Abstract
Background
BRCA1/BRCA2 germ line (GL) mutation carriers with pancreatic adenocarcinoma (PDAC) may have distinct outcomes. We recently described an apparent more favourable prognosis of surgically resected BRCA-associated PDAC patients in a single-arm, uncontrolled, retrospective study. However, the prognostic impact of GL BRCA1/2 mutations in surgically resected PDAC has not been compared with a matched control population.Methods
A larger multi-centre, case-control retrospective analysis was performed. Cases were patients with surgically resected, BRCA1/2-associated PDAC from 2004 to 2013. Controls included surgically resected PDAC cases treated during the same time period that were either BRCA non-carriers, or had no family history of breast, ovarian or pancreatic cancers. Cases and controls were matched by: age at diagnosis (within ±5-year period) and institution. Demographics, clinical history, overall survival (OS) and disease-free survival (DFS) were abstracted from patient records. Statistical comparisons were assessed using χ2- and Fisher's exact test, and median DFS/OS using Kaplan-Meier method and log-rank testing.Results
Twenty-five patients with BRCA1-(n=4) or BRCA2 (N=21)-associated resectable PDAC were identified. Mean age was 55.7 years (range, 34-78 years), 48% (n=12) were females and 76% (n=19) were Jewish. Cases were compared (1 : 2) with 49 resectable PDAC controls, and were balanced for age, ethnicity and other relevant clinical and pathological features. BRCA-associated PDAC patients received neoadjuvant, or adjuvant platinum-based treatment more frequently than controls (7 out of 8 vs 6 out of 14) and (7 out of 21 vs 3 out of 44), respectively. No significant difference in median OS (37.06 vs 38.77 months, P=0.838) and in DFS (14.3 vs 12.0 months, P=0.303) could be demonstrated between cases and controls. A trend to increased DFS was observed among BRCA-positive cases treated with neoadjuvant/adjuvant platinum-containing regimens (n=10) compared with similarly treated controls (n=7) (39.1 vs 12.4 months, P=0.255).Conclusions
In this retrospective analysis, the prognosis of surgically resectable BRCA-associated PDAC is no different than that of sporadic PDAC from the same institution. The role of platinum-based adjuvant therapy in this setting requires prospective investigation.Free full text

Overall survival and clinical characteristics of BRCA mutation carriers with stage I/II pancreatic cancer
Associated Data
Abstract
Background:
BRCA1/BRCA2 germ line (GL) mutation carriers with pancreatic adenocarcinoma (PDAC) may have distinct outcomes. We recently described an apparent more favourable prognosis of surgically resected BRCA-associated PDAC patients in a single-arm, uncontrolled, retrospective study. However, the prognostic impact of GL BRCA1/2 mutations in surgically resected PDAC has not been compared with a matched control population.
Methods:
A larger multi-centre, case–control retrospective analysis was performed. Cases were patients with surgically resected, BRCA1/2-associated PDAC from 2004 to 2013. Controls included surgically resected PDAC cases treated during the same time period that were either BRCA non-carriers, or had no family history of breast, ovarian or pancreatic cancers. Cases and controls were matched by: age at diagnosis (within ±5-year period) and institution. Demographics, clinical history, overall survival (OS) and disease-free survival (DFS) were abstracted from patient records. Statistical comparisons were assessed using χ2- and Fisher's exact test, and median DFS/OS using Kaplan–Meier method and log-rank testing.
Results:
Twenty-five patients with BRCA1-(n=4) or BRCA2 (N=21)-associated resectable PDAC were identified. Mean age was 55.7 years (range, 34–78 years), 48% (n=12) were females and 76% (n=19) were Jewish. Cases were compared (1 :
: 2) with 49 resectable PDAC controls, and were balanced for age, ethnicity and other relevant clinical and pathological features. BRCA-associated PDAC patients received neoadjuvant, or adjuvant platinum-based treatment more frequently than controls (7 out of 8 vs 6 out of 14) and (7 out of 21 vs 3 out of 44), respectively. No significant difference in median OS (37.06 vs 38.77 months, P=0.838) and in DFS (14.3 vs 12.0 months, P=0.303) could be demonstrated between cases and controls. A trend to increased DFS was observed among BRCA-positive cases treated with neoadjuvant/adjuvant platinum-containing regimens (n=10) compared with similarly treated controls (n=7) (39.1 vs 12.4 months, P=0.255).
2) with 49 resectable PDAC controls, and were balanced for age, ethnicity and other relevant clinical and pathological features. BRCA-associated PDAC patients received neoadjuvant, or adjuvant platinum-based treatment more frequently than controls (7 out of 8 vs 6 out of 14) and (7 out of 21 vs 3 out of 44), respectively. No significant difference in median OS (37.06 vs 38.77 months, P=0.838) and in DFS (14.3 vs 12.0 months, P=0.303) could be demonstrated between cases and controls. A trend to increased DFS was observed among BRCA-positive cases treated with neoadjuvant/adjuvant platinum-containing regimens (n=10) compared with similarly treated controls (n=7) (39.1 vs 12.4 months, P=0.255).
