Abstract
Free full text

A Phase I/II Multicenter Study of Single-Agent Foretinib as First-Line Therapy in Patients with Advanced Hepatocellular Carcinoma
Abstract
Purpose
This phase I/II single-arm study evaluated the safety, pharmacokinetics, pharmacodynamics, and activity of foretinib, an oral multikinase inhibitor of MET, ROS, RON, AXL, TIE-2, and VEGFR2, in the first-line setting in advanced hepatocellular carcinoma (HCC) patients.
Methods
In the phase I part, advanced HCC patients were dose-escalated on foretinib (30–60 mg) once daily (QD) using the standard 3+3 design. Once the maximum tolerated dose (MTD) was determined, an additional 32 patients were dosed at the MTD in the phase II expansion cohort for assessment of efficacy and safety. Exploratory analyses were conducted to assess potential biomarkers that might correlate with clinical efficacy and survival.
Results
The MTD of foretinib was established as 30 mg QD. The most frequent adverse events were hypertension, decreased appetite, ascites, and pyrexia. When dosed at 30 mg QD in the first-line setting, foretinib demonstrated promising anti-tumor activity. According to the modified Response Evaluation Criteria in Solid Tumors (mRECIST), the objective response rate was 22.9%, the disease stabilization rate 82.9% and the median duration of response 7.6 months. The median time to progression was 4.2 months and the median overall survival (OS) was 15.7 months. Fifteen candidate biomarkers whose levels in the circulation were significantly altered in response to foretinib treatment were elucidated. Multivariate analyses identified IL6 and IL8 as independent predictors of OS.
Conclusion
Foretinib demonstrated promising anti-tumor activity and good tolerability in the first-line setting in Asian advanced HCC patients. Baseline plasma levels of IL6 or IL8 might predict the response to foretinib.
Introduction
Advanced hepatocellular carcinoma (HCC) has a poor prognosis, and systemic therapy with cytotoxic agents demonstrates no survival benefit [1]. Two phase III randomized trials conducted in Western [2] and Asian [3] populations with advanced HCC demonstrated improved survival with sorafenib monotherapy, which led to regulatory approval for the use of sorafenib in advanced HCC. Nevertheless, the survival benefit associated with sorafenib is generally modest.
MET is a receptor tyrosine kinase (RTK) that is widely expressed in epithelial and endothelial cells. Its cognate ligand, hepatocyte growth factor (HGF), is secreted primarily by cells of mesenchymal origin. HGF/MET mitogenic signalling is fundamentally important in hepatic development and biology [4]. Notably, MET has also been implicated as a mediator of many aspects of tumor pathobiology, including tumor survival, growth, angiogenesis, invasion, and dissemination [5, 6]. Additionally, amplification, activating mutations and overexpression of the MET gene have been associated with poor prognosis and a metastatic phenotype in various human cancers [6]. The reported incidence of MET gene amplification in HCC is variable: 1.7% of 350 samples assessed using fluorescence in situ hybridization (FISH) or chromogenic in situ hybridization (CISH) [7]; 0.9% of 231 samples as assessed by single nucleotide polymorphism (SNP) array [8]; 3% of 440 samples assessed by SNP array (TCGA provisional HCC data set); and 24% of 255 samples by SNP array [9]. MET mutation frequency is relatively low (0.9% of 440 samples in TCGA provisional HCC data set), but MET overexpression is more common: 7% of 440 in TCGA provisional HCC data set and 28% of 237 samples [9]. MET may thus be an attractive molecular target for HCC therapy.
Cabozantinib is an inhibitor of MET and vascular endothelial growth factor receptor (VEGFR)–2 that is currently in development for the treatment of HCC. In a phase II trial, Verslype and colleagues [10] reported that cabozantinib had preliminary activity in sorafenib-refractory advanced HCC. A randomized phase III study of cabozantinib vs. placebo is now recruiting HCC patients with prior sorafenib therapy (NCT01908426). Tivantinib [11], an agent believed to act in part through MET inhibition, demonstrated encouraging activity in a phase II setting in patients with advanced HCC tumors that displayed MET overexpression who had progressed on or were unable to tolerate first-line systemic therapy [12]. Although these prior studies suggest that MET inhibitors may provide clinical benefit in advanced HCC, they were conducted in the second-line setting, and the impact of MET inhibition in patients with advanced HCC without prior sorafenib treatment remains unevaluated.
Foretinib (GSK1363089) is an oral multikinase inhibitor of MET, ROS, RON, AXL, TIE-2, and VEGFR2 that has demonstrated efficacy and acceptable tolerability in papillary renal cancer [13]. The objective of this phase I/II single-arm, multicenter study was to identify the maximum tolerated dose (MTD) of foretinib in Asian patients with advanced HCC and to assess its clinical activity, safety and pharmacokinetics (PK) in the first-line setting. Importantly, both pharmacogenomics and biomarkers potentially correlated with clinical efficacy and survival were explored.
