mmp19−/− mice lung fibroblasts
| Unique ID | Entrez Symbol | Description | Fold Change | Entrez ID |
|---|---|---|---|---|
| A_51_P223569 | Ddx4 | DEAD (Asp-glu-ala-asp) box polypeptide-4 | 96 | 13206 |
| A_51_P239673 | Hprt | Hypoxanthine guanine phosphoribosyl transferase | 72 | 15452 |
| A_55_P2027900 | LOC100047443 | Similar to γ-aminobutyric-acid receptor subunit α2 precursor | 62 | 100047443 |
| A_55_P2124736 | Col14a1 | Collagen, Type XIV, α1 | 49 | 7373 |
| A_55_P2378486 | Kcnma1 | Potassium large-conductance calcium-activated channel, subfamily M α member 1 | 40 | 16531 |
| A_52_P151240 | Fam150a | Family with sequence similarity 150, member A | 38 | 620393 |
| A_55_P1953402 | Mlana | Melan-A | 35 | 77836 |
| A_51_P372393 | Angpt4 | Angiopoietin 4 | 34 | 11602 |
| A_52_P235347 | Fgf21 | Fibroblast growth factor 21 | 34 | 56636 |
| A_52_P113537 | Xist | Inactive X-specific transcripts | 31 | 213742 |
| A_55_P2107765 | Col28a1 | Collagen type XXVIII α1 | 30 | 213945 |
| A_55_P2097219 | Gbp1 | Guanylate-binding protein 1, interferon-inducible | 29 | 2633 |
| A_51_P457196 | Sfrp4 | Secreted frizzled-related protein 4 | 28 | 20379 |
| A_51_P281778 | 2210010C17Rik | Immunoglobulin superfamily, member 23 | 26 | 70080 |
| A_55_P2037585 | Trf | Transferrin | 24 | 22041 |
| A_55_P2167803 | Abi3bp | ABI gene family, member 3 (NESH)-binding protein | 22 | 320712 |
| A_52_P63905 | Ddc | Dopa decarboxylase | 19 | 13195 |
| A_51_P417720 | Itga11 | Integrin α11 | 17 | 319480 |
| A_52_P101990 | Hils1 | Histone H1-like protein in spermatids 1 | 17 | 54388 |
| A_55_P2142226 | Serpina3f | Serine (or cysteine) peptidase inhibitor, clade A, member 3F | 17 | 238393 |
| A_52_P645862 | Agtr1a | Angiotensin II receptor, type 1a | 16 | 11607 |
| A_55_P1972034 | Muc16 | Mucin 16 | 16 | 73732 |
| A_52_P105537 | Nov | Nephroblastoma overexpressed gene | 16 | 18133 |
| A_55_P1965101 | Xlr3b | X-linked lymphocyte-regulated 3C | 15 | 22446 |
| A_51_P508259 | Ppm1n | Protein phosphatase, Mg2+/Mn2+-dependent, 1N | 15 | 232941 |
| A_55_P2018697 | Ldhd | Lactate dehydrogenase D | 15 | 52815 |
| A_55_P2088625 | Fbln7 | Fibulin 7 | 15 | 70370 |
| A_52_P676819 | Bnc1 | Basonuclin 1 | 14 | 12173 |
| A_55_P2027386 | Gpr156 | G protein-coupled receptor 156 | 14 | 239845 |
| A_51_P315666 | Nid2 | Nidogen 2 | 14 | 18074 |
| A_55_P1999962 | Podn | Podocan | 14 | 242608 |
| A_55_P2173947 | Zar1l | Zygote arrest 1-like | 13 | 646799 |
| A_55_P1967776 | Slc4a4 | Solute carrier family 4 (anion exchanger), member 4 | 13 | 54403 |
| A_55_P2000595 | LOC674948 | Similar to X-linked lymphocyte-regulated protein 3A | 13 | 674948 |
| A_55_P1975370 | Apoc1 | Apolipoprotein C-I | 12 | 11812 |
| A_51_P295085 | Ogn | Osteoglycin | 12 | 18295 |
| A_51_P142421 | Rspo1 | R-spondin homolog | 11 | 192199 |
| A_52_P278336 | Fbln5 | Fibulin 5 | 11 | 28376 |
| A_55_P1970324 | Gm5366 | Tubulin, α 1A pseudogene | 10 | 384954 |