Conclusions:
In this retrospective analysis, the prognosis of surgically resectable BRCA-associated PDAC is no different than that of sporadic PDAC from the same institution. The role of platinum-based adjuvant therapy in this setting requires prospective investigation.
Somatic genomic analysis has identified four specific subtypes of pancreatic adenocarcinoma (PDAC): stable, locally rearranged, scattered and unstable (Biankin and Maitra, 2015). The unstable subtype is associated with inactivation of DNA damage homologous repair genes: BRCA1, BRCA2 or PALB2 exhibiting a unique mutational signature reflecting DNA damage repair deficiency and reportedly displaying platinum sensitivity.
In an estimated 5% of PDAC, germ line (GL) BRCA mutation can be detected (Easton et al, 1996), with higher rates in Ashkenazi Jewish PDAC patients (10–15%), an ethnic group where one of three predominant mutations in BRCA1 (185delAG, 5382InsC) and BRCA2 (6174delT) can be detected in 2.5% of the general population and in the majority of high-risk breast/ovarian cancer families (Oddoux et al, 1996; Roa et al, 1996).
In our previous retrospective analysis of BRCA-associated PDAC (n=71 patients at stages I–IV), an improved overall survival (OS) was noted with platinum-based agents compared with other chemotherapeutic modalities. The median OS for patients with stage I/II disease had not been reached at 60 months when the data were retrieved, and the median OS for stage III/IV was 12 months (95% CI 6–15) (Golan et al, 2014). These results suggested that BRCA mutation status is a favourable prognostic marker in early-stage disease and that BRCA-associated PDAC patients may benefit from the addition of platinum agents to standard therapy. However, one major limitation of that study was the lack of an institutional control population. The purpose of this study was to investigate the impact of GL BRCA1 and BRCA2 mutations on natural history in patients with early-stage PDAC, using a larger cohort of genetically characterised and clinically annotated patients.
Materials and methods
Patient identification and data collection
A multi-centre case–control retrospective analysis was performed with eligible cases identified from six participating institutions: Rambam Medical Institute, Haifa; Princess Margaret Cancer Centre, Toronto, Ontario; Memorial Sloan Kettering Cancer Center (MSKCC), New York; MD Anderson (MDACC), Houston; The University of Chicago Medicine, Chicago and Sheba Medical Center, Tel Hashomer. ‘Cases' were defined as patients diagnosed between January 2004 to December 2013 with surgically resectable stage I or II PDAC with pathogenic GL BRCA1/2 mutations. Controls were patients diagnosed with PDAC during the same time period in the same institutions who were either BRCA GL mutation non-carriers, or who had no discernable personal or family history of breast, ovarian or PDAC. Data retrieved included demographics, clinical history, family history of cancer, past surgical procedures specifically pertaining to PDAC, type of systemic chemotherapy and response to treatment. Clinical stage (based on pathology report) was classified according to the seventh edition of the American Joint Committee on Cancer staging criteria (Edge and Compton, 2010). We attempted to match each case with two institutional control patients with similar stage, age, ethnicity and therapy. In case the participating institution lacked a control population fulfilling all the matching criteria, preference was given to stage-based matching. The institutional review board of each participating institute approved this study.
Germ line BRCA mutation analysis (GenBank reference sequences: NM_007294.3 for BRCA1 and NM_000059.3 for BRCA2).
At the Rambam and Sheba Medical Centers, mutational analyses for the three predominant Ashkenazi Jewish mutations (185delAG, 5382InsC in BRCA1 and 6174delT in BRCA2) were carried out by restriction enzyme digest of PCR products, and analysis of the digested PCR products on agarose gels, as described previously (Abeliovich et al, 1997). For each of these three mutations, a known mutation carrier was used as a positive control in each experiment. All abnormally migrating fragments were confirmed by Sanger sequencing.
At the Princess Margaret Cancer Centre in Toronto, mutational analysis of all exons and flanking regions of the BRCA1 and BRCA2 loci was carried out at the Advanced Molecular Diagnostics Laboratory at Mount Sinai Hospital. PCR was carried out in 12 μl reaction volumes. PCR primers and conditions are available upon request. Following PCR clean-up, BigDyeTerminator Cycle Sequencing Version 3.1 (Applied Biosystems, Toronto, ON, USA) was used for sequencing reactions using 2
μl reaction volumes. PCR primers and conditions are available upon request. Following PCR clean-up, BigDyeTerminator Cycle Sequencing Version 3.1 (Applied Biosystems, Toronto, ON, USA) was used for sequencing reactions using 2 μl of the cleaned up PCR products and using the recommended protocol for cycle sequencing, and analysed on an ABI 3730XL Genetic Analyzer (Applied Biosystems, Toronto, ON, USA). Sequencing files were processed using SequenceAnalysis software (Applied Biosystems, Toronto, ON, USA), and assembled and analysed using Mutation Surveyor (version 4.0.7, SoftGenetics, LLC, State College, PA, USA).