Materials and Methods
Study Design
This was a single-arm, phase 1/2 study performed at seven centers in Asia (Hong Kong, Taiwan and Thailand). The study protocol was approved by the institutional review boards or human research ethics committees of participating centers and complied with country-specific regulatory requirements. The study was performed in accordance with both the Declaration of Helsinki and the International Conference of Harmonisation Good Clinical Practice. All patients provided informed consent before treatment was started. The trial was registered at ClinicalTrials.gov (NCT00920192).
The aim of the phase I dose-escalation component of the study was to determine the MTD and safety of foretinib. It was then further evaluated for efficacy, tolerability, pharmacokinetics, pharmacogenomic and potential biomarkers in a phase II dose expansion cohort.
Patient Eligibility
Patients aged at least 18 years with advanced (unresectable or metastatic) HCC diagnosed according to current guidelines [14, 15] with measurable disease according to RECIST v1.0 and/or mRECIST [16]. Prior local-regional therapies were allowed, provided that 4 weeks had elapsed since surgery or radiotherapy, 6 weeks since prior chemoembolisation, and 8 weeks since prior radiofrequency ablation. If a target lesion was within the field of prior local therapy, an increase in size of ≥25% in that lesion had to be observed following local therapy. Patients were also required to have at most a Child-Pugh A classification, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, no signs of poorly controlled portal hypertension, and a life expectancy of ≥12 weeks. Adequate hematologic (absolute neutrophil count ≥1.5 × 109/L, hemoglobin ≥9 g/dL, platelets ≥80 × 109/L, and PT/PTT/INR ≤1.3 × upper limit of normal; ULN), hepatic (albumin ≥2.8 g/dL, serum bilirubin ≤2.0 mg/dL or ≤2 × ULN, and aspartate aminotransferase and alanine aminotransferase ≤5.0 × ULN), renal (urine-protein-creatinine ratio <1 from a urine sample or <1.0 g of protein determined by 24-hour urine protein analysis and calculated creatinine clearance ≥50 mL/min), and adrenal (cortisol level after ACTH injection in ACTH stimulation test at or above the level required by institutional guidelines for the ACTH stimulation test or adequate cortisol levels according to the package insert of the specific ACTH-stimulation test kit used) function was also required.
The main exclusion criteria included history of main portal-vein thrombosis; poorly controlled systemic hypertension; history of cerebrovascular accident, including transient ischemic attack, pulmonary embolism, or untreated deep venous thrombosis within the past 6 months; recent haemoptysis; and history of oesophageal or gastric variceal bleeding. No prior sorafenib, investigational tyrosine kinase inhibitor, or other systemic therapies for advanced HCC were allowed.
Phase I Dose-escalation
In the dose-escalation phase to determine the MTD in advanced HCC, three doses were initially planned: foretinib 30 mg once daily (QD) (50% of MTD in other solid tumors), 45 mg QD, and 60 mg QD. Patients received increasing doses of foretinib in a standard 3+3 design with at least six patients treated at the MTD.
Dose-limiting toxicities (DLTs) were defined as 1) any grade 3 or 4 clinically significant non-haematological toxicity except alopecia; grade 3 nausea, vomiting, or diarrhea for which adequate supportive therapy was not instituted; grade 3 hypertension despite optimalantihypertensive medication(s); grade 3 proteinuria without associated hypertension and/or renal impairment that improved to grade 2 or lower upon interruption of foretinib; or liver toxicity for which clinical and radiologic criteria supported either progressive disease or viral reactivation as the cause of increased hepatic dysfunction; 2) grade 3 neutropenia with a duration of at least 7 days or the occurrence of neutropenic fever; 3) grade 4 neutropenia; 4) grade 3 or 4 thrombocytopenia. The MTD was defined as the highest daily dose of foretinib at which no more than one of six patients experienced DLTs.
Phase II Expansion Cohort
Once the MTD was determined, an additional 32 patients were recruited to receive foretinib at the MTD in the phase II expansion cohort to determine the anti-tumor activity (objective response rate, ORR, disease stabilization rate, duration of response, and time to progression, TTP) by modified Response Evaluation Criteria in Solid Tumors (mRECIST), overall survival (OS), effect on alpha fetoprotein (AFP) levels, safety and tolerability, and PK profile of foretinib. Disease stabilization rate was defined as the proportion of patients achieving best overall response of CR or PR or stable disease (SD) per mRECIST. SD was defined as neither sufficient shrinkage to qualify for PR nor a sufficient increase to qualify for progressive disease.
Disease Evaluation and Safety Assessment
Tumor assessments were performed at baseline, every subsequent 6 weeks and at the time of progression. Patients were assessed according to mRECIST criteria to align with the guidelines of the American Association for the Study of Liver Disease (AASLD) [15, 16], with RECIST criteria serving as an additional form of analysis.
Stable disease was considered the best response, if it was demonstrated for ≥12 weeks after baseline. Best overall response was considered not evaluable when PD had not been documented, and a best overall response of CR, PR, or SD could not be established.
Safety was assessed through standard clinical and laboratory tests and reports of adverse events (AEs) and serious AEs (SAEs) were documented. AEs were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) v3.0.
