Abstract
Free full text

Immunological properties of hepatitis B core antigen fusion proteins.
Abstract
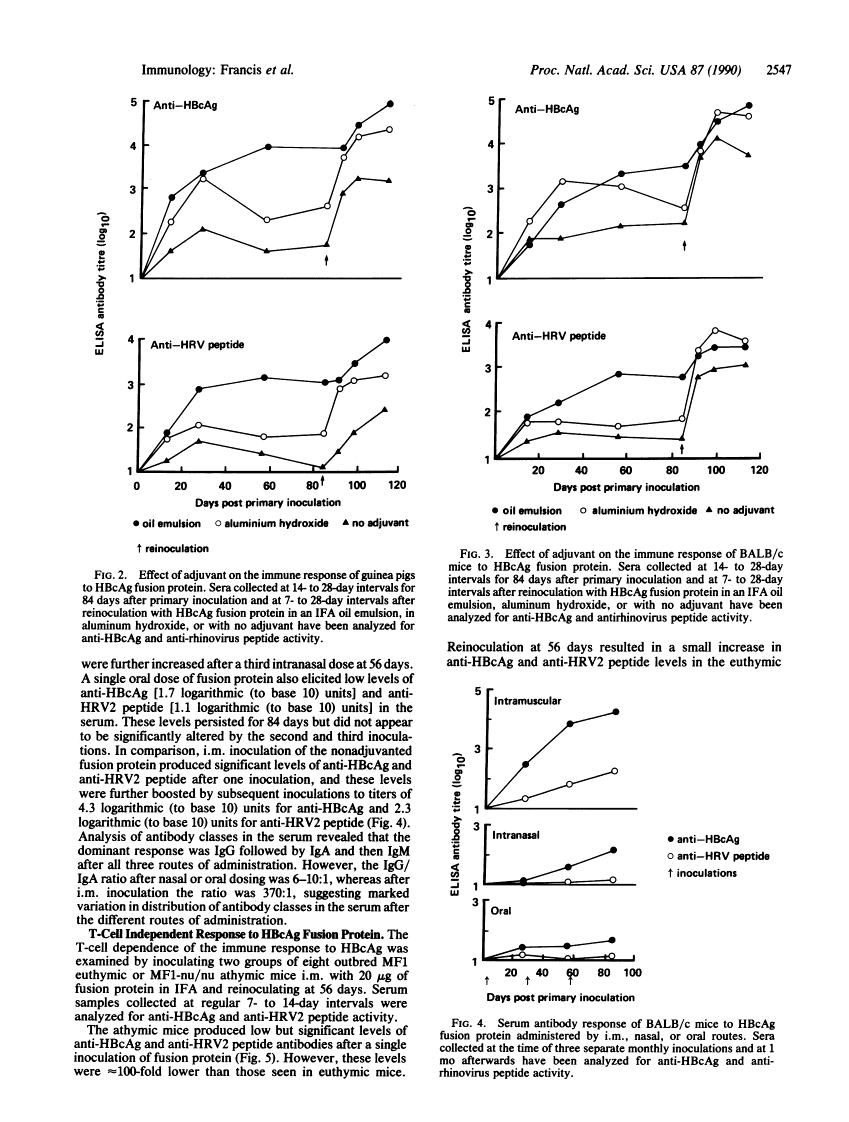
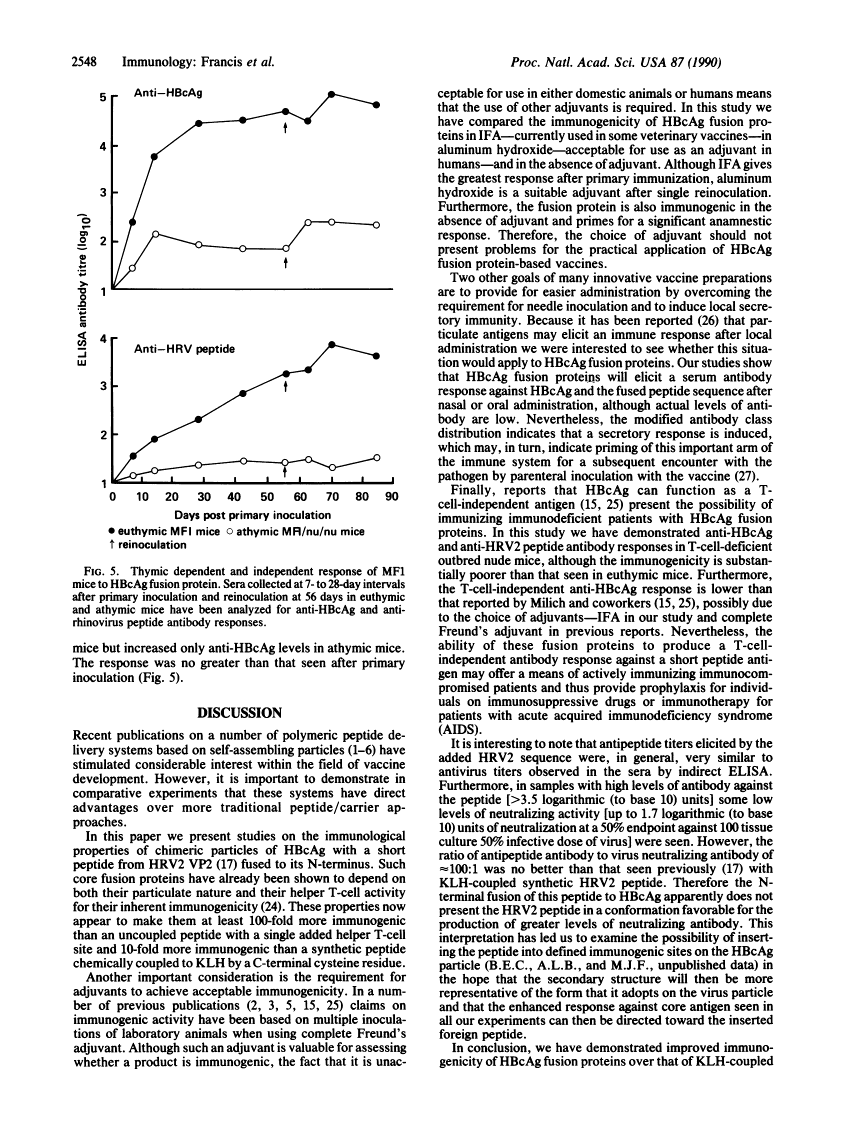
The immunogenicity of a 19 amino acid peptide from foot-and-mouth disease virus has previously been shown to approach that of the inactivated virus from which it was derived after multimeric particulate presentation as an N-terminal fusion with hepatitis B core antigen. In this report we demonstrate that rhinovirus peptide-hepatitis B core antigen fusion proteins are 10-fold more immunogenic than peptide coupled to keyhole limpet hemocyanin and 100-fold more immunogenic than uncoupled peptide with an added helper T-cell epitope. The fusion proteins can be readily administered without adjuvant or with adjuvants acceptable for human and veterinary application and can elicit a response after nasal or oral dosing. The fusion proteins can also act as T-cell-independent antigens. These properties provide further support for their suitability as presentation systems for "foreign" epitopes in the development of vaccines.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (967K), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clarke BE, Newton SE, Carroll AR, Francis MJ, Appleyard G, Syred AD, Highfield PE, Rowlands DJ, Brown F. Improved immunogenicity of a peptide epitope after fusion to hepatitis B core protein. Nature. 330(6146):381–384. [Abstract] [Google Scholar]
- Stahl SJ, Murray K. Immunogenicity of peptide fusions to hepatitis B virus core antigen. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6283–6287. [Europe PMC free article] [Abstract] [Google Scholar]
- Delpeyroux F, Chenciner N, Lim A, Malpièce Y, Blondel B, Crainic R, van der Werf S, Streeck RE. A poliovirus neutralization epitope expressed on hybrid hepatitis B surface antigen particles. Science. 1986 Jul 25;233(4762):472–475. [Abstract] [Google Scholar]
- Burke KL, Dunn G, Ferguson M, Minor PD, Almond JW. Antigen chimaeras of poliovirus as potential new vaccines. Nature. 1988 Mar 3;332(6159):81–82. [Abstract] [Google Scholar]
- Adams SE, Dawson KM, Gull K, Kingsman SM, Kingsman AJ. The expression of hybrid HIV:Ty virus-like particles in yeast. Nature. 1987 Sep 3;329(6134):68–70. [Abstract] [Google Scholar]
- Budkowska A, Shih JW, Gerin JL. Immunochemistry and polypeptide composition of hepatitis B core antigen (HBc Ag). J Immunol. 1977 Apr;118(4):1300–1305. [Abstract] [Google Scholar]