| A_51_P346964 | Mrap | Melanocortin 2 receptor accessory protein | 10 | 77037 |
| A_52_P29743 | 5033411D12Rik | RIKEN cDNA 5033411D12 gene | 10 | 192136 |
| A_51_P450888 | Rtn4r | Reticulon 4 receptor | 10 | 65079 |
| A_52_P546513 | Ppyr1 | Neuropeptide Y receptor Y4 | 10 | 19065 |
| A_55_P2023818 | Cysltr1 | Cysteinyl leukotriene receptor 1 | 10 | 58861 |
| A_51_P338998 | Prim2 | DNA primase, p58 subunit | 9 | 19076 |
| A_51_P146560 | Msln | Mesothelin | 9 | 56047 |
| A_51_P117739 | Figf | c-Fos-induced growth factor | 9 | 14205 |
| A_52_P536494 | Mycn | v-Myc myelocytomatosis viral-related oncogene, neuroblastoma derived | 9 | 18109 |
| A_51_P497985 | C2 | Complement component 2 | 9 | 12263 |
| A_51_P225224 | Htra1 | HtrA serine peptidase 1 | 9 | 56213 |
| A_52_P292792 | Col8a1 | Collagen, type VIII, α1 | 8 | 12837 |
| A_51_P309920 | Itga8 | Integrin α8 | 8 | 241226 |
| A_52_P454183 | Olfml2b | Olfactomedin-like 2B | 8 | 320078 |
| A_52_P349182 | Gria4 | Glutamate receptor, ionotropic, AMPA4 | 8 | 14802 |
| A_55_P2010216 | 1700008P20Rik | Tescalcin-like | 8 | 69301 |
| A_51_P461319 | Gatm | Glycine amidinotransferase | 8 | 67092 |
| A_55_P2053561 | Gm13011 | Predicted gene 13011 | 8 | 242711 |
| A_51_P207622 | Fmod | Fibromodulin | 8 | 14264 |
| A_55_P1978681 | Tspan8 | Tetraspanin 8 | 8 | 216350 |
| A_51_P467110 | Dpp4 | Dipeptidylpeptidase 4 | 8 | 13482 |
| A_51_P379409 | Cuedc1 | CUE domain containing 1 | 8 | 404093 |
| A_55_P2286176 | C030009O12Rik | RIKEN cDNA C030009O12 | 8 | 77328 |
| A_55_P2161450 | Serpina3b | Serine (or cysteine) peptidase inhibitor, clade A, member 3B | 8 | 271047 |
| A_51_P240019 | Gyg | Glycogenin | 7 | 27357 |
| A_55_P2020072 | LOC100044365 | Similar to Bex4 protein | 7 | 100044365 |
| A_51_P153423 | Fndc1 | Fibronectin type III domain containing 1 | 7 | 68655 |
| A_52_P52849 | Cpxm2 | Carboxypeptidase X 2 (M14 family) | 5 | 55987 |
| A_55_P2015994 | Fgf9 | Fibroblast growth factor 9 | 5 | 14180 |
| A_52_P413646 | Bmp6 | Bone morphogenetic protein 6 | 4 | 12161 |
| A_55_P2073754 | Adam23 | A disintegrin and metallopeptidase domain 23 | 4 | 23792 |
| A_55_P2022678 | C1qtnf1 | C1q and tumor necrosis factor-related protein 1 | 3 | 56745 |
| A_55_P2154387 | Bmp4 | Bone morphogenetic protein 4 | 3 | 12159 |
| A_55_P2031636 | Igf1 | Insulin-like growth factor 1 | 3 | 16000 |
| A_52_P516409 | Col4a6 | Collagen, type IV, α6 | 3 | 94216 |
| A_55_P2100375 | Col11a1 | Collagen, type XI, α1 | 3 | 12814 |
| A_51_P304239 | Loxl1 | Lysyl oxidase-like 1 | 3 | 16949 |
| A_55_P2132651 | Wisp1 | WNT1-inducible signaling pathway protein 1 | 3 | 22402 |
| A_52_P304128 | Mmp14 | Matrix metallopeptidase 14 (membrane-inserted) | 3 | 17387 |
| A_51_P282144 | Adam23 | A disintegrin and metallopeptidase domain 23 | 3 | 23792 |
| A_55_P2098805 | Adamtsl3 | ADAMTS-like 3 | 3 | 269959 |
| A_55_P2118520 | Col1a1 | collagen, type I, α1 | 3 | 12842 |
Table 2.
Most downregulated genes in Mmp19−/− mice lung fibroblasts
| Unique ID | Entrez Symbol | Description | Fold Change | Entrez ID |
|---|---|---|---|---|
| A_51_P31838 1 | Pgf | Placental growth factor | 8 | 18654 |
| A_55_P1961099 | Atg4c | Autophagy-related 4C, cysteine peptidase | 8 | 611339 |
| A_55_P2394308 | Fst | Follistatin | 8 | 14313 |
| A_52_P424767 | Rbbp4 | Retinoblastoma-binding protein 4 | 8 | 19646 |
| A_55_P2067707 | Mep1a | Meprin 1α | 8 | 17287 |
| A_51_P193686 | 1700012B09Rik | RIKEN cDNA 1700012B09 gene | 8 | 69325 |
| A_51_P500661 | Rnf182 | Ring finger protein 182 | 8 | 328234 |
| A_55_P1975874 | Bcl2l15 | BCLl2-like 15 | 8 | 229672 |
| A_51_P438841 | Ctnna2 | Catenin (cadherin associated protein), α2 | 8 | 12386 |
| A_52_P549973 | 4933404M02Rik | Glutamate-rich 2 | 8 | 66748 |
| A_55_P2053236 | LOC100045988 | Similar to OPR | 8 | 100045988 |
| A_51_P111962 | Bean | Brain expressed, associated with Nedd4, 1 | 8 | 65115 |
| A_55_P2037050 | 2610305D13Rik | RIKEN cDNA 2610305D13 gene | 9 | 112422 |
| A_52_P274496 | Tspan18 | Tetraspanin 18 | 9 | 241556 |
| A_52_P331762 | Lmo1 | LIM domain only 1 | 9 | 109594 |
| A_55_P1956497 | Serpinf2 | Serine (or cysteine) peptidase inhibitor, clade F, member 2 | 10 | 18816 |
| A_55_P2040026 | Itga4 | Integrin α4 | 10 | 16401 |
| A_55_P2081105 | AI607873 | Expressed sequence AI607873 | 10 | 226691 |
| A_66_P119034 | Pla2 g7 | Phospholipase A2, group VII (platelet-activating factor acetylhydrolase, plasma) | 10 | 22726 |
| A_66_P125777 | Slc43a2 | Solute carrier family 43, member 2 | 10 | 225213 |
| A_55_P2004370 | Car6 | Carbonic anhydrase 6 | 10 | 12353 |
| A_55_P2017677 | Cap2 | CAP, adenylate cyclase-associated protein, 2 | 11 | 67252 |
| A_55_P2069891 | 1700007E05Rik | RIKEN cDNA 1700007E05 gene | 11 | 114672 |
| A_55_P1952920 | AI854703 | Expressed sequence AI854703 | 12 | 273373 |
| A_55_P1964896 | Gm3014 | Predicted gene 3014 | 14 | 100040872 |
| A_51_P501897 | Pitx2 | Paired-like homeodomain transcription factor 2 | 14 | 18741 |
| A_51_P279038 | Ppargc1a | Peroxisome proliferative-activated receptor, γ, coactivator 1α | 14 | 19017 |
| A_51_P371051 | Glipr1 | GLI pathogenesis-related 1 | 14 | 73690 |
| A_55_P2137023 | Glud2 | Glutamate receptor, ionotropic, δ2 | 15 | 14804 |
| A_55_P2125557 | Irx2 | Iroquois-related homeobox 2 | 17 | 16372 |
| A_55_P1976278 | Prkcb | Protein kinase C, β | 17 | 18751 |
| A_55_P1988368 | Upp1 | Uridine phosphorylase 1 | 17 | 22271 |
| A_55_P2132512 | Ngef | Neuronal guanine nucleotide exchange factor | 18 | 53972 |
| A_55_P2086433 | Oasl1 | 2′-5′ Oligoadenylate synthetase-like 1 | 18 | 231655 |
| A_55_P2347976 | Mir17 hg | Mir17 host gene 1 | 18 | 75957 |
| A_55_P2105944 | Olfr224 | Olfactory receptor 224 | 20 | 258198 |
| A_51_P317695 | Trpc3 | Transient receptor potential cation channel, subfamily C, member 3 | 21 | 22065 |
| A_55_P2035946 | Penk | Preproenkephalin | 21 | 18619 |
| A_51_P269166 | Mmp19 | Matrix metallopeptidase 19 | 21 | 58223 |
| A_65_P20249 | Prkcb | Protein kinase C, β | 22 | 18751 |
| A_55_P1974432 | Gm5067 | Predicted gene 5067 | 23 | 272714 |
| A_55_P2037081 | 2610305D13Rik | RIKEN cDNA 2610305D13 gene | 27 | 112422 |
| A_55_P2099363 | Stac2 | SH3 and cysteine-rich domain 2 | 29 | 217154 |
| A_55_P2163128 | 0610042G04Rik | RIKEN cDNA 0610042G04 gene | 30 | 68380 |
| A_55_P2033308 | Six2 | Sine oculis-related homeobox 2 | 35 | 20472 |
| A_52_P619248 | Prl2c5 | Prolactin family 2, subfamily c, member 5 | 72 | 107849 |
MMP-19-deficient fibroblasts showed a significant increase of genes associated with secreted proteins, many of them related with ECM, including fibrillar, fiber-associated, and basement membrane collagens such as Col XIVα1, Col XXVIIIα1, Col VIIIα1, Col IVα6, Col XIα1, and Col Iα1. Two members of the a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) family, ADAMTS-2 and -3, that are involved in procollagen processing were also upregulated. Other increased ECM genes were fibulin 5, fibulin 7, nephroblastoma overexpressed gene (Ccn3), nidogen 2, osteoglycin, podocan, and fibronectin type III domain 1.
There was a modest upregulation of Mmp14, a membrane-bound MMP (MT1-MMP), without increase of any other member of the MMP family, indicating that there was no compensation for the lack of MMP-19. Several myosin heavy-chain genes were strongly upregulated in MMP-19-deficient fibroblasts, suggesting that an increased number of them were myofibroblasts.
A network examination using IPA software showing the molecular networks altered by the absence of MMP-19 in mouse fibroblasts is illustrated in Fig. 2.