Pharmacokinetics
Blood samples for PK analysis were obtained before and after dosing (within 60 minutes before administration, as well as 1, 2, 3, 4, 6, 8, and 24 hours post dose), on days 1 and 15, in both the dose-escalation and expansion cohort phases. PK endpoints included maximum plasma concentration (Cmax), time of maximum concentration (Tmax), area under the concentration-time curve from time 0 (predose) to 24 hours (AUC0-24), area under the concentration-time curve extrapolated to infinity (AUC0-∞), elimination half-life (T½), and plasma concentration 24 hours after dose administration (C24). PK parameters were calculated from plasma concentrations of foretinib by noncompartmental analysis using WinNonlin software version 5.1.1 (Pharsight Corporation, Mountain View, CA, USA) and SAS® software version 9.1.3 (SAS Institute Inc., Cary, NC, USA).
Pharmacogenomics
Venous blood was collected from consenting patients and DNA was extracted using Qiagen Autopure automated DNA extraction by Covance (Indianapolis, IN, USA) and genotyped for 20 single nucleotide polymorphisms (SNPs) with potential functional consequence from 14 candidate genes. Genotyping was achieved using Sanger sequencing or custom TaqMan® SNP genotyping assays (Applied Biosystems, Foster City, CA, USA) at GlaxoSmithKline or via TaqMan®Assay on Demand genotyping assays (Applied Biosystems) by Gen-Probe (Wythenshawe, Manchester, UK).
Exploratory Biomarkers
Blood samples were collected at baseline (day 1) and post-treatment on days 8, 15, and 22. The following 29 circulating markers were measured using the Searchlight platform (Aushon BioSystems): interleukin-6 (IL6), placental growth factor (PlGF), thrombomodulin (TM), transforming growth factor beta 1 (TGFB1), hepatocyte growth factor (HGF), Fas ligand (FASL), granulocyte colony–stimulating factor (GCSF), IL8, TNF-related apoptosis-inducing ligand (TRAIL), angiopoietin 2 (ANG2), fibroblast growth factor 2 (FGFb), stem cell factor (SCF), vascular endothelial growth factor (VEGF), bone morphogenetic protein 9 (BMP9), osteopontin (OPN), E-cadherin, epidermal growth factor (EGF), E-selectin, insulin-like growth factor–binding protein 1 (IGFBP1), leptin, thrombospondin 2 (TSP2), vascular endothelial growth factor 2 (VEGFR2), insulin-like growth factor–binding protein 3 (IGFBP3), matrix metalloproteinase 9 (MMP9), tissue inhibitor of metalloproteinase 2 (TIMP2), vascular cell adhesion molecule 1 (VCAM1), clusterin, and fibronectin. Levels of circulating sMET and HGF were determined using electrochemiluminescent two-site immunoassays, as described previously [13].
Statistical Analysis
The expansion cohort phase was to accrue 33 patients at the identified MTD. No formal statistical hypothesis was tested, because there were no published tumor response data in the advanced disease setting applying prospective evaluation according to mRECIST upon which to base a hypothesis. Instead, an estimation approach was used with the point estimate and corresponding 95% exact CI for all efficacy variables. In addition, the ORR was summarized for all patients enrolled in the dose-escalation phase and for each cohort separately. Survival analysis was computed by the Kaplan-Meier method. TTP was calculated from the date of commencement of study drugs to the date of documented progression or death. All statistical analysis was performed using SAS version 8.2 (SAS Institute Inc.).
For the pharmacogenomic analyses, the association with ORR was evaluated using Fisher’s exact test; the association with TTP and OS was tested using time-to-event (progression) models and the Score (log-rank) test. Because of the limited sample size, genetic analyses were performed without adjustment for baseline demographics or potential covariates, and p values were not adjusted for multiple testing.
In exploratory biomarker analyses, the correlation of baseline (day 1) circulating biomarker levels and tumor burden—as measured by the sum of the longest diameter (SLD)—was assessed using the Spearman rank-correlation test. The association between baseline circulating biomarker levels and clinical response was assessed using univariate and multivariate (covariate-adjusted) logistic regression analysis. The association between baseline circulating biomarker levels and survival (both TTP and OS) was assessed using univariate and multivariate (covariate-adjusted) proportional hazard regression analysis. Multivariate analyses were conducted with the following covariates: ECOG performance status, hepatitis status, cirrhotic status, sex, age, and baseline tumor burden.
Results
Between August 2009 and August 2012, 45 patients with advanced HCC were enrolled. Thirty-nine patients received foretinib 30 mg QD (dose-escalation phase, n=7; expansion phase, n=32), and six patients received foretinib 45 mg QD in the dose-escalation phase.
Dose-escalation Phase
Thirteen patients were enrolled in the dose-escalation phase. At the starting dose of foretinib 30 mg QD, three patients were initially enrolled and no DLTs were reported. The dose was subsequently escalated to 45 mg QD and three patients were recruited, with one experiencing a DLT (grade 3 proteinuria); three additional patients were recruited at this dose, and an additional DLT was observed (grade 3 renal impairment and hyperkalaemia). Four additional patients were then dosed at 30 mg QD: one patient was removed from the study due to ineligibility and was not evaluable for DLTs, whereas the remaining three patients did not report DLTs. Thus, the MTD of foretinib in Asian patients with advanced HCC was established as 30 mg QD. This dose was used in the expansion phase.