μl of the cleaned up PCR products and using the recommended protocol for cycle sequencing, and analysed on an ABI 3730XL Genetic Analyzer (Applied Biosystems, Toronto, ON, USA). Sequencing files were processed using SequenceAnalysis software (Applied Biosystems, Toronto, ON, USA), and assembled and analysed using Mutation Surveyor (version 4.0.7, SoftGenetics, LLC, State College, PA, USA).
In MSKCC, genetic testing was performed by either Myriad (Salt Lake City, UT, USA) genetics, or using an in-house MSKCC Ashkenazi Jewish panel (agreement with Myriad). At MDACC and University of Chicago, genetic testing was performed by Myriad, Ambry (Aliso Viejo, CA, USA) or GeneDX (Gaithersburg, MD, USA). Only patients with deleterious BRCA1/2 mutations were enrolled.
Statistical analysis
Overall Survival was defined as either the time from the date of diagnosis (imaging/biopsy/surgery in patients who did not undergo neoadjuvant treatment) to the date of death from any cause, or censored until the date of last follow-up. Disease-free survival (DFS) was defined as the time from the date of surgery to the date of recurrence (imaging or clinical evidence recurrence), or death in stage I and II patients. If a patient did not have an event, DFS was censored at the date of last follow-up.
Distributions for categorical variables were compared and analysed using the χ2- and Fisher's exact test. The Kaplan–Meier (KM) model was used to calculate OS and DFS as a function of time. The difference between the KM curves was tested for significance with the use of the log-rank test. In order to accommodate for a potential immortal time bias sensitivity analysis including only patients who went on to receive adjuvant therapy was performed.
The effects of nodal status, vascular resection, stage, involved margins, lymphvascular involvement (LVI), perineural involvement, BRCA status, platinum treatment, and gender and age variables on DFS and OS were tested with a Cox univariate analysis. Multivariate analysis was performed with a Cox proportional hazard model. A P-value of 0.05 in the univariate survival analysis was adopted as the limit for inclusion in the multivariate model, and cases with missing values were excluded from this analysis. All tests were two-sided, and a P<0.05 was considered statistically significant. The SPSS statistical package (SPSS 20, SPSS, Chicago, IL, USA) was used for all statistical analyses.
Results
Patient characteristics
At the outset, 30 BRCA-positive surgically resectable PDAC cases and 49 PDAC controls, diagnosed between 2004 and 2013, and matched by age, treatment and institution were identified at the six participating institutions. Of the BRCA-positive cases, five were excluded from final analysis due to advanced disease at diagnosis (one patient metastatic at presentation of disease and four patients had stage III locally advanced disease at presentation and were down-staged following neoadjuvant treatment). The relevant characteristics for both groups are shown in Table 1. Twenty-six of the 49 controls who underwent limited or extended BRCA genotyping were BRCA wild type. Notably, 8 of the 26 controls who tested negative for GL BRCA had a familial or personal family history of BRCA-associated tumours (one had a personal history of breast or ovarian cancer and seven had family histories of breast, ovarian or PDAC). The remaining 23 controls had an unknown BRCA status but no first degree family history of breast, ovarian or PDAC.
Table 1
| Cases (n=25) | Controls (n=49) | ||||
|---|---|---|---|---|---|
| Variable | n | % | n | % | P |
| Center | |||||
| Chicago | 1 | 4.0% | 2 | 4.1% | |
| MDACC | 5 | 20.0% | 10 | 20.4% | |
| MSKCC | 5 | 20.0% | 10 | 20.4% | |
| Sheba | 5 | 20.0% | 9 | 18.4% | |
| Toronto | 6 | 24.0% | 12 | 24.5% | |
| Rambam | 3 | 12.0% | 6 | 12.2% | |
| Gender | |||||
| Male | 13 | 52.0% | 20 | 40.8% | 0.541 |
| Female | 12 | 48.0% | 28 | 57.1% | |
| Unknown | 0 | 0.0% | 1 | 2.0% | |
| Ethnicity | |||||
| Caucasian | 22 | 88.0% | 41 | 83.7% | 0.772 |
| Black | 0 | 0.0% | 1 | 2.0% | |
| Asian | 1 | 4.0% | 1 | 2.0% | |
| Other | 1 | 4.0% | 5 | 10.2% | |
| Unknown | 1 | 4.0% | 1 | 2.0% | |
| Age (average, s.d.) | 55.7 | 10.35 | 57.7 | 8.99 | 0.400 |
| Jewish | |||||
| Ashkenazi | 16 | 64.0% | 12 | 24.5% | 0.002 |
| Non-Ashkenazi | 3 | 12.0% | 4 | 8.2% | |
| Other/unknown | 6 | 24.0% | 33 | 67.3% | |
Abbreviations: MDACC=MD Anderson Cancer Center; MSKCC=Memorial Sloan Kettering Cancer Center.