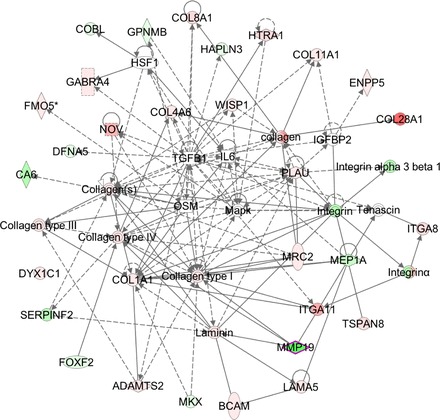
Molecular networks altered by the absence of Mmp19 in mouse fibroblasts. The molecular network generated by Ingenuity Pathway Analysis (IPA) software (Ingenuity Systems, www.ingenuity.com) displays interactions between the target genes that were differentially regulated after Mmp19 ablation. Red indicates upregulated, green indicates downregulated, white indicates genes that were not in the data set but form part of this network, and gray indicates genes that were added manually. Solid lines indicates direct relationships between molecules, and dashed lines represent indirect interactions. Results represent data obtained from lung fibroblasts derived from 3 Mmp19−/− and 3 Mmp19+/+ mice.
The expression of several genes associated with proliferation and migration was increased in MMP-19-deficient lung fibroblasts (Table 1). As illustrated in Fig. 3, one of the dysregulated transduction pathways leading to the change in the proliferation rate was ERK1 pathway, which in this model was specifically linked to an increase of the cytokine fibroblast growth factor 21, proliferation receptor interleukin 6, and the cytokine cardiotrophin 1. Other molecules dysregulated in MMP-19-deficient fibroblasts and associated to cellular proliferation were integrin α-11, insulin-like growth factor-1 (IGF1), v-Myc avian myelocytomatosis viral oncogene neuroblastoma-derived homolog (MYCN), and hypoxanthine phosphoribosyltransferase 1, this latter possibly regulating glucose metabolism and nitric oxide. Several genes involved in p38 MAPK pathway and cell migration were also upregulated, including nephroblastoma overexpressed, dipeptidyl-peptidase 4, c-Fos-induced growth factor, angiopoietin 2, plasminogen activator urokinase, laminin α5 IGF, ezrin, and WNT1-inducible signaling pathway protein 1 (WISP1) (Fig. 4).
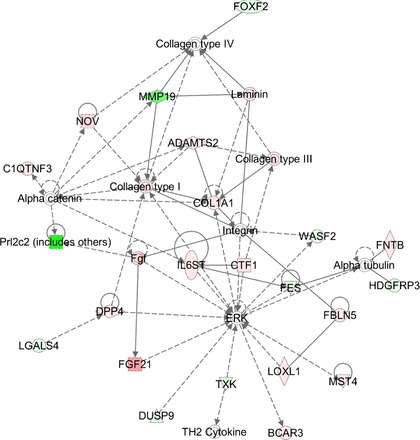
Cell proliferation network of Mmp19−/− vs. Mmp19+/+ fibroblasts. The molecular network generated by IPA software shows genes differentially regulated related to ERK transduction pathway. Red indicates upregulated, green indicates downregulated, white indicates genes that were not in the data set but form part of this network, and gray indicates genes that were added manually. Solid lines indicate direct relationships between molecules, and dashed lines represent indirect interactions. Results represent data obtained from lung fibroblasts derived from 3 Mmp19−/− and 3 Mmp19+/+ mice.
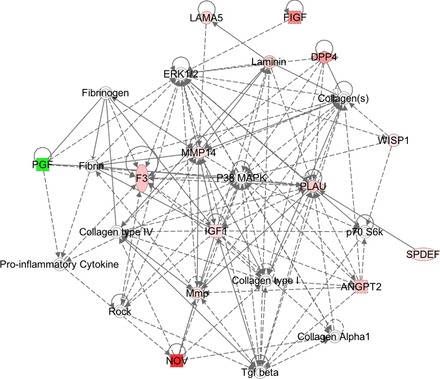
Cell migration network deregulated in MMP-19-deficient fibroblasts. The molecular network generated by IPA software shows differentially regulated genes related to p38/MAPK pathway. Red indicates upregulated, green indicates downregulated, white indicates genes that were not in the data set but form part of this network, and gray indicates genes that were added manually. Solid lines indicate direct relationships between molecules, and dashed lines represent indirect interactions. Results represent data obtained from lung fibroblasts derived from 3 mice of each genotype.
We corroborated by quantitative RT-PCR the level of expression of several differentially expressed genes. As shown in Fig. 5A, Col14α1, Itga11, Nid2, Mmp14, and Fndc1 were significantly upregulated under basal conditions in the Mmp19-deficient mice.
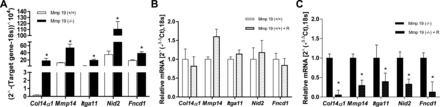
Gene expression by Mmp19−/− and Mmp19+/+ fibroblasts. A: expression of α1-collagen XIV, nidogen 2, fibronectin type III domain containing 1, integrin α 11, matrix metalloprotease 14; mRNA was evaluated by quantitative RT-PCR. The expression was normalized to the level of 18S rRNA. B and C: Mmp19+/+ and Mmp19−/− fibroblasts were incubated with or without recombinant MMP-19 (200 ng/ml) for 18 h. Data are expressed as means ± SD from lung fibroblasts derived from 3 Mmp19−/− and 3 Mmp19+/+ mice performed by triplicate; *P < 0.01.