Patient Demographics
Demographics and baseline characteristics are described in Table 1. Most enrolled patients had Child Pugh A cirrhosis. Only 2 (5.1%) and 1 (16.7%) enrolled patients had no underlying cirrhosis at baseline in the 30 mg QD and 45 mg QD cohorts, respectively.
Table 1
Patient demographics and baseline characteristics (N=45).
| Characteristic | 30 mg (n=39) | 45 mg (n=6) |
|---|---|---|
| Age, median (range), y | 56.7 (31–82) | 60.2 (50–68) |
| Sex, n (%) | ||
 Female Female | 8 (20.5) | 2 (33.3) |
 Male Male | 31 (79.5) | 4 (66.7) |
| ECOG performance status at baseline, n (%) | ||
 0 0 | 34 (87.2) | 5 (83.3) |
 1 1 | 5 (12.8) | 1 (16.7) |
| Child-Pugh status, n (%) | ||
 A A | 37 (94.9) | 5 (83.3) |
 B B | 0 | 0 |
 C C | 0 | 0 |
| Hepatitis serology, n (%) | ||
 Hepatitis B surface antigen positive Hepatitis B surface antigen positive | 20 (51.3) | 6 (100) |
  On lamivudine On lamivudine | 7 (17.9) | 3 (50) |
  On entecavir On entecavir | 10 (25.6) | 2 (33.3) |
  On telbivudine On telbivudine | 1 (2.6) | 0 |
  On lamivudine + adefovir On lamivudine + adefovir | 1 (2.6) | 1 (16.7) |
 Anti-hepatitis C antibody positive Anti-hepatitis C antibody positive | 9 (23.1) | 0 |
| Baseline AFP level, n (%) | ||
 <200 ng/mL <200 ng/mL | 22 (56.4) | 4 (66.7) |
 ≥200 ng/mL ≥200 ng/mL | 17 (43.6) | 2 (33.3) |
| EASL diagnostic criteria, n (%) | ||
 Cytohistological criteria Cytohistological criteria | 14 (35.9) | 3 (50) |
 Noninvasive criteria Noninvasive criteria | 25 (64.1) | 3 (50) |
| BCLC stage at screening, n (%) | ||
 A (Early) A (Early) | 0(0) | 0(0) |
 B (Intermediate) B (Intermediate) | 4 (10.3) | 1 (16.7) |
 C (Advanced) C (Advanced) | 35 (89.7) | 5 (83.3) |
| Receiving at least 1 course of prior local anticancer therapy, n (%) | 12 (30.8) | 5 (83.3) |
 Local ablative therapy Local ablative therapy | 12 (30.8) | 5 (83.3) |
  1–3 courses of chemoembolisation/trans-catheter therapy 1–3 courses of chemoembolisation/trans-catheter therapy | 7 (17.9) | 2 (33.3) |
  >3 courses of chemoembolisation/trans-catheter therapy >3 courses of chemoembolisation/trans-catheter therapy | 5 (12.8) | 3 (50) |
| Prior radiotherapy, n (%) | 1 (2.6) | 1 (16.7) |
AFP=alpha fetoprotein. BCLC=Barcelona Clinic Liver Cancer. EASL=European Association for the Study of the Liver. ECOG=Eastern Cooperative Oncology Group.
Safety
In addition to two DLTs, two other patients who received 45 mg foretinib had dose reductions. In contrast, no patients dosed with 30 mg foretinib had dose reductions due to an AE. These observations contributed to the determination of the MTD for foretinib at 30 mg QD.
AEs for patients dosed with 30 mg foretinib are summarized in Table 2. Twenty-two patients treated at 30 mg experienced an SAE; seven (17.9%) patients had treatment-related grade 3 AEs, and one (2.6%) patient had a treatment-related grade 4 AE (Table 2). Eleven (28.2%) patients dosed with 30 mg foretinib had dose interruptions due to AEs: increased ALT (three patients), thrombocytopenia, urinary tract infection, and hepatic encephalopathy (two events each), and ascites, gingival bleeding, peritoneal haemorrhage, pyrexia, and hyponatremia (one event each). Three (7.7%) patients experienced an AE leading to study treatment discontinuation (one patient each due to increased ALT, ascites, and decreased appetite).