- Pasek M, Goto T, Gilbert W, Zink B, Schaller H, MacKay P, Leadbetter G, Murray K. Hepatitis B virus genes and their expression in E. coli. Nature. 1979 Dec 6;282(5739):575–579. [Abstract] [Google Scholar]
- Almeida JD, Rubenstein D, Stott EJ. New antigen-antibody system in Australia-antigen-positive hepatitis. Lancet. 1971 Dec 4;2(7736):1225–1227. [Abstract] [Google Scholar]
- Kniskern PJ, Hagopian A, Montgomery DL, Burke P, Dunn NR, Hofmann KJ, Miller WJ, Ellis RW. Unusually high-level expression of a foreign gene (hepatitis B virus core antigen) in Saccharomyces cerevisiae. Gene. 1986;46(1):135–141. [Abstract] [Google Scholar]
- Roossinck MJ, Jameel S, Loukin SH, Siddiqui A. Expression of hepatitis B viral core region in mammalian cells. Mol Cell Biol. 1986 May;6(5):1393–1400. [Europe PMC free article] [Abstract] [Google Scholar]
- Lanford RE, Luckow V, Kennedy RC, Dreesman GR, Notvall L, Summers MD. Expression and characterization of hepatitis B virus surface antigen polypeptides in insect cells with a baculovirus expression system. J Virol. 1989 Apr;63(4):1549–1557. [Europe PMC free article] [Abstract] [Google Scholar]
- Hoofnagle JH, Gerety RJ, Barker LF. Antibody to hepatitis-B-virus core in man. Lancet. 1973 Oct 20;2(7834):869–873. [Abstract] [Google Scholar]
- Milich DR, McLachlan A, Moriarty A, Thornton GB. Immune response to hepatitis B virus core antigen (HBcAg): localization of T cell recognition sites within HBcAg/HBeAg. J Immunol. 1987 Aug 15;139(4):1223–1231. [Abstract] [Google Scholar]
- Milich DR, McLachlan A. The nucleocapsid of hepatitis B virus is both a T-cell-independent and a T-cell-dependent antigen. Science. 1986 Dec 12;234(4782):1398–1401. [Abstract] [Google Scholar]
- Francis MJ, Clarke BE. Peptide vaccines based on enhanced immunogenicity of peptide epitopes presented with T-cell determinants or hepatitis B core protein. Methods Enzymol. 1989;178:659–676. [Abstract] [Google Scholar]
- Francis MJ, Hastings GZ, Sangar DV, Clark RP, Syred A, Clarke BE, Rowlands DJ, Brown F. A synthetic peptide which elicits neutralizing antibody against human rhinovirus type 2. J Gen Virol. 1987 Oct;68(Pt 10):2687–2691. [Abstract] [Google Scholar]
- Houghten RA. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5131–5135. [Europe PMC free article] [Abstract] [Google Scholar]
- Voller A, Bartlett A, Bidwell DE, Clark MF, Adams AN. The detection of viruses by enzyme-linked immunosorbent assay (ELISA). J Gen Virol. 1976 Oct;33(1):165–167. [Abstract] [Google Scholar]
- Milich DR, McLachlan A, Stahl S, Wingfield P, Thornton GB, Hughes JL, Jones JE. Comparative immunogenicity of hepatitis B virus core and E antigens. J Immunol. 1988 Nov 15;141(10):3617–3624. [Abstract] [Google Scholar]
- O'Hagan DT, Palin K, Davis SS, Artursson P, Sjöholm I. Microparticles as potentially orally active immunological adjuvants. Vaccine. 1989 Oct;7(5):421–424. [Abstract] [Google Scholar]
- Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987 Jul;7(4):265–276. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.87.7.2545
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc53726?pdf=render
Citations & impact
Impact metrics
Article citations
Immune response induced by recombinant pres2/S-protein and a pres2-S-protein fused with a core 18-27 antigen fragment of hepatitis B virus compared to conventional HBV vaccine.
Virus Genes, 59(4):499-514, 04 May 2023
Cited by: 0 articles | PMID: 37140777
Oral and Subcutaneous Immunization with a Plant-Produced Mouse-Specific Zona Pellucida 3 Peptide Presented on Hepatitis B Core Antigen Virus-like Particles.
Vaccines (Basel), 11(2):462, 17 Feb 2023
Cited by: 1 article | PMID: 36851339 | PMCID: PMC9963689
Plant-derived VLP: a worthy platform to produce vaccine against SARS-CoV-2.
Biotechnol Lett, 44(1):45-57, 27 Nov 2021
Cited by: 17 articles | PMID: 34837582 | PMCID: PMC8626723
Review Free full text in Europe PMC
Peptide-based supramolecular vaccine systems.
Acta Biomater, 133:153-167, 16 May 2021
Cited by: 22 articles | PMID: 34010691 | PMCID: PMC8497425
Review Free full text in Europe PMC
Hepatitis B core-based virus-like particles: A platform for vaccine development in plants.
Biotechnol Rep (Amst), 29:e00605, 28 Feb 2021
Cited by: 21 articles | PMID: 33732633 | PMCID: PMC7937989
Review Free full text in Europe PMC
Go to all (37) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Presentation and immunogenicity of viral epitopes on the surface of hybrid hepatitis B virus core particles produced in bacteria.
J Gen Virol, 71 ( Pt 5):1109-1117, 01 May 1990
Cited by: 33 articles | PMID: 1693163
Hybrid hepatitis B virus core antigen as a vaccine carrier moiety: I. presentation of foreign epitopes.
J Biotechnol, 44(1-3):91-96, 01 Jan 1996
Cited by: 31 articles | PMID: 8717391
Review
Foreign epitopes in immunodominant regions of hepatitis B core particles are highly immunogenic and conformationally restricted.
Vaccine, 9(8):595-601, 01 Aug 1991
Cited by: 35 articles | PMID: 1722937
Immunogenicity of peptide fusions to hepatitis B virus core antigen.
Proc Natl Acad Sci U S A, 86(16):6283-6287, 01 Aug 1989
Cited by: 63 articles | PMID: 2474830 | PMCID: PMC297822