Abstract
Objective
To compare the efficacy of zinc acetate lozenges with zinc gluconate lozenges in common cold treatment and to examine the dose-dependency of the effect.Design
Meta-analysis.Setting
Placebo-controlled zinc lozenge trials, in which the zinc dose was > 75 mg/day. The pooled effect of zinc lozenges on common cold duration was calculated by using inverse-variance random-effects method.Participants
Seven randomised trials with 575 participants with naturally acquired common colds.Main outcome measure
Duration of the common cold.Results
The mean common cold duration was 33% (95% CI 21% to 45%) shorter for the zinc groups of the seven included trials. Three trials that used lozenges composed of zinc acetate found that colds were shortened by 40% and four trials that used zinc gluconate by 28%. The difference between the two salts was not significant: 12 percentage points (95% CI: -12 to + 36). Five trials used zinc doses of 80-92 mg/day, common cold duration was reduced by 33%, and two trials used zinc doses of 192-207 mg/day and found an effect of 35%. The difference between the high-dose and low-dose zinc trials was not significant: 2 percentage points (95% CI: -29 to + 32).Conclusions
Properly composed zinc gluconate lozenges may be as effective as zinc acetate lozenges. There is no evidence that zinc doses over 100 mg/day might lead to greater efficacy in the treatment of the common cold. Common cold patients may be encouraged to try zinc lozenges for treating their colds. The optimal lozenge composition and dosage scheme need to be investigated further.Free full text

Zinc lozenges and the common cold: a meta-analysis comparing zinc acetate and zinc gluconate, and the role of zinc dosage
Abstract
Objective
To compare the efficacy of zinc acetate lozenges with zinc gluconate lozenges in common cold treatment and to examine the dose-dependency of the effect.
Design
Meta-analysis.
Setting
Placebo-controlled zinc lozenge trials, in which the zinc dose was >
> 75
75 mg/day. The pooled effect of zinc lozenges on common cold duration was calculated by using inverse-variance random-effects method.
mg/day. The pooled effect of zinc lozenges on common cold duration was calculated by using inverse-variance random-effects method.
Participants
Seven randomised trials with 575 participants with naturally acquired common colds.
Main outcome measure
Duration of the common cold.
Results
The mean common cold duration was 33% (95% CI 21% to 45%) shorter for the zinc groups of the seven included trials. Three trials that used lozenges composed of zinc acetate found that colds were shortened by 40% and four trials that used zinc gluconate by 28%. The difference between the two salts was not significant: 12 percentage points (95% CI: −12 to +
+ 36). Five trials used zinc doses of 80–92
36). Five trials used zinc doses of 80–92 mg/day, common cold duration was reduced by 33%, and two trials used zinc doses of 192–207
mg/day, common cold duration was reduced by 33%, and two trials used zinc doses of 192–207 mg/day and found an effect of 35%. The difference between the high-dose and low-dose zinc trials was not significant: 2 percentage points (95% CI: −29 to
mg/day and found an effect of 35%. The difference between the high-dose and low-dose zinc trials was not significant: 2 percentage points (95% CI: −29 to +
+ 32).
32).
Conclusions
Properly composed zinc gluconate lozenges may be as effective as zinc acetate lozenges. There is no evidence that zinc doses over 100 mg/day might lead to greater efficacy in the treatment of the common cold. Common cold patients may be encouraged to try zinc lozenges for treating their colds. The optimal lozenge composition and dosage scheme need to be investigated further.
mg/day might lead to greater efficacy in the treatment of the common cold. Common cold patients may be encouraged to try zinc lozenges for treating their colds. The optimal lozenge composition and dosage scheme need to be investigated further.
Background
Interest in zinc lozenges for the treatment of the common cold arose when the common cold symptoms of a 3-year-old girl with leukaemia disappeared within a few hours after she had slowly dissolved a therapeutic zinc tablet in her mouth instead of immediately swallowing it as instructed.1 The benefit seemed to be obtained from the slow dissolution of the tablet in her mouth, which implied that zinc has local effects in the pharyngeal region. This observation prompted the father of the child, George Eby, to conduct the first randomised double-blind placebo-controlled trial on zinc lozenges. Eby et al.1 used zinc gluconate lozenges, providing 207 mg/day of elemental zinc, and they significantly shortened the duration of colds.
mg/day of elemental zinc, and they significantly shortened the duration of colds.
A series of zinc lozenge trials have been carried out since Eby’s study but with varying results, which could be partly ascribed to the seven-fold variation in the daily dosage of elemental zinc in those trials.2 The composition of the zinc lozenges between the trials also differed as some lozenges contained substances that tightly bind zinc, which prevented the release of zinc ions. The variation in the levels of free zinc ions in the oro-pharyngeal region has been proposed as a factor that might explain the wide divergence between those results.3–9 Eby hypothesised that zinc acetate might be a better constituent for lozenges than zinc gluconate, since acetate binds zinc ions less strongly.7,8 Nevertheless, it is not clear whether the difference between acetate and gluconate has practical importance at the clinical level.
A previous meta-analysis showed that five low-dose trials of zinc lozenges (<75 mg/day zinc) uniformly found no effect on the duration of colds. Three high-dose (>75
mg/day zinc) uniformly found no effect on the duration of colds. Three high-dose (>75 mg/day) zinc acetate trials found a 42% reduction in the duration of colds, whereas five high-dose zinc gluconate trials found just a 20% reduction in cold duration. Such a difference was consistent with Eby’s proposal; however, there was significant heterogeneity in the five high-dose zinc gluconate studies but not in the three high-dose zinc acetate studies.2 Thus, there may be factors that could confound the comparison of the efficacy of zinc acetate and zinc gluconate.
mg/day) zinc acetate trials found a 42% reduction in the duration of colds, whereas five high-dose zinc gluconate trials found just a 20% reduction in cold duration. Such a difference was consistent with Eby’s proposal; however, there was significant heterogeneity in the five high-dose zinc gluconate studies but not in the three high-dose zinc acetate studies.2 Thus, there may be factors that could confound the comparison of the efficacy of zinc acetate and zinc gluconate.
Although the zinc lozenges dissolved in the oro-pharyngeal region led to the highest zinc levels in that region, a meta-analysis found no evidence that zinc acetate lozenges have a greater effect on cold symptoms that originate in lower anatomical regions compared with those of the nasal region.10 Another meta-analysis of zinc acetate trials found that there was no difference in the efficacy by gender, age, ethnic background, or smoking.11 Further systematic reviews on zinc and the common cold have been published,12–14 but some of them have methodological problems,15–17 and a Cochrane review was recently withdrawn.18
The goal of this meta-analysis was to compare zinc acetate and zinc gluconate in their efficacy in the treatment of the common cold, and to examine the dose-response relationship between the daily dose of elemental zinc and the efficacy of zinc lozenges in treating the common cold.
Methods
Selection of the trials
This meta-analysis was restricted to placebo-controlled trials on zinc lozenges for patients with naturally acquired common cold infections in which the zinc dosage was >75 mg/day. Previous searches of the literature2,8,12–14 identified eight trials that fulfilled these search criteria.1,19–25 Turner25 published a trial of three zinc arms: two arms used zinc acetate <75
mg/day. Previous searches of the literature2,8,12–14 identified eight trials that fulfilled these search criteria.1,19–25 Turner25 published a trial of three zinc arms: two arms used zinc acetate <75 mg/day and one arm used zinc gluconate >75
mg/day and one arm used zinc gluconate >75 mg/day; only the latter was included in this meta-analysis. The methods and characteristics of the eight trials are shown in Supplementary file 1. No additional zinc lozenge trials were found by searching PubMed and Scopus using the free search terms ‘zinc’ and ‘lozenge*’ (15 November 2016).
mg/day; only the latter was included in this meta-analysis. The methods and characteristics of the eight trials are shown in Supplementary file 1. No additional zinc lozenge trials were found by searching PubMed and Scopus using the free search terms ‘zinc’ and ‘lozenge*’ (15 November 2016).
The lozenge of the Smith et al. trial19 contained mannitol and sorbitol.9 There is experimental evidence that mannitol and sorbitol bind zinc ions in the presence of saliva,9 which may explain the negative findings in the Smith et al. trial. Furthermore, Dr Smith was one of the authors of the Godfrey et al.20 trial, which stated in its introduction (p.235) that ‘it has been demonstrated that …
… mannitol/sorbitol inactivate zinc by chelation in saliva’ and ‘mannitol/sorbitol [zinc lozenge] formulations release no zinc ions when dissolved in the mouth’ referring to the Smith et al. trial.19 This indicates that afterwards Dr Smith did not trust the lozenge formulation of his 1989 trial. Therefore, the Smith et al. trial was excluded from the current analysis.
mannitol/sorbitol inactivate zinc by chelation in saliva’ and ‘mannitol/sorbitol [zinc lozenge] formulations release no zinc ions when dissolved in the mouth’ referring to the Smith et al. trial.19 This indicates that afterwards Dr Smith did not trust the lozenge formulation of his 1989 trial. Therefore, the Smith et al. trial was excluded from the current analysis.
There are no studies that directly compare high-dose zinc acetate lozenges versus high-dose zinc gluconate lozenges head-to-head. In addition, there are no studies that compared the same salt by using different doses in the high-dose range. The comparisons in this analysis are indirect.
Outcomes and extraction of data
The outcome in this meta-analysis was the duration of colds. The data reported by Eby et al.,1 Mossad et al.,21 and Turner25 had censored observations that had been previously imputed.2 This study used the mean values and standard deviations for the Eby and the Turner trials based on those previously imputed data.2 The Mossad et al. data were reanalysed for this study leading to small differences compared with the previous imputation (Supplementary file 2, p. 7). The duration of the longest cold symptom was used as the outcome for the Petrus et al. trial22; Dr Petrus kindly made available the dataset of his trial.
Statistical methods
Variation in the recorded cold durations between different patient groups can be caused by differences in the distributions of viruses over time and by differences in the operational outcome definitions. Therefore, the relative effect of zinc lozenges on the common cold duration was calculated in percentages because the relative effect adjusts for variations between the patient groups and outcome definitions26 (see transformation to the relative scale in Supplementary file 2).
The pooled effects of zinc lozenges on common cold duration were calculated by using the inverse-variance random-effects option of the RevMan program.27 Heterogeneity between the studies and subgroups was assessed by Cochran Q test (the χ2-test) and the I2-statistic.28 The I2-statistic estimates the percentage of total variation across studies that is due to true heterogeneity rather than due to chance. Values of I2 greater than about 75% indicate a high level of heterogeneity. The 95% CI for the difference between the estimates was calculated by summing the variances of the subgroup estimates.
Results
Seven zinc lozenge trials with natural common cold infections that had administered >75 mg/day of elemental zinc were included in this meta-analysis. The zinc doses ranged from 80 to 207
mg/day of elemental zinc were included in this meta-analysis. The zinc doses ranged from 80 to 207 mg/day, with three trials using zinc acetate22–24 and four using zinc gluconate.1,20,21,25 All the identified trials were randomised, placebo-controlled, and double-blind. Their characteristics are described in Supplementary file 1. There were 575 participants among the seven trials.
mg/day, with three trials using zinc acetate22–24 and four using zinc gluconate.1,20,21,25 All the identified trials were randomised, placebo-controlled, and double-blind. Their characteristics are described in Supplementary file 1. There were 575 participants among the seven trials.
The effect of zinc lozenges on common cold duration in the seven included trials, and the pooled effects for zinc acetate and zinc gluconate trials are shown in Figure 1. The pooled estimate over the seven included trials indicates a reduction in the common cold duration by 33% (95% CI 21% to 45%). The estimate of zinc acetate indicates a 40% (95% CI 31% to 50%) and the estimate of zinc gluconate indicates a 28% (95% CI 6% to 50%) reduction in common cold duration. There is no difference in the estimates of effect between the two subgroups, with I2 =
= 0% (p
0% (p =
= 0.3). The difference between zinc acetate trials and zinc gluconate trials is 12 percentage points (95% CI:
0.3). The difference between zinc acetate trials and zinc gluconate trials is 12 percentage points (95% CI: −
− 12 to
12 to +
+ 36); thus, the two salts do not differ significantly. Therefore, the pooled estimate of 33% for all the seven trials appears to be a valid estimate for both zinc acetate and zinc gluconate lozenges.
36); thus, the two salts do not differ significantly. Therefore, the pooled estimate of 33% for all the seven trials appears to be a valid estimate for both zinc acetate and zinc gluconate lozenges.
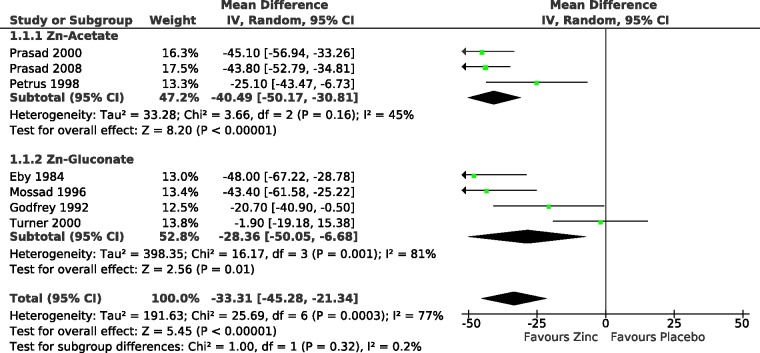
Effect of high-dose zinc lozenges on the duration of the common cold. Trials with zinc acetate are separated from trials with zinc gluconate. The duration of symptoms was transformed to the relative scale, thus the duration in the respective placebo group was given the value of 100%. The estimate of effect over all seven trials indicates a 33% (95% CI: 21% to 45%) reduction in common cold duration. The high level of heterogeneity among all the seven trials (I2 =
= 77%; p
77%; p =
= 0.0003) is explained by the Turner trial. If the Turner trial is excluded, there is no significant heterogeneity over the remaining six trials (I2
0.0003) is explained by the Turner trial. If the Turner trial is excluded, there is no significant heterogeneity over the remaining six trials (I2 =
= 39%; p
39%; p =
= 0.15), see Supplementary file 2. In the forest plots on the right hand side, the vertical line indicates the placebo level. The horizontal lines indicate the 95% CI for the zinc effect and the square in the middle of the horizontal line indicates the point estimate of the effect in the particular trial. The diamond shapes indicate the pooled effects and their 95% CI.
0.15), see Supplementary file 2. In the forest plots on the right hand side, the vertical line indicates the placebo level. The horizontal lines indicate the 95% CI for the zinc effect and the square in the middle of the horizontal line indicates the point estimate of the effect in the particular trial. The diamond shapes indicate the pooled effects and their 95% CI.
Analysis of the dose-dependency between the daily elemental zinc level and the effect of the lozenges is shown in Figure 2. The thick horizontal line indicates the pooled effect and the thick vertical line shows its 95% CI. Five trials used zinc doses from 80 to 92 mg/day and found a mean 33% (95% CI 18% to 48%) reduction in common cold duration. Two trials used substantially higher doses of zinc, 192 and 207
mg/day and found a mean 33% (95% CI 18% to 48%) reduction in common cold duration. Two trials used substantially higher doses of zinc, 192 and 207 mg/day, with a pooled estimate of 35% (95% CI 8% to 61%) reduction in common cold duration. There is no difference in the estimates of effect between the low-dose and high-dose trials, with I2
mg/day, with a pooled estimate of 35% (95% CI 8% to 61%) reduction in common cold duration. There is no difference in the estimates of effect between the low-dose and high-dose trials, with I2 =
= 0% (p
0% (p =
= 0.9) (see Supplementary file 2). The difference between the high dose and the low-dose zinc trials is 2 percentage points (95% CI: −29 to
0.9) (see Supplementary file 2). The difference between the high dose and the low-dose zinc trials is 2 percentage points (95% CI: −29 to +
+ 32). Thus, the pooled estimate of 33% for all the seven trials appears to be a reasonable estimate of the efficacy of zinc lozenges over the dose range that has been tested. Such an effect seems to be reached with doses of 80 to 92
32). Thus, the pooled estimate of 33% for all the seven trials appears to be a reasonable estimate of the efficacy of zinc lozenges over the dose range that has been tested. Such an effect seems to be reached with doses of 80 to 92 mg/day of zinc. So far, there is no evidence that doses higher than 100
mg/day of zinc. So far, there is no evidence that doses higher than 100 mg/day of elemental zinc might lead to greater benefits.
mg/day of elemental zinc might lead to greater benefits.
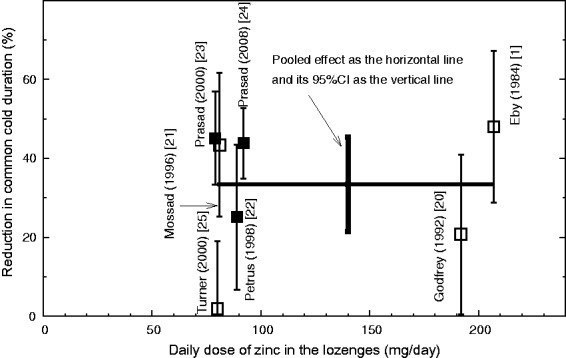
Effect of dosage on the zinc lozenge efficacy on the duration of the common cold. Seven randomised trials are shown in which >75 mg/day of elemental zinc was administered per day. The observed effect in each study is indicated by the square in the middle of the vertical line, and the vertical line indicates the accuracy of the measurement as the 95% CI. The thick horizontal line indicates the pooled effect of the seven trials that were included and the thick vertical line denotes the 95% CI for the pooled effect; on average 33% (95% CI 21% to 45%) shorter colds in the zinc groups. Zinc acetate studies are indicated by filled squares and zinc gluconate studies by open squares.
mg/day of elemental zinc was administered per day. The observed effect in each study is indicated by the square in the middle of the vertical line, and the vertical line indicates the accuracy of the measurement as the 95% CI. The thick horizontal line indicates the pooled effect of the seven trials that were included and the thick vertical line denotes the 95% CI for the pooled effect; on average 33% (95% CI 21% to 45%) shorter colds in the zinc groups. Zinc acetate studies are indicated by filled squares and zinc gluconate studies by open squares.
When all the seven trials are included in the meta-analysis, there is very strong evidence of heterogeneity over the trials with I2 =
= 77% (p
77% (p =
= 0.0003). This high level heterogeneity is accounted for by the Turner trial25 (Figures 1 and 2). If the Turner trial is excluded as an outlier, the level of heterogeneity decreases to I2
0.0003). This high level heterogeneity is accounted for by the Turner trial25 (Figures 1 and 2). If the Turner trial is excluded as an outlier, the level of heterogeneity decreases to I2 =
= 39% (p
39% (p =
= 0.15). When the Turner trial is excluded, the difference between zinc acetate and zinc gluconate trials shrinks to 2 percentage points (95% CI −17 to 21), and the difference between the high-dose and low-dose trials increases to 7 (95% CI −20 to +35) percentage points; neither of these differences is significant. If the Turner trial is excluded, the estimated effect of zinc lozenges based on the remaining six trials increases to 40% (95% CI 31% to 47%) reduction in common cold duration (see Supplementary file 2 for the forest plots).
0.15). When the Turner trial is excluded, the difference between zinc acetate and zinc gluconate trials shrinks to 2 percentage points (95% CI −17 to 21), and the difference between the high-dose and low-dose trials increases to 7 (95% CI −20 to +35) percentage points; neither of these differences is significant. If the Turner trial is excluded, the estimated effect of zinc lozenges based on the remaining six trials increases to 40% (95% CI 31% to 47%) reduction in common cold duration (see Supplementary file 2 for the forest plots).
The dose-dependency in Figure 2 was analysed by the total daily dose of elemental zinc. However, the total daily dose of zinc has two components: (1) the amount of zinc per lozenge and (2) the number of zinc lozenges dissolved in the mouth per day. Both of them are independent variables. Figure 3 shows these two components for the seven trials which were used to calculate the estimated 33% reduction in cold duration. The dose of elemental zinc per lozenge ranged from 9 to 23.7 mg, and the average frequency of lozenge use ranged from 6 to 9.9 times per day.
mg, and the average frequency of lozenge use ranged from 6 to 9.9 times per day.
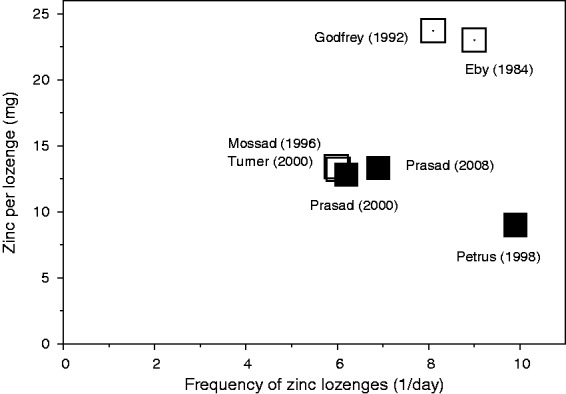
The dose of zinc per lozenge and the number of lozenges per day in the seven trials that were included in the analysis. Zinc acetate lozenges are indicated by filled squares and zinc gluconate lozenges by open squares. The Mossad et al. and the Turner trials both had 13.3 mg zinc per lozenge and six lozenges per day.
mg zinc per lozenge and six lozenges per day.
Discussion
Eby considered the chemical properties of zinc acetate and zinc gluconate and proposed that the acetate might be a better constituent for zinc lozenges than the gluconate.7,8 The first goal of this study was to carry out a meta-analysis to compare the two zinc salts. The comparison of three zinc acetate trials against four zinc gluconate trials did not find a meaningful difference between the two salts in their effects on common cold duration (Figure 1). The second goal of this study was to analyse the dose-dependency of zinc lozenge effect by the total daily dose of elemental zinc. No evidence was found that doses higher than 80–92 mg/day of zinc might cause greater effects (Figure 2). Thus, it seems that the maximal effects of zinc lozenges may be reached by doses of some 80
mg/day of zinc might cause greater effects (Figure 2). Thus, it seems that the maximal effects of zinc lozenges may be reached by doses of some 80 mg/day, if the lozenge composition is optimal, i.e. the lozenge does not contain substances that bind zinc.
mg/day, if the lozenge composition is optimal, i.e. the lozenge does not contain substances that bind zinc.
Zinc ion can form complexes with a number of substances, which has caused substantial problems with the lozenge formulations in a few trials. Farr et al.29 used a lozenge that contained 2% citric acid, which binds zinc ions and very little or no free zinc is released from such lozenges.3,4,6–9 The low-dose (<75 mg/day) zinc lozenges studied by Douglas et al.30 contained tartaric acid and sodium bicarbonate, which bind zinc ions.7,8 The Turner trial25 had two low-dose zinc acetate trial arms. The zinc acetate lozenges contained hydrogenated palm-kernel oil, cotton-seed oil, and soy lecithin, and Eby pointed out that most probably those substances formed insoluble reaction products with zinc at the high preparation temperatures used in the production.31 These concerns were not refuted by Turner. Finally, the first author of the Smith et al. study19 stated in a 1992 paper that the negative findings of the Smith et al. study most probably were explained by the presence of mannitol and sorbitol in the lozenges.20 The negative findings of the above studies were thus explained by flaws in the composition of the zinc lozenges. Evidently, problems in lozenge formulation cannot be compensated for by appropriate methodology at the level of randomisation and blinding. Furthermore, problems in lozenge formulation can lead to false negative conclusions about the potential effects of zinc lozenges but cannot lead to false positive conclusions.
mg/day) zinc lozenges studied by Douglas et al.30 contained tartaric acid and sodium bicarbonate, which bind zinc ions.7,8 The Turner trial25 had two low-dose zinc acetate trial arms. The zinc acetate lozenges contained hydrogenated palm-kernel oil, cotton-seed oil, and soy lecithin, and Eby pointed out that most probably those substances formed insoluble reaction products with zinc at the high preparation temperatures used in the production.31 These concerns were not refuted by Turner. Finally, the first author of the Smith et al. study19 stated in a 1992 paper that the negative findings of the Smith et al. study most probably were explained by the presence of mannitol and sorbitol in the lozenges.20 The negative findings of the above studies were thus explained by flaws in the composition of the zinc lozenges. Evidently, problems in lozenge formulation cannot be compensated for by appropriate methodology at the level of randomisation and blinding. Furthermore, problems in lozenge formulation can lead to false negative conclusions about the potential effects of zinc lozenges but cannot lead to false positive conclusions.
The divergence between the Turner trial25 and the three other zinc gluconate studies1,20,21 is statistically so great that the Turner trial may be considered separately (Figures 1 and and2).2). It is possible that the zinc gluconate lozenges of the Turner trial were non-optimal, though no specific concerns about those lozenges have been expressed. If the Turner trial is excluded from the comparison of zinc acetate against zinc gluconate in Figure 1, the difference between the two salts substantially diminishes. If the Turner trial is excluded from the calculation of overall effect of zinc lozenges, the overall estimate increases from 33% reduction to 40% reduction in common cold duration. In any case, the inclusion or exclusion of the Turner trial does not influence the comparison of the two salts or the analysis of dose-dependency.
The doses of zinc used in the studies included in this meta-analysis were over 75 mg/day, but this dosage level should not be interpreted as the minimal effective dose. In addition to the dosage, the published five low-dose trials have other problems, and their negative findings should not be attributed unambiguously to the nominal zinc dosage per day. As described above, lozenges were not properly formulated in three of the low-dose zinc comparisons,25,30 and it is unlikely that free zinc was released.7,8,31 The fourth trial used a particularly low dose of zinc, 45
mg/day, but this dosage level should not be interpreted as the minimal effective dose. In addition to the dosage, the published five low-dose trials have other problems, and their negative findings should not be attributed unambiguously to the nominal zinc dosage per day. As described above, lozenges were not properly formulated in three of the low-dose zinc comparisons,25,30 and it is unlikely that free zinc was released.7,8,31 The fourth trial used a particularly low dose of zinc, 45 mg/day.32 The fifth low-dose trial used children as subjects.33 Compliance may be a concern in studies that recruit children because they can be less likely than adults to follow instructions to slowly dissolve a lozenge in the mouth. Thus, the five low-dose trials are not valid for comparing the efficacy of, say, 50–70
mg/day.32 The fifth low-dose trial used children as subjects.33 Compliance may be a concern in studies that recruit children because they can be less likely than adults to follow instructions to slowly dissolve a lozenge in the mouth. Thus, the five low-dose trials are not valid for comparing the efficacy of, say, 50–70 mg/day of zinc against 80–90
mg/day of zinc against 80–90 mg/day of zinc for treating colds in adults. Estimation of the dose-dependency in the region below 80
mg/day of zinc for treating colds in adults. Estimation of the dose-dependency in the region below 80 mg/day thus requires further research.
mg/day thus requires further research.
The dose-response analysis in Figure 2 is based on the total daily dose of zinc, which is a simplification. The daily dosage of elemental zinc is determined by the amount of zinc per lozenge and the number of lozenges used per day (Figure 3). Mossad et al.21 and Prasad et al.23 administered lozenges only six times per day and used 13 mg of zinc per lozenge and both found a 45% reduction in common cold duration. There are no indications that a higher frequency or a higher dose of zinc per lozenge might increase the effect over that reached by those two studies (Figure 3). It is not clear how the efficacy of zinc depends on these two variables; however, it seems unlikely that dissolving a lozenge that contains 80
mg of zinc per lozenge and both found a 45% reduction in common cold duration. There are no indications that a higher frequency or a higher dose of zinc per lozenge might increase the effect over that reached by those two studies (Figure 3). It is not clear how the efficacy of zinc depends on these two variables; however, it seems unlikely that dissolving a lozenge that contains 80 mg of zinc in the mouth once per day equals dissolving eight lozenges that each contain 10
mg of zinc in the mouth once per day equals dissolving eight lozenges that each contain 10 mg of zinc over the entire day time, even though the nominal daily dose is the same. Evidently, the optimal lozenge composition and dosage scheme should be investigated.
mg of zinc over the entire day time, even though the nominal daily dose is the same. Evidently, the optimal lozenge composition and dosage scheme should be investigated.
This analysis was based on the calculation of the relative effect, percentages, of zinc lozenges on the common cold duration because the relative effect partly adjusts for variations between the patient groups and outcome definitions in the trials. Nevertheless, the absolute effect on days is also an important measure of the zinc lozenge effect. On average, the three zinc acetate trials22–24 found that zinc lozenges shortened colds by 2.7 days from the mean duration of 7.3 days in the placebo groups.11 In the zinc gluconate trials, zinc lozenges shortened colds by 3.6 days from 7.5 days in the placebo group,1 by 4.0 days from 9.2 days,21 by 1.3 days from 6.1 days,20 but Turner25 did not find any effect of zinc.
Farr and Gwaltney34 speculated that the benefit reported by Eby et al.1 might have been explained by the bad taste of the zinc lozenges. However, they did not provide evidence that bad taste might shorten the duration of colds. The early findings of Eby are consistent with a number of later trials, and bad taste does not seem a reasonable explanation for the benefits. For example, none of the three high-dose zinc acetate lozenge trials reported bad taste to be a problem, there was no substantial difference between the zinc and placebo groups in the recorded adverse effects, and only a few drop-outs occurred.22–24 In the most recent trial by Prasad et al.,24 a few participants identified the lozenges, but when the analysis was restricted to those who remained blinded at the end of the study, the efficacy of zinc lozenges was no less.
Zinc doses of 100 to 150 mg/day have been administered for certain patient groups for months with few adverse effects.2,10,35,36 Therefore, a zinc dose of some 80
mg/day have been administered for certain patient groups for months with few adverse effects.2,10,35,36 Therefore, a zinc dose of some 80 mg/day for 1–2 weeks starting at the early symptoms of the common cold is unlikely to cause long-term adverse effects. Nevertheless, even though there is strong evidence that properly formulated zinc lozenges can shorten the duration of colds, the majority of zinc lozenges in the market seem to have either too low doses of zinc or they contain substances such as citric acid that bind zinc.8 Therefore, the findings of this study are not directly applicable to the wide variety of formulations of zinc lozenges on the market.
mg/day for 1–2 weeks starting at the early symptoms of the common cold is unlikely to cause long-term adverse effects. Nevertheless, even though there is strong evidence that properly formulated zinc lozenges can shorten the duration of colds, the majority of zinc lozenges in the market seem to have either too low doses of zinc or they contain substances such as citric acid that bind zinc.8 Therefore, the findings of this study are not directly applicable to the wide variety of formulations of zinc lozenges on the market.
In conclusion, the trials included in this study were of high methodological quality: randomised, double-blind, and placebo-controlled. They were carried out over three decades by six different research groups. The evidence is thus very strong that zinc lozenges may shorten the duration of colds by approximately 33%. The optimal composition of zinc lozenges should be investigated in addition to the optimum frequency of their administration. Nevertheless, the current evidence of efficacy for zinc lozenges, in particular zinc acetate lozenges, is so strong that common cold patients may be encouraged to try them for treating their colds.
Supplementary Material
Declarations
Competing interests
None declared.
Funding
None declared.
Ethics approval
This is a secondary analysis of published controlled trials: ethical approval not needed.
Guarantor
HH.
Contributorship
Sole authorship.
Acknowledgements
The author is grateful to Dr Petrus for providing the data set for the 1998 study.22
Provenance
Not commissioned; peer-reviewed by Ricardo José.
References
 mg zinc sulfate twice daily in the treatment of rosacea. Int J Dermatol
2012; 51: 459–462. DOI: 10.1111/j.1365-4632.2011.05353.x. [Abstract] [Google Scholar]
mg zinc sulfate twice daily in the treatment of rosacea. Int J Dermatol
2012; 51: 459–462. DOI: 10.1111/j.1365-4632.2011.05353.x. [Abstract] [Google Scholar]Articles from JRSM Open are provided here courtesy of SAGE Publications
Full text links
Read article at publisher's site: https://doi.org/10.1177/2054270417694291
Read article for free, from open access legal sources, via Unpaywall:
https://journals.sagepub.com/doi/pdf/10.1177/2054270417694291
Citations & impact
Impact metrics
Article citations
Nutrition and Golf Performance: A Systematic Scoping Review.
Sports Med, 30 Sep 2024
Cited by: 0 articles | PMID: 39347918
Review
Shortcomings in the Cochrane review on zinc for the common cold (2024).
Front Med (Lausanne), 11:1470004, 16 Oct 2024
Cited by: 0 articles | PMID: 39478818 | PMCID: PMC11521859
Zinc for prevention and treatment of the common cold.
Cochrane Database Syst Rev, 5:CD014914, 09 May 2024
Cited by: 3 articles | PMID: 38719213
Review
Explorations on the antiviral potential of zinc and magnesium salts against chikungunya virus: implications for therapeutics.
Front Cell Infect Microbiol, 14:1335189, 04 Jun 2024
Cited by: 0 articles | PMID: 38895735 | PMCID: PMC11183322
Cellular zinc metabolism and zinc signaling: from biological functions to diseases and therapeutic targets.
Signal Transduct Target Ther, 9(1):6, 03 Jan 2024
Cited by: 15 articles | PMID: 38169461 | PMCID: PMC10761908
Review Free full text in Europe PMC
Go to all (54) article citations
Other citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Zinc acetate lozenges for treating the common cold: an individual patient data meta-analysis.
Br J Clin Pharmacol, 82(5):1393-1398, 28 Jul 2016
Cited by: 16 articles | PMID: 27378206 | PMCID: PMC5061795
Zinc lozenges may shorten the duration of colds: a systematic review.
Open Respir Med J, 5:51-58, 23 Jun 2011
Cited by: 45 articles | PMID: 21769305 | PMCID: PMC3136969
Zinc Acetate Lozenges May Improve the Recovery Rate of Common Cold Patients: An Individual Patient Data Meta-Analysis.
Open Forum Infect Dis, 4(2):ofx059, 03 Apr 2017
Cited by: 20 articles | PMID: 28480298 | PMCID: PMC5410113
Zinc lozenges as cure for the common cold--a review and hypothesis.
Med Hypotheses, 74(3):482-492, 10 Nov 2009
Cited by: 34 articles | PMID: 19906491 | PMCID: PMC7173295
Review Free full text in Europe PMC