Table 2
Most frequent adverse events experienced by at least 10% of patients dosed with 30 mg foretinib.
| Foretinib 30 mg QD (N=39) | |||
|---|---|---|---|
| All Patients, n (%) | Grade 3, n (%) | Grade 4, n (%) | |
| Total number of AEs | 341 | — | — |
| Patients with any AE | 39 (100) | 22 (55·4) | 2 (5·1) |
 Hypertension* Hypertension* | 17 (43·6) | 5 (12·8) | 0 |
 Decreased appetite* Decreased appetite* | 11 (28·2) | 0 | 0 |
 Ascites Ascites | 10 (25·6) | 3 (7·7) | 0 |
 Pyrexia Pyrexia | 10 (25·6) | 1 (2.6) | 0 |
 ALT increased* ALT increased* | 9 (23·1) | 5 (12·8) | 0 |
 Constipation Constipation | 8 (25·0) | 0 | 0 |
 Oedema peripheral Oedema peripheral | 8 (25·0) | 0 | 1 (2.6) |
 Hypoalbuminaemia Hypoalbuminaemia | 7 (17·9) | 5 (12.8) | 0 |
 Cough Cough | 6 (15·4) | 0 | 0 |
 Diarrhoea* Diarrhoea* | 6 (15·4) | 0 | 0 |
 Insomnia Insomnia | 6 (15·4) | 1 (2.6) | 0 |
 Abdominal pain Abdominal pain | 5 (12·8) | 3 (7·7) | 0 |
 Hyperbilirubinaemia Hyperbilirubinaemia | 5 (12·8) | 3 (7·7) | 0 |
 Urinary tract infection Urinary tract infection | 5 (12·8) | 1 (2.6) | 0 |
 Abdominal pain upper Abdominal pain upper | 4 (10·3) | 1 (2.6) | 0 |
 Dyspnoea Dyspnoea | 4 (10·3) | 2 (5·1) | 0 |
 Fatigue* Fatigue* | 4 (10·3) | 0 | 0 |
 Haemoptysis Haemoptysis | 4 (10·3) | 0 | 0 |
 Hepatic encephalopathy Hepatic encephalopathy | 4 (10·3) | 2 (5·1) | 2 (5·1) |
 Hypoglycaemia Hypoglycaemia | 4 (10·3) | 0 | 0 |
 Hyponatraemia Hyponatraemia | 4 (10·3) | 4 (10·3) | 0 |
 Palmar-plantar erythrodysaesthesia syndrome Palmar-plantar erythrodysaesthesia syndrome | 4 (10·3) | 0 | 0 |
 Vomiting Vomiting | 4 (10·3) | 0 | 0 |
AE=adverse event. ALT=alanine aminotransferase. QD=once daily. AEs were coded using MeDRA v11·0 or later. The incidence of the following pairs of preferred terms cannot be summed because at least one patient reported at least one of each event: cachexia or decreased appetite, hyperbilirubinaemia or jaundice, and abdominal pain or abdominal pain upper. Asterisk (*) indicates foretinib treatment-related adverse events.
SAEs reported by more than one patient were hepatic encephalopathy (four patients; 10.3%, including two patients who experienced grade 4 hepatic encephalopathy) and ascites (three patients; 7.7%). Most other SAEs were reported by only a single patient, apart from abdominal pain, increased alanine aminotransferase (ALT), decreased appetite, hyponatremia, and haemoptysis (each reported by two patients; 5.1%).
During the study among patients treated with 30 mg foretinib, there were three fatal events: haemoptysis (patient was no longer on foretinib and was receiving sorafenib and radiotherapy), possible brain infarction, and cardiopulmonary arrest in relation to intubation for sepsis and associated septic shock; none of these events were considered related to study treatment.
Efficacy and Survival
Thirty-five patients treated with 30 mg foretinib were evaluable for efficacy using mRECIST. The ORR was 22.9% (95% CI 10.4–40.1), and eight patients achieved a PR (Figure 1; Table 3). For three of the 35 subjects meeting the clinical criteria for inclusion in the mRECIST evaluable population, a mRECIST radiological assessment could not be made, reducing the number of subjects in the waterfall plot (Fig. 1) to 32. The disease stabilization rate (defined as the proportion of patients achieving best overall response of CR or PR or SD per mRECIST, where SD was defined as neither sufficient shrinkage to qualify for PR nor a sufficient increase to qualify for progressive disease) was 82.9% (95% CI 66.4–93.4); and the median duration of response was 7.6 months (95% CI 5.32–not available). A lower response rate (7.9%; N=38) was observed when evaluated according to RECIST (Table 3). The median TTP was 4.24 months (95% CI 2.79–9.59).

Maximum percentage change from baseline in mRECIST tumor measurement for patients receiving 30 mg foretinib. mRECIST=modified Response Evaluation Criteria in Solid Tumors; PD=progressive disease; PR=partial response; QD=once daily; SD=stable disease. PR, PD, and SD are best overall response. Asterisks indicate the four patients who were still on treatment at the time of the analysis (August 2012).
Table 3
Tumor response in patients receiving foretinib 30 mg QD.
| mRECIST Population (n=35) | RECIST Population (n=38) | |
|---|---|---|
| Criteria applied for assessing tumor response | mRECIST | RECIST |
| Patients achieving best overall response, n (%) | ||
 Complete response Complete response | 0 | 0 |
 Partial response Partial response | 8 (22·9) | 3 (7.9) |
 Stable disease Stable disease | 21 (60·0) | 21 (55.3) |
 Disease progression Disease progression | 6 (17·1) | 14 (36.8) |
| Objective response rate | ||
 n (%) n (%) | 8 (22·9) | 3 (7·9) |
 95% CI* 95% CI* | 10·4–40·1 | 1·7–21·4 |
| Disease stabilization rate | ||
 n (%) n (%) | 29 (82·9) | 24 (63·2) |
 95% CI* 95% CI* | 66·4–93·4 | 46·0–78·2 |
| Patients with baseline AFP ≥200 ng/mL, n | 15 | — |
| Patients achieving ≥50% reduction from baseline AFP, n (%) | 7 (46·7) | — |
AFP=alpha fetoprotein. mRECIST=modified Response Evaluation Criteria in Solid Tumors. QD=once daily. The Evaluable Population included all patients who received at least one dose of study treatment, who met all eligibility criteria and completed at least one treatment period (3 weeks), and underwent at least one post-baseline radiological evaluation of disease (ie, baseline and on-study disease assessment). The mRECIST-evaluable population excluded patients from the evaluable population who discontinued treatment due to disease progression according to RECIST but would not have discontinued treatment had mRECIST been applied.
Note: Denominators for percentages are N, the total number of patients. Best overall response was assessed by the central reader per mRECIST. Objective response rate was defined as the proportion of patients achieving best overall response of complete response (CR) or partial response (PR) across all evaluations. Disease stabilization rate was defined as the proportion of patients achieving best overall response of CR or PR or stable disease (SD) per mRECIST. SD was defined as neither sufficient shrinkage to qualify for PR nor a sufficient increase to qualify for progressive disease.
Overall, 46.7% (95% CI 21.3–73.4; N=35) of the patients dosed at 30 mg and with a baseline alpha fetoprotein (AFP) level ≥ 200 ng/mL had a 50% decrease from baseline at at least one time point during foretinib treatment. Three patients received foretinib (30 mg) for more than 2 years, two of whom received drug for more than 3.7 years. A Kaplan-Meier curve of all enrolled patients treated at the MTD (N=39) revealed that the median OS was 15.7 months (95% CI 7.9–not available).
Pharmacokinetics and Pharmacogenomics
PK parameters obtained from both phases of the study are summarized in Supplementary Table 1. Foretinib oral clearance (CL/F) on day 15 was 50% higher with the 45-mg dose compared with the 30-mg dose, but this finding should be interpreted with caution due to the small sample size (N=6) and between-subject variability (CV% 40%–60%). During the expansion cohort phase (N=31 at the 30-mg dose), median Tmax was 3 hours on both days 1 and 15 and the mean T1/2 on day 1 was 38 hours at the 30-mg dose, consistent with previous data [17]. During the dose-escalation phase (N=6 at both the 30- and 45-mg doses), the time of maximum concentration (Tmax) of foretinib ranged from 3 to 3.5 hours. Results from the dose-proportionality assessment suggested that foretinib exposures appeared to increase with increasing dose from 30 to 45 mg on day 1, whereas on day 15 there was little increase in exposures, suggesting dose proportionality was not achieved.
Thirty-one patients treated at the MTD of 30 mg QD provided consent and a sample for pharmacogenomic research. No statistically significant associations were detected between ORR and any of the genetic variants or haplotypes evaluated. The CAT haplotype (a unique combination of the C, A, and T alleles of SNPs rs2307424, rs2307418, and rs4073054, respectively) in NR1I3/CAR (nuclear receptor subfamily 1, group I, member 3/ Constitutive Androstane Receptor) was significantly associated with TTP in foretinib-treated patients. The presence of two copies of CAT in patients receiving foretinib was associated with inferior TTP (median TTP: ≈2 months) compared with patients with one or no copy (median TTP: ≈6 months; p=0·024; Supplementary Figure 1). None of the genetic variants or combinations of variants (haplotypes) were significantly associated with OS (p>0·05), although there was a non-significant trend toward inferior OS with the CAT haplotype. NR1I3/CAR, a nuclear receptor, regulates the expression of CYP3A4 (cytochrome P450, subfamily IIIA, polypeptide 4) and ABCB1, which code for proteins responsible for foretinib metabolism and efflux, respectively.
Exploratory Biomarker Analyses
Thirty-eight patients had samples available for biomarker analysis; they all received foretinib at 30 mg, had pharmacodynamic data available at baseline and for at least one post-baseline time point, and received at least 75% of the planned doses up to the time of the last pharmacodynamics sample. There was a significant change from baseline at one or more post-baseline time points in 15 of 30 biomarkers analysed (all p<0.01; Supplementary Table 2). Additionally, five biomarkers (ANG2, IGFBP1, IL8, OPN and TSP2) had a statistically significant positive association with baseline tumor burden (Table 4). No significant correlations were observed between baseline tumor burden and plasma levels of either soluble MET (sMET) or HGF (data not shown). No significant correlations were observed between plasma levels of sMET and HGF and tumor response (data not shown). For the association between baseline (day 1) circulating cytokine and angiogenic factor (CAF) levels and tumor response, there were no significant associations in the univariate or multivariate models.
Table 4
Circulating biomarkers with statistically significant correlation of baseline levels and baseline tumor burden (N=35).
| Circulating Biomarkers | Correlation | p Value |
|---|---|---|
| ANG2 | 0·62 | 0·0001 |
| IGFBP1 | 0·56 | 0·0005 |
| IL8 | 0·56 | 0·0005 |
| OPN | 0·48 | 0·0035 |
| TSP2 | 0·45 | 0·0064 |
ANG2=angiopoietin 2. IGFPB1=insulin-like growth factor–binding protein 1. IL8 =interleukin 8; OPN=osteopontin. TSP2=thrombospondin 2.
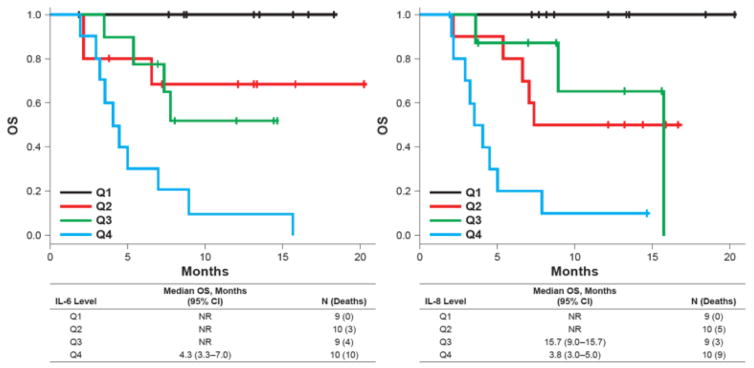
Interestingly, higher baseline levels of MMP9 and IL6 were associated with shorter TTP in univariate models (p=0.0109, hazard ratio [HR] 1.75; and p=0.0024, HR 1.44). The effect of IL6 was retained in multivariate models. Using a median split, patients with lower baseline IL6 levels (median-split) had a 6.7-month longer TTP than did those with higher IL6 levels (9.6 vs 2.9 months). Shorter OS was associated with higher baseline levels of MMP9 (p=0.0059, HR 2.18), IL6 (p=0.0002, HR 1.79), IL8 (p<0.0001, HR 2.38), TSP2 (p=0.0024, HR 2.21), and IGFBP1 (p=0.0071, HR 1.48). IL6 and IL8 were independent predictors of OS in multivariate models. Using quartile splits, the effect of IL6 and IL8 levels on OS was evident (Supplementary Table 3 and Figure 2).
Discussion
Single agent sorafenib is the only standard of care to treat advanced HCC patients. Prior attempts to develop additional small molecule inhibitors—including alternative tyrosine kinase inhibitors (TKIs) and mTOR inhibitors—that combat advanced HCC more effectively than sorafenib have proven unsuccessful [18–20]. Notably, the MET inhibitors cabozantinib [10] and tivantinib [11, 12] have shown activity as potential second-line therapies in advanced HCC, and, in the case of tivantinib, immunohistochemical analysis showed that TTP was higher in patients with tumors expressing high levels of MET. When evaluated in conjunction with the known associations of MET status with HCC pathogenesis [6, 21–23], these observations provide the rationale for development of foretinib—a multikinase inhibitor of MET, ROS, RON, AXL, TIE-2, and VEGFR2—to treat advanced HCC.
The MTD for foretinib in Asian patients with advanced HCC was determined to be 30 mg QD lower than the previously reported MTD (i.e., 60 mg QD) in other tumor types in predominantly non-Asian populations [24]. However, foretinib exposures at the 30 mg dose in patients with advanced HCC were similar to exposures at a dose of 60 mg QD in patients with other solid tumors. The lower MTD in this study may be attributable to compromised hepatic function due to underlying chronic liver disease in patients with advanced HCC (foretinib is metabolized primarily in the liver), lower mean body weight in the Asian patients evaluated in this study compared with the North American–based patients analyzed previously, or pharmacogenomic differences between patient populations. The MTD of 30 mg QD in this study had acceptable safety and tolerability; the AE profile of foretinib was consistent with results from other foretinib cancer studies [13, 24]. The AE profile of hypertension, increased ALT, and decreased appetite was also consistent with VEGFR inhibition, but lesser frequencies of hand-foot syndrome and rash were observed than in Asian subjects with HCC exposed to either sorafenib or sunitinib [25, 26]. A number of the AEs seen in this study were consistent with the underlying liver disease and cirrhosis seen in this study population. Increased ALT, ascites, and hepatic encephalopathy were seen in at least 10% of subjects. Upon treatment with 30 mg QD foretinib, there were no dose reductions and only 8% of patients discontinued due to an AE. These results are particularly encouraging relative to the pivotal study of sorafenib in Asia-Pacific patients with HCC, where 30.9% of sorafenib-treated patients required dose reduction and the discontinuation rate was 19.5% [3]. Relevant patient demographics in the current study (n = 45 total) were similar to those in the pivotal sorafenib study (n = 226 total [3]): median age (range), 57 (31 – 82) in our study vs. 52 (23 – 79) [3]; male/female, 78%/22% in our study vs. 85%/15% [3]; Child Pugh A, 93% in our study vs. 97% [3]; Child Pugh B: 0% in our study vs. 3% [3]; Hepatitis B positive: 58% in our study vs. 73% [3]; and Hepatitis C positive: 20% in our study vs. 8% [3].