The mean age at diagnosis for patients with BRCA-positive PDAC was 55.7 years (range, 34–78 years) and was similar to the group of PDAC controls (mean, 57.7 years; range, 41–80 years, P=0.4). In the BRCA-positive group, there were 4 BRCA1 and 21 BRCA2 mutations (the specific mutations are shown in Table 2). Thirteen of the 25 BRCA-positive patients tested positive for one of the founder Jewish mutations, and 19 of the BRCA-positive patients were Jewish.
Table 2
| BRCA1 | |
| 185delAG | 2 |
| 5382insC | 1 |
| c.181T>G, p.C61G | 1 |
| BRCA2 | |
| 4075delGT | 1 |
| 6174delT | 11 |
| 7525delA | 1 |
| 9631delc | 1 |
| c.2041delA | 1 |
| c.3393delC | 1 |
| c.3398del5 | 1 |
| c.3447_3848delGT | 1 |
| c.3967delA | 1 |
| c.4150G>T | 1 |
| c.5164del4 | 1 |
Clinical and treatment characteristics
Relevant clinical and surgical characteristics are summarised in Table 3. All analysed patients had stage I or II resectable disease at presentation. There were no significant differences in tumour location, stage, vascular resection, nodal involvement and involved margins, or other pathological characteristics between cases and controls. A relatively high percentage (16.2%) of patients underwent vascular resection; this is compatible with high-volume centres (Muller et al, 2009). Treatment characteristics are described in Table 4. Neoadjuvant treatment was administered to 32% of BRCA-positive group and 28.6% of controls (P=NS). In addition, 87.5% (7 out of 8) of BRCA-positive cases received a platinum-containing neoadjuvant regimen compared to 42.9% (6 out of 14) controls (P=0.074). With one patient achieving a cPR following neoadjuvant platinum-based chemotherapy (gemcitabine and oxaliplatin). The majority of patients in both groups received adjuvant therapy. However, 33% (7 out of 21) of the BRCA-positive group received platinum-containing adjuvant regimen compared with 6.8% (3 out of 44) controls, a statistically significant difference (P=0.012). Overall, there was a significantly higher percentage of patients receiving adjuvant or neoadjuvant platinum-containing therapy in the BRCA-positive group compared with the control group (40% vs 14.9%, P=0.012). Recurrence was reported among 60% and 79.6% of cases and controls, respectively (P=0.111). Upon recurrence, less than half of the study population received platinum-based chemotherapy.
Table 3
| Cases (n=25) | Controls (n=49) | ||||
|---|---|---|---|---|---|
| Variable | n | % | n | % | P |
| Presenting symptoms | |||||
| Asymptomatic | 2 | 8.0% | 2 | 4.1% | 0.600 |
| Weight loss | 9 | 36.0% | 25 | 51.0% | 0.220 |
| Pain | 11 | 44.0% | 27 | 55.1% | 0.366 |
| Jaundice | 10 | 40.0% | 28 | 57.1% | 0.163 |
| Tumour site | |||||
| Head | 21 | 84.0% | 41 | 83.7% | 0.925 |
| Head–body | 1 | 4.0% | 1 | 2.0% | |
| Body | 1 | 4.0% | 4 | 8.2% | |
| Body–tail | 1 | 4.0% | 2 | 4.1% | |
| Tail | 1 | 4.0% | 1 | 2.0% | |
| Surgery type | |||||
| Classic whipple | 17 | 68.0% | 39 | 79.6% | 0.230 |
| Pylorus preserving | 3 | 12.0% | 5 | 10.2% | |
| Total | 2 | 8.0% | 0 | 0.0% | |
| Distal | 3 | 12.0% | 5 | 10.2% | |
| Vascular resection | |||||
| Yes | 4 | 16.0% | 8 | 16.3% | 0.971 |
| Nodal involvement | |||||
| Yes | 13 | 52.0% | 28 | 57.1% | 0.674 |
| TNM stage | |||||
| 1 | 1 | 4.0% | 4 | 8.2% | 0.304 |
| 2 | 23 | 92.0% | 45 | 91.8% | |
| cPR | 1 | 4.0% | |||
| Grade | |||||
| Well | 3 | 12.0% | 9 | 18.4% | 0.785 |
| Moderate | 13 | 52.0% | 23 | 46.9% | |
| Poor | 9 | 36.0% | 16 | 32.7% | |
| Unknown | 0 | 0.0% | 1 | 2.0% | |
| Involved margins | |||||
| Yes | 2 | 8.0% | 5 | 10.2% | 0.359 |
| No | 22 | 88.0% | 44 | 89.8% | |
| Unknown | 1 | 4.0% | 0 | 0.0% | |
| Lymphvascular invasion | |||||
| Yes | 7 | 28.0% | 23 | 46.9% | 0.117 |
| No | 17 | 68.0% | 21 | 42.9% | |
| Unknown | 1 | 4.0% | 5 | 10.2% | |
| Perineural invasion | |||||
| Yes | 21 | 84.0% | 38 | 79.6% | 0.610 |
| No | 3 | 12.0% | 10 | 18.4% | |
| Unknown | 1 | 4.0% | 1 | 2.0% | |
Abbreviations: cPR=complete pathological response; TNM=tumour, node, metastasis.
Table 4
| Cases (n=25) | Controls (n=49) | ||||
|---|---|---|---|---|---|
| Variable | n | % | n | % | P |
| Neoadjuvant | |||||
| Chemotherapy | 6 | 24.0% | 5 | 10.2% | 0.427 |
| Chemoradiation | 1 | 4.0% | 4 | 8.2% | |
| Chemo>chemorad | 1 | 4.0% | 5 | 10.2% | |
| None | 17 | 68.0% | 34 | 69.4% | |
| Unknown | 0 | 0.0% | 1 | 2.0% | |
| Neoadjuvant regimen | |||||
| Platinum based | 7 | 87.5% | 6 | 42.9% | 0.074 |
| Non-platinum | 1 | 12.5% | 8 | 57.1% | |
| Adjuvant | |||||
| Chemotherapy | 16 | 64.0% | 30 | 61.2% | 0.804 |
| Chemoradiation | 5 | 20.0% | 14 | 28.6% | |
| None | 3 | 12.0% | 4 | 8.2% | |
| Unknown | 1 | 4.0% | 1 | 2.0% | |
| Adjuvant regimen | |||||
| Platinum based | 7 | 33.3% | 3 | 6.8% | 0.010 |
| Non-platinum | 14 | 66.7% | 41 | 93.2% | |
| Neoadjuvant or adjuvant platinum | |||||
| Yes | 10 | 40.0% | 7 | 14.9% | 0.012 |
| No | 14 | 56.0% | 40 | 85.1% | |
| Recurrence | |||||
| Yes | 15 | 60.0% | 39 | 79.6% | 0.111 |
| No | 10 | 40.0% | 9 | 18.4% | |
| Unknown | 0 | 0.0% | 1 | 2.0% | |
| Treatment on recurrence | |||||
| Platinum | 7 | 46.7% | 17 | 43.6% | 0.732 |
| PARP | 7 | 46.7% | 0 | 0.0% | 0.001 |
| Death | |||||
| Yes | 15 | 60.0% | 30 | 61.2% | 0.919 |
Abbreviation: PARP=Poly (ADP-ribose) polymerase.
DFS
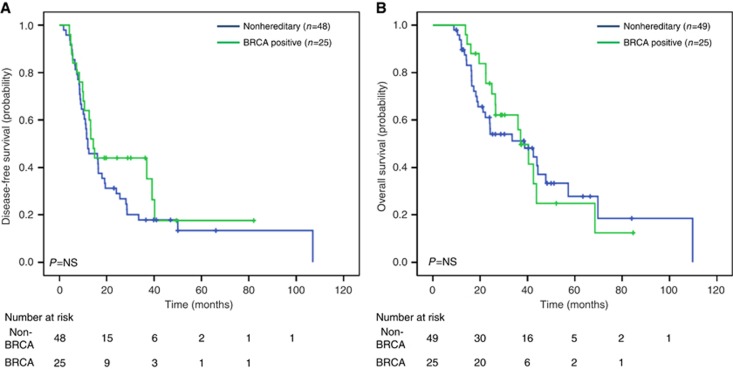
All study patients had an imaging recurrence except four patients with clinical evidence of recurrence as ascertained by treating physician. Our analysis of DFS using the log-rank test revealed no significant differences between outcome for BRCA-positive cases and the matched controls (P=0.303). Median DFS for the GL BRCA-positive group was 14.3 months (95% CI 11.6–17.1 months), and 12.0 months (95% CI 6.5–17.5) for the controls (Figure 1A). When comparing DFS in all analysed patients treated with neoadjuvant/adjuvant platinum-containing treatment, there was a trend to increased DFS among the BRCA-positive cases (n=10) compared with controls (n=7) (39.1 vs 12.4 months, P=0.255) (Figure 2A). In the subgroup of patients receiving a non-platinum-containing agent, no differences in DFS was observed for the BRCA-positive group (n=14) in comparison with the control group (n=40) (10.1 vs 12.0 months, P=0.916) (Figure 2B). In a sensitivity analysis among 65 patients who received adjuvant chemotherapy, similar results were observed for platinum-treated (cases: 39.1 months vs controls: 12.4 months, P=0.223) and non-platinum-treated (cases: 10.1 months vs controls: 12.0 months, P=0.860) patients. For DFS, univariate analysis using Cox regression, identified only LVI (HR 1.87, CI 1.08–3.24, P=0.025) as a significant prognostic factor. However, the prognostic effect of receiving a neoadjuvant/adjuvant platinum treatment demonstrated borderline significance (HR 0.54, CI 0.27–1.08, P=0.081). LVI remained significant in multivariate analysis (Supplementary Table 1).

DFS and OS based on exposure to platinum-based treatments. (A) DFS in patients exposed to platinum-based neoadjuvant/adjuvant treatment. (B) DFS in patients not exposed to platinum-containing neoadjuvant/adjuvant treatment. (C) OS in patients exposed to platinum-based neoadjuvant/adjuvant treatment. (D) OS in patients not exposed to platinum-containing neoadjuvant/adjuvant treatment.
OS
The KM curve estimates from diagnosis showed no difference in OS for the BRCA-positive group (median survival 37.1 months, 95% CI 30.3–43.8) in comparison with control (median survival 38.8 months, 95% CI 15.6–61.9, P=0.838) (Figure 1B). In addition, when comparing OS of all analysed patients treated with neoadjuvant/adjuvant platinum-containing treatment, no differences were observed in BRCA-positive cases (43.8 months) compared with controls (44.4 months, P=0.775) (Figure 2C). Similarly, in the subgroup of patients analysed not receiving a platinum-containing regimen, no differences in OS were observed between the BRCA-positive compared to the control group (Figure 2D). Similar results were observed for platinum-treated (cases: 43.8 months vs controls: 44.4 months, P=0.914) and non-platinum-treated (cases: 36 months vs controls: 38 months, P=0.983) patients on sensitivity analysis. Only LVI involvement was significantly associated with poor OS on univariate (HR 2.19, CI 1.16–4.13, P=0.015) and multivariate (Supplementary Table 1) analyses.
Discussion
In this retrospective case–control, multi-institution study, we found no differences in OS between early-stage GL BRCA-associated PDAC patients compared with matched ‘sporadic' PDAC matched controls. Similarly, Ferrone et al (2009) analysed 145 Jewish Ashkenazi patients diagnosed with resectable PDAC between 1986 and 2004, 8 of whom (5.5%) were BRCA mutation carriers (2 BRCA1, 6 BRCA2), and reported no statistically significant differences in OS between carriers and non-carriers (median, 6 vs 16 months; P=0.35). These results are difficult to interpret as the study was conducted before the introduction of modern surgical techniques, improved post-operative care and Level 1 evidence for routine use of adjuvant therapy (Hartwig et al, 2013). Several authors have reported a superior OS and response to platinum-based treatments in advanced GL BRCA-positive PDAC disease compared with historically reported data (Fogelman et al, 2011; Lowery et al, 2011). In the largest retrospective study to date of 71 BRCA-positive PDAC cases, we reported the median OS for patients with stage 1–2 PDAC had not been reached at 60 months, while median OS for stage 3–4 was 12 months (95% CI 6–15) (Golan et al, 2014).
The more favourable prognosis in BRCA-positive PDAC that was previously reported, including our prior series, may be due to an ascertainment bias. The patients who were selected for genetic testing during the study period (2004–2013) were more likely ‘outliers' with durable responses in advanced disease. These retrospective studies with limited data sets and no comparison arm were the first to identify BRCA carriers as a subset of the PDAC population, illustrating a favourable clinical response to platinum agents or PARPi, thus supporting the hypothesis that BRCA-positive PDAC may be a predictive biomarker for platinum/PARPi treatments. In order to better investigate the prognostic value of BRCA-positive PDAC, we specifically chose early-stage disease in a case-matched study.
The main drawbacks to the study are the retrospective nature of the data and the difficulty of eliminating bias in the case–control design. The population size is limited and insufficiently powered to report on the differential efficacy between platinum-treated BRCA-positive vs sporadic cases. Nevertheless, this was an extensive multi-institutional international effort, stressing the challenge in assembly a large cohort of these relatively rare patients. The superior median OS for both controls and cases in comparison to historic controls (Oettle et al, 2013) is notable and may be due to the young age of our cohort (Worni et al, 2013), advances in standard of care therapy, as well as the fact that participating institutions are high-volume tertiary academic hospitals.
We observed a similar OS among BRCA-positive and sporadic cases treated with neoadjuvant/adjuvant platinum-containing regimens. DFS in patients receiving platinum-based treatment was 40.3 months for cases vs 12.4 months for controls (P=0.233). While not reaching statistical significance, the apparent increased DFS among BRCA-positive subjects treated with platinum compounds is in line with our previous findings (Golan et al, 2014). The lack of statistically significant differences in both OS and DFS in the current study population may be attributed to the limited number of patients enrolled in the study: only 17 patients in our study were treated with neoadjuvant or adjuvant platinum, as determined by institution-based protocols (MDACC recruits the majority of their patients in neoadjuvant/adjuvant protocols). Notably, exposure to platinum (cisplatinum or oxaliplatinum) varied considerably, from several weeks to months. The majority of our patients cases/controls did not receive platinum-based chemotherapies at advanced disease as the study population was collected before the era of newer combinatorial treatment protocols (Conroy et al, 2011).
In conclusion, in this case–control study, we did not identify BRCA mutations as a prognostic factor in early-stage PDAC. Current guidelines recommend considering platinum-based treatments for advanced-stage BRCA-positive PDAC (2016), however, it is unclear whether BRCA carriers should be offered platinum-based neoadjuvant or adjuvant chemotherapy. Preliminary results have recently been reported with PARP inhibition in BRCA-mutant PDAC, supporting the mutation's predictive role (Kaufman et al, 2015). Based on our limited data set, we believe that neoadjuvant or adjuvant platinum-based therapy may be worth considering in early-stage PDAC, however prospective studies are required to more clearly address this question for this population.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
The authors declare no conflict of interest.
References
- NCCN Clinical Practice Guidelines in Oncology–Pancreatic Adenocarcinoma. National Comprehensive Cancer Network. 2016.
- Abeliovich D, Kaduri L, Lerer I, Weinberg N, Amir G, Sagi M, Zlotogora J, Heching N, Peretz T (1997) The founder mutations 185delAG and 5382insC in BRCA1 and 6174delT in BRCA2 appear in 60% of ovarian cancer and 30% of early-onset breast cancer patients among Ashkenazi women. Am J Hum Genet 60: 505–514. [Europe PMC free article] [Abstract] [Google Scholar]
- Biankin AV, Maitra A (2015) Subtyping pancreatic cancer. Cancer Cell 28: 411–413. [Abstract] [Google Scholar]
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, De La Fouchardiere C, Bennouna J, Bachet JB, Khemissa-Akouz F, Pere-Verge D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364: 1817–1825. [Abstract] [Google Scholar]
- Easton DF, Matthews FE, Ford D, Swerdlow AJ, Peto J (1996) Cancer mortality in relatives of women with ovarian cancer: the OPCS Study. Office of Population Censuses and Surveys. Int J Cancer 65: 284–294. [Abstract] [Google Scholar]
- Edge SB, Compton CC (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17: 1471–1474. [Abstract] [Google Scholar]
- Ferrone CR, Levine DA, Tang LH, Allen PJ, Jarnagin W, Brennan MF, Offit K, Robson ME (2009) BRCA germline mutations in Jewish patients with pancreatic adenocarcinoma. J Clin Oncol 27: 433–438. [Europe PMC free article] [Abstract] [Google Scholar]
- Fogelman DR, Wolff RA, Kopetz S, Javle M, Bradley C, Mok I, Cabanillas F, Abbruzzese JL (2011) Evidence for the efficacy of Iniparib, a PARP-1 inhibitor, in BRCA2-associated pancreatic cancer. Anticancer Res 31: 1417–1420. [Abstract] [Google Scholar]
- Golan T, Kanji ZS, Epelbaum R, Devaud N, Dagan E, Holter S, Aderka D, Paluch-Shimon S, Kaufman B, Gershoni-Baruch R, Hedley D, Moore MJ, Friedman E, Gallinger S (2014) Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br J Cancer 111: 1132–1138. [Europe PMC free article] [Abstract] [Google Scholar]
- Hartwig W, Werner J, Jager D, Debus J, Buchler MW (2013) Improvement of surgical results for pancreatic cancer. Lancet Oncol 14: e476–e485. [Abstract] [Google Scholar]
- Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmana J, Mitchell G, Fried G, Stemmer SM, Hubert A, Rosengarten O, Steiner M, Loman N, Bowen K, Fielding A, Domchek SM (2015) Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 33: 244–250. [Abstract] [Google Scholar]
- Lowery MA, Kelsen DP, Stadler ZK, Yu KH, Janjigian YY, Ludwig E, D'Adamo DR, Salo-Mullen E, Robson ME, Allen PJ, Kurtz RC, O'Reilly EM (2011) An emerging entity: pancreatic adenocarcinoma associated with a known BRCA mutation: clinical descriptors, treatment implications, and future directions. Oncologist 16: 1397–1402. [Europe PMC free article] [Abstract] [Google Scholar]
- Muller SA, Hartel M, Mehrabi A, Welsch T, Martin DJ, Hinz U, Schmied BM, Buchler MW (2009) Vascular resection in pancreatic cancer surgery: survival determinants. J Gastrointest Surg 13: 784–792. [Abstract] [Google Scholar]
- Oddoux C, Struewing JP, Clayton CM, Neuhausen S, Brody LC, Kaback M, Haas B, Norton L, Borgen P, Jhanwar S, Goldgar D, Ostrer H, Offit K (1996) The carrier frequency of the BRCA2 6174delT mutation among Ashkenazi Jewish individuals is approximately 1%. Nat Genet 14: 188–190. [Abstract] [Google Scholar]
- Oettle H, Neuhaus P, Hochhaus A, Hartmann J, Geller TK, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J, Arning M, Sinn M, Hinke A, Riess H (2013) Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 310: 1473–1481. [Abstract] [Google Scholar]
- Roa BB, Boyd AA, Volcik K, Richards CS (1996) Ashkenazi Jewish population frequencies for common mutations in BRCA1 and BRCA2. Nat Genet 14: 185–187. [Abstract] [Google Scholar]
- Worni M, Guller U, White R, Castleberry A, Pietrobon R, Cerny T, Gloor B, Koeberle D (2013) Modest improvement in overall survival for patients with metastatic pancreatic cancer: a trend analysis using the surveillance, epidemiology, and end results registry from 1988 to 2008. Pancreas 42: 1157–1163. [Abstract] [Google Scholar]
Articles from British Journal of Cancer are provided here courtesy of Cancer Research UK
Full text links
Read article at publisher's site: https://doi.org/10.1038/bjc.2017.19
Read article for free, from open access legal sources, via Unpaywall:
https://www.nature.com/articles/bjc201719.pdf
Citations & impact
Impact metrics
Citations of article over time
Article citations
The role of germline BRCA1 & BRCA2 mutations in familial pancreatic cancer: A systematic review and meta-analysis.
PLoS One, 19(5):e0299276, 29 May 2024
Cited by: 0 articles | PMID: 38809921 | PMCID: PMC11135687
Review Free full text in Europe PMC
Mutational profiling of 103 unresectable pancreatic ductal adenocarcinomas using EUS-guided fine-needle biopsy.
Endosc Ultrasound, 13(3):154-164, 01 May 2024
Cited by: 0 articles | PMID: 39318643 | PMCID: PMC11419524
Predictive Value and Therapeutic Significance of Somatic BRCA Mutation in Solid Tumors.
Biomedicines, 12(3):593, 06 Mar 2024
Cited by: 0 articles | PMID: 38540206 | PMCID: PMC10967875
Review Free full text in Europe PMC
Advances in artificial intelligence for the diagnosis and treatment of ovarian cancer (Review).
Oncol Rep, 51(3):46, 19 Jan 2024
Cited by: 2 articles | PMID: 38240090 | PMCID: PMC10828921
Review Free full text in Europe PMC
Targeting BRCA and PALB2 in Pancreatic Cancer.
Curr Treat Options Oncol, 25(3):346-363, 04 Jan 2024
Cited by: 0 articles | PMID: 38311708
Review
Go to all (43) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
RefSeq - NCBI Reference Sequence Database (2)
- (1 citation) RefSeq - NM_000059.3
- (1 citation) RefSeq - NM_007294.3
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
BRCA1/BRCA2 Germline Mutation Carriers and Sporadic Pancreatic Ductal Adenocarcinoma.
J Am Coll Surg, 226(4):630-637.e1, 05 Jan 2018
Cited by: 43 articles | PMID: 29309945 | PMCID: PMC6178809
Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers.
Br J Cancer, 111(6):1132-1138, 29 Jul 2014
Cited by: 226 articles | PMID: 25072261 | PMCID: PMC4453851
Adjuvant versus neoadjuvant chemotherapy in triple-negative breast cancer patients with BRCA mutations.
Breast Cancer Res Treat, 170(1):101-109, 22 Feb 2018
Cited by: 14 articles | PMID: 29470805 | PMCID: PMC5994186
Overall Survival and Clinical Characteristics of BRCA-Associated Cholangiocarcinoma: A Multicenter Retrospective Study.
Oncologist, 22(7):804-810, 09 May 2017
Cited by: 54 articles | PMID: 28487467 | PMCID: PMC5507643
Review Free full text in Europe PMC