To examine whether the addition of recombinant MMP-19 modifies the expression of these upregulated genes, WT and MMP-19-deficient fibroblasts were incubated in serum-free medium with or without the recombinant protein (200 ng/ml per 18 h). As shown in Fig. 5B, addition of MMP-19 did not modify the expression of Col14α1, Itga11, Nid2, Mmp14, and Fndc1 in WT fibroblasts. In contrast, in MMP-19-deficient fibroblasts, the recombinant protein significantly reduces the expression of these genes (Fig. 5C).
Mmp19 deletion increases collagen expression.
To evaluate the effects of MMP-19 deficiency on matrix production, we measured the expression and production of collagen I by quantitative RT-PCR and Sircol assay. As illustrated in Fig. 6A, under basal conditions, Mmp19−/− lung fibroblasts showed significantly higher levels of α1-collagen I gene expression (P < 0.001). Consistent with higher mRNA levels, increased levels of collagen protein in the cell culture supernatant were also detected (P < 0.05) (Fig. 6B). Similar results were obtained when lung fibroblasts were stimulated with 3 ng/ml of TGF-β.
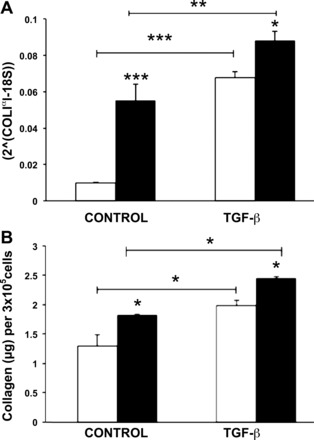
Collagen expression by Mmp19−/− and Mmp19+/+ fibroblasts under basal conditions and after stimulation with TGF-β. A: quantitative RT PCR of α1-collagen I gene mRNA expression normalized to the level of 18S rRNA. B: collagen concentration in conditioned media was quantified by Sircol collagen soluble assay. Data are expressed as means ± SD from 2 independent experiments. Gene and protein results are expressed as means ± SD and represent data obtained from lung fibroblasts derived from 3 Mmp19−/− and 3 Mmp19+/+ mice performed by triplicate. *P < 0.05; **P < 0.01; ***P < 0.001. Open bars, Mmp19+/+; solid bars, Mmp19−/−.
Expression of α-SMA is upregulated in Mmp19−/− lung fibroblasts.
Fibroblast to myofibroblast differentiation, which is often evaluated according to α-SMA expression, plays an important role in tissue remodeling and fibrogenesis. Therefore, we assessed whether the absence of Mmp19 gene expression has an effect on this process. Fibroblast lysate proteins derived from four different Mmp19−/− and Mmp19+/+ mice were analyzed for α-SMA expression by Western blotting. As shown in Fig. 7, MMP-19-deficient mice spontaneously expressed significantly higher amounts of α-SMA compared with the WT littermates (P < 0.05). After stimulation with TGF-β1, a known inducer of myofibroblast differentiation, both Mmp19+/+ and Mmp19−/− lung fibroblasts showed a significant increase in α-SMA.
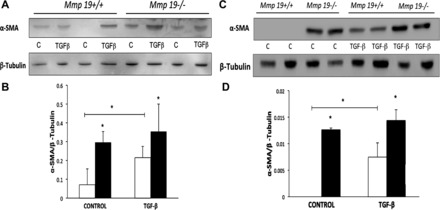
α-Smooth muscle actin (α-SMA) expression by Mmp19−/− and Mmp19+/+ fibroblasts. A and C: Western blot of α-SMA and β-tubulin used as loading control from 4 different fibroblasts from each genotype. B and D: densitometric analysis; each bar represents mean ± SD of 2 different primary lung fibroblasts, *P < 0.05. Open bars, Mmp19+/+; solid bars, Mmp19−/−. C, control.
MMP-19-deficient mice exhibit diminished ATG4C protein expression.
Among the downregulated genes is autophagy related 4C, cysteine peptidase (Atg4c), which participates in lipidation of LC3-I to phosphatidylethanolamine (PE) to expand the autophagosomal membrane. A decreased amount of Atg4c may provoke an impaired autophagic response.
To validate the results obtained in the microarrays, we analyzed the amount of ATG4C protein by Western blot in lung fibroblasts obtained from five different Mmp19−/− and Mmp19+/+ mice. As illustrated in Fig. 8, a marked decrease of Atg4c (~52 kDa) was detected in the Mmp19-deficient mice. A decrease of a nonidentified ~30-kDa band was also observed.
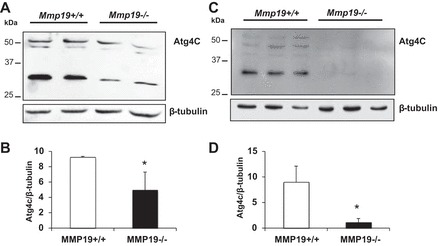
Expression of autophagy-related protein 4C (ATG4C) by Mmp19−/− and Mmp19+/+ fibroblasts. A and C: Western blot of ATG4C and β-tubulin used as loading control from 5 different fibroblasts from each genotype. B and D: densitometric analysis; each bar represents mean ± SD of 2 (B) and 3 (D) different primary lung fibroblasts. *P < 0.05; open bars, Mmp19+/+; solid bars, Mmp19−/−.
Mmp19 deletion increases lung fibroblast growth rate.
Because several genes implicated in cell proliferation were upregulated in Mmp19-deficient fibroblasts, cell growth was examined in the Mmp19−/− and Mmp19+/+ cells using the WST-1 assay. As illustrated in Fig. 9, primary lung fibroblasts from Mmp19−/− mice cultured with DMEM supplemented with 10% FBS displayed a significantly higher growth rate, as measured by WST-1 assay, compared with Mmp19+/+ cells from the second day on; *P < 0.05 at 48 h; **P < 0.01 at 72 and 96 h. We also evaluated cell proliferation by CyQUANT assay in fibroblasts cultured in DMEM supplemented with 2% FBS in the presence or absence of MMP-19 recombinant protein (100 ng/ml). As shown in Fig. 9B, recombinant MMP-19 significantly decreased cell proliferation at 72 h in MMP-19-deficient fibroblasts, whereas it had no effect on WT fibroblasts.
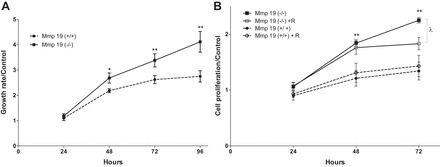
Growth rate analysis. A: lung fibroblasts from Mmp19+/+ and Mmp19−/− mice (n = 5 in each group) were grown in 96-well plates in DMEM supplemented with 10% FBS, and cell growth was analyzed by water-soluble tetrazolium salt assay. B: lung fibroblasts from Mmp19+/+ and Mmp19−/− mice (n = 3) were cultured in DMEM supplemented with 2% FBS with or without the presence of the recombinant MMP-19 protein (100 ng/ml), and proliferation was evaluated by CyQUANT assay. Each point represents the mean ± SD of the different fibroblasts assayed in triplicate; *P < 0.05; **P < 0.01; λ < 0.05.
Mmp19 deletion increases transmigration through ECM components.
To analyze the effects of Mmp19 deficiency on fibroblast migration, we used collagen I- or Matrigel-coated Boyden chambers. Cell migration was examined in the presence or absence of PDGF-B, a fibroblast-chemotactic mediator. As seen in Fig. 10, fibroblast transmigration through collagen I was significantly higher in Mmp19−/− compared with Mmp19+/+ cells either spontaneously (P < 0.01) or in response to PDGF-B (P < 0.01). Similar results were observed when transmigration was examined using Matrigel both under basal conditions and with PDGF.
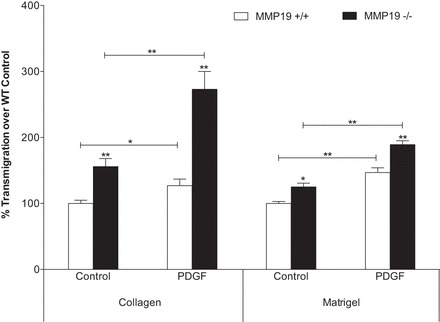
Transmigration through extracellular matrix components. Fibroblasts were seeded on collagen-coated or Matrigel-coated Boyden chambers, and the migrating cells were quantified after 8 or 24 h of incubation, respectively, in the presence of PDGF (20 ng/ml). Each bar represents the mean ± SD of 6 primary cell fibroblasts. *P < 0.05; **P < 0.01. WT, wild-type.
To examine whether PDGF also has chemokinetic properties, checkerboard was performed. As illustrated in Fig. 11, PDGF showed both chemotactic (11A) and chemokinetic (11B) activities although its effect was higher in MMP-19-deficient fibroblasts.
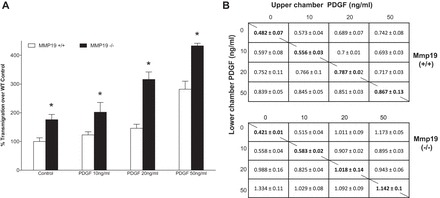
Checkerboard analysis. A: migration (chemotaxis) of fibroblasts Mmp19+/+ and Mmp19−/− in response to PDGF (10, 20, and 50 ng/ml in lower chamber) in collagen-coated Boyden chambers. B: checkerboard analysis (chemokinesis) of fibroblasts. Fibroblasts were added in the upper chamber, and different concentrations of PDGF (10, 20, and 50 ng/ml) were placed in the upper and lower chambers. Each datum in the table represents the mean ± SD of absorbance values at 560 nm of 3 independent experiments.
DISCUSSION
IPF is a progressive and lethal disease, characterized by epithelial cell dysfunction resulting in the increase of the fibroblast/myofibroblast population and the excessive accumulation of ECM, mainly fibrillar collagens. The abnormal remodeled matrix may lead to a positive feedback of mesenchymal cell activation and progressive fibrosis. In these biopathological processes, a variety of mediators, including several MMPs contributes to the pathogenesis of IPF, but their specific roles are not fully understood (5).
We recently demonstrated that MMP-19, an MMP that shows some unique structural features, expression pattern, and functional characteristics (14), is increased in alveolar epithelial cells adjacent to fibrotic regions of IPF lungs. Interestingly, we found that the MMP-19-deficient mice displayed a more severe bleomycin-induced fibrotic lung disorder, suggesting that this enzyme may have a protective antifibrogenic role (25). Supporting this notion, we also observed that MMP-19 induces in alveolar epithelial cells the expression of cyclooxygenase-2 the enzyme responsible for the synthesis of the prostaglandin E2, a potent antifibrotic mediator. An important observation in the fibrotic response in the MMP-19-deficient mice was the remarkable increase of fibroblasts/myofibroblasts that formed typical foci in the lungs. However, the role of MMP-19 in mesenchymal cells was not explored.
In the present study, we evaluated the influence of MMP-19 deficiency in a number of fibroblast functions relevant for fibrogenesis. We first examined the gene expression profiles of MMP-19-deficient and MMP-19-sufficient lung fibroblasts through microarray analysis. Our results revealed that several genes and pathways involved in relevant fibrogenic activities, such as proliferation, migration, collagens synthesis, cell-matrix interaction, and autophagy, were dysregulated in MMP-19-deficient fibroblasts, conferring them a profibrotic phenotype.
Migration and proliferation are two essential mechanisms for the clustering of fibroblasts in damaged tissues during the fibrotic response, and several growth factors that participate in these processes were strongly dysregulated in MMP-19-deficient mice. For example, the α11 integrin (ITGA11), a major collagen receptor involved in collagen reorganization, was one of the most upregulated genes. Importantly, this integrin induces cell proliferation regulating the expression of members of the IGF family, and one of them, IGF1, a multifunctional regulator of cell proliferation, survival, and differentiation in multiple cell types, was also upregulated in the MMP-19-deficient fibroblasts (26). IGF1 overexpression has been associated with increased fibrogenesis in some experimental models (22). Furthermore, recent work demonstrated that ITGA11 is induced by TGF-β1 contributing to the differentiation of fibroblasts to myofibroblasts, a process that was also revealed in our study, where MMP-19-deficient fibroblasts showed higher levels of α-SMA, a major characteristic of myofibroblasts (9, 23). Curiously, the protooncogene MYCN, which is dysregulated in diverse cancers, was highly upregulated in MMP-19-deficient fibroblasts. Upregulation of this gene has been noticed in the exaggerated fibroblast proliferation associated with hypertrophic scar formation (17). The upregulation of this gene may be related to the increase of SET and MYND domain-containing protein 3, a histone methyltransferase that may activate the transcription of a set of downstream genes, including MYCN, and that also induces cell migration and proliferation (18). On the other hand, several genes that inhibit cell proliferation were downregulated. Of note, interferon-inducible protein, p200, which has a modular organization consisting of one (p203) or two (p202 and p204) 200-amino-acid motifs and displays an antiproliferative activity, was strongly downregulated (3). Another interesting downregulated gene was the histone methyltransferase SUV39H1, whose encoded protein trimethylates lysine 9 of histone H3, resulting in transcriptional gene silencing. Recent studies have shown that telomeres in SUV39H1 knockdown cells are longer compared with those of the control without modifications in telomerase activities but likely associated to loss of DNA methylation at subtelomeric regions (2).
On the basis of these findings, we evaluated fibroblast proliferation by two assays and fibroblast chemotaxis and chemokinesis through a migration chamber assay, which confirmed that MMP-19-deficient fibroblasts have a marked increase in proliferative and migratory activities. As a proof of concept, we examined the effect of adding back MMP-19 recombinant protein on gene expression and fibroblast proliferation. Gene expression levels of Colα1, Mmp14, Itga11, Nid2, and Fncd1 that were originally increased in MMP-19-deficient fibroblasts were significantly decreased when the recombinant was added, reaching similar levels to the WT fibroblasts. Similarly, recombinant protein decreased the exaggerated cell proliferation of MMP-19-deficient fibroblasts. These results indicate that the observed differences in fibroblast behavior are specifically related to MMP-19 gene modification.
Scar deposition resulting from an altered wound-healing response is characterized by increased production of matrix proteins, mainly fibrillar collagens. In this context, several collagens, including collagen type I, the prototype of increased fibrillar collagens in fibrosis, were upregulated in MMP-19-deficient fibroblasts. The increase of the α1 type I collagen was corroborated by quantitative RT-PCR and the increase of secreted collagens by the Sircol assay, a dye-binding method for the analysis of soluble collagens. In addition, several genes that increase collagen synthesis were upregulated in the MMP-19-deficient fibroblasts, including WISP1. It has been shown that stimulation of mouse and human lung fibroblasts with recombinant WISP1 enhanced the production of ECM components, primarily type I collagen and fibronectin (6); both molecules increased in MMP-19-deficient fibroblasts.
Network analysis showed that expression of collagens and MMP-14, the only “compensatory” upregulated MMP, was likely associated to ERK activation.
Another interesting upregulated gene was Loxl1, a member of the lysyl oxidase gene family, which are all ECM-embedded copper-dependent amine oxidases that play critical roles in the formation of crosslinks in collagens and elastin and which are usually highly expressed in fibrotic processes (8). The increased expression of fibrillar and fiber-associated collagens and a member of the Lox family confirms the profibrotic phenotype of the MMP-19-deficient fibroblasts.
Autophagy is a fundamental cell process that involves the sequestration of cytoplasmic components in double-membraned autophagosomes that fuse with lysosomes, where their cargoes are delivered for degradation and recycling. Alterations in autophagy have been implicated in several chronic-degenerative processes, and recent data indicate that it is decreased in IPF lungs and fibroblasts (1, 12, 19). In the initial steps of autophagy, the proteolytic processing of ATG8, by the cysteine protease ATG4, is critical for conjugation of ATG8 with phosphatidylethanolamine and, subsequently, autophagosome formation. We found by gene expression and protein analysis a marked decrease of ATG4, suggesting that lack of MMP-19 may cause alterations in the autophagy-lysosomal pathway. The effect of autophagic inhibition on fibroblasts in the context of a fibrotic response is presently unclear, but, interestingly, it may lead to increased α-SMA, fibronectin, and collagen expression (12), as we found in our microarray data, and to increased ECM accumulation because of decreased intracellular degradation.
In summary, our findings demonstrate that MMP-19 has strong regulatory effects on fibroblast migration and proliferation as well as the fibroblast to myofibroblast differentiation and the synthesis of key ECM components. These effects on fibroblast behavior may represent a promising therapeutic approach in fibrotic disorders.
GRANTS
Paul Jara acknowledges the scholarship and financial support provided by Consejo Nacional de Ciencia y Tecnología, México (CONACyT), and UNAM. This study was supported by CONACyT 80473 and PAPIIT IN214612-3.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: P.J., J. Calyeca, Y.R., L.P., G.Y., and J. Cisneros performed experiments; P.J., G.Y., N.K., V.M., J. Cisneros, and M.S. analyzed data; P.J., J. Calyeca, Y.R., L.P., G.Y., N.K., V.M., M.S., and A.P. approved final version of manuscript; M.S. and A.P. conception and design of research; A.P. drafted manuscript.
ACKNOWLEDGMENTS
We thank Carlos López Otin for kindly donating MMP-19-deficient mice and for critical review of the manuscript.
This paper constitutes a partial fulfillment of the Graduate Program in Biomedical Sciences of the Universidad Nacional Autónoma de México (UNAM) for Paul Jara.
REFERENCES
Articles from American Journal of Physiology - Lung Cellular and Molecular Physiology are provided here courtesy of American Physiological Society
Full text links
Read article at publisher's site: https://doi.org/10.1152/ajplung.00043.2014
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc5243210
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1152/ajplung.00043.2014
Article citations
MMP19 Variants in Familial and Sporadic Idiopathic Pulmonary Fibrosis.
Lung, 201(6):571-580, 16 Nov 2023
Cited by: 2 articles | PMID: 37971547
An integrated cell atlas of the lung in health and disease.
Nat Med, 29(6):1563-1577, 08 Jun 2023
Cited by: 151 articles | PMID: 37291214 | PMCID: PMC10287567
Amelioration of Pulmonary Fibrosis by Matrix Metalloproteinase-2 Overexpression.
Int J Mol Sci, 24(7):6695, 03 Apr 2023
Cited by: 7 articles | PMID: 37047672 | PMCID: PMC10095307
Endothelial cell-derived MMP19 promotes pulmonary fibrosis by inducing E(nd)MT and monocyte infiltration.
Cell Commun Signal, 21(1):56, 13 Mar 2023
Cited by: 5 articles | PMID: 36915092 | PMCID: PMC10009991
Identification of immune biomarkers associated with basement membranes in idiopathic pulmonary fibrosis and their pan-cancer analysis.
Front Genet, 14:1114601, 02 Mar 2023
Cited by: 2 articles | PMID: 36936416 | PMCID: PMC10017543
Go to all (31) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Genes & Proteins (4)
- (1 citation) UniProt - P29743
- (1 citation) UniProt - P20249
- (1 citation) UniProt - P63905
- (1 citation) UniProt - P52849
GEO - Gene Expression Omnibus
- (1 citation) GEO - GSE49643
Nucleotide Sequences (2)
- (2 citations) ENA - AI854703
- (1 citation) ENA - AI607873
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Novel differences in gene expression and functional capabilities of myofibroblast populations in idiopathic pulmonary fibrosis.
Am J Physiol Lung Cell Mol Physiol, 315(5):L697-L710, 09 Aug 2018
Cited by: 17 articles | PMID: 30091381
Extracellular matrix proteins: a positive feedback loop in lung fibrosis?
Matrix Biol, 34:170-178, 27 Nov 2013
Cited by: 54 articles | PMID: 24291458
Matrix metalloproteinase-19 is a key regulator of lung fibrosis in mice and humans.
Am J Respir Crit Care Med, 186(8):752-762, 02 Aug 2012
Cited by: 64 articles | PMID: 22859522 | PMCID: PMC5450991
Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis.
Am J Respir Cell Mol Biol, 53(5):585-600, 01 Nov 2015
Cited by: 238 articles | PMID: 26121236 | PMCID: PMC4742954
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NHLBI NIH HHS (2)
Grant ID: R01 HL095397
Grant ID: U01 HL112707
 1
1