With regard to efficacy, a major limitation of the study is inherent in its phase I/II design and the lack of sorafenib as a comparative control arm. Nevertheless, at the MTD of 30 mg QD, foretinib did show evidence of anti-tumor activity. Disease stabilization rates according to mRECIST and RECIST criteria were 82.9% and 63.2, respectively. Moreover, the median duration of response was 7.6 months and the median TTP 4.2 months. Importantly, the median OS in the current study for foretinib-treated patients was 15.7 months in contrast to the median OS of 6.5 months observed in the pivotal Asia-Pacific sorafenib study [3]. The magnitude of response and survival data observed in Asian advanced HCC patients makes it is unlikely that our results were biased by patient selection and subsequent therapies. Most enrolled patients had advanced disease: ~90% patients were in BCLC stage C and ~62% had distant metastases. Moreover, only 10 (25.6%) patients in the current study went on to receive sorafenib as second-line therapy, thereby potentially contributing to the observed OS. As with most phase II efficacy studies, cross trial comparison of treatment efficacy should be interpreted cautiously as the comparison might be confounded by the different demographics of the enrolled patients in different trials and also might be due to stage migration.
Pharmacogenomic analyses suggested that a haplotype of three SNPs in NR1I3/CAR was significantly associated with TTP in foretinib-treated patients. Notably, CAT haplotype was previously reported to be associated with worse progression-free survival (PFS) in sunitinib-treated patients with renal cell carcinoma [27]. Our exploratory biomarker studies also identified candidate biomarkers whose levels were associated with foretinib treatment, baseline tumor burden, TTP, and OS. Of note, multivariate analyses revealed that baseline IL6 and IL8 were independent predictors of OS; these data reinforce a prior report that found that higher baseline plasma levels of IL6 and IL8 were associated with tumor progression and mortality in sunitinib-treated patients with advanced HCC [28]. High levels of IL6 and IL8 were also associated with shorter PFS in pazopanib-treated patients with renal cell carcinoma, suggesting that IL6 and IL8 may represent broad prognostic indicators that act across multiple tumor types and angiogenesis inhibitors [29]. We also identified 13 other candidate biomarkers whose levels in the circulation were significantly altered in response to foretinib treatment. The possibility that IL6 and/or IL8 levels may predict response to foretinib warrants testing in a randomized, placebo-controlled study. Unfortunately, a second major limitation of the current study was that insufficient archival tumor samples were available to assess the link between target inhibition (MET, ROS, RON, AXL, TIE-2, and VEGFR2) and response; or the relationship between foretinib activity and intratumoral MET expression levels and gene copy number. Moving forward, improved, uniform tumor sample collection will be necessary to evaluate these factors, as well as other tumor correlative analyses. Although foretinib and sorafenib have many common targets, an extended analysis of those unique to each in future trials may shed light on activity differences and inform patient selection. Recent studies reveal certain targets unique to foretinib that deserve further interrogation: TYRO3 was shown to be overexpressed in HCC tumors and linked to HCC cell growth in vitro [30], AXL protein abundance was positively correlated with lymph node metastasis and HCC clinical stage [31], and AXL pathway activation promoted autocrine transforming growth factor-β signaling [32] and invasiveness through activation of SNAI2 [33] in HCC cell lines, as well as HCC xenograft growth in mice [31].
In summary, this phase I/II study of foretinib as monotherapy in first-line advanced HCC in Asian patients showed promising anti-tumor activity and acceptable safety, tolerability, and PK characteristics. These data warrant additional testing in a randomized setting to evaluate the relative efficacy of foretinib and the current standard of care (sorafenib) in patients with HCC.
Acknowledgments
Financial Support: This study (NCT00920192) was funded, initiated and sponsored by GlaxoSmithKline. PPD administered the study and also provided data analysis services. Biomarker studies were supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. The study was designed by some of the investigators in collaboration with the sponsor. Data were obtained by the sponsor and investigators, and all authors had access to the study data. The manuscript was written by authors as noted in collaboration with the sponsor, with editorial support from Clinical Thinking. All listed authors meet the criteria for authorship set forth by the International Committee for Medical Journal Editors.
We thank all of the patients and their families for their participation. We also thank T-T Chang (National Cheng Kung University Hospital, Taiwan). This work was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. Editorial support in the form of writing parts of the first draft, collating comments, fact-checking, and graphic services was provided by Clinical Thinking and was funded by GlaxoSmithKline.
Footnotes
Contributions: TY, WS, YC, C-JY, WL, P-JC, TS, YL, LHO, RTTP, KER and DPB collected the data. TY, YC, DSC, YL, DK, HK, LHO and RTTP designed the study. TY, RL, YC, AC, DSC, RG, KER, YL, DK, HK, LHO and DPB analysed the data. TY, RL, YC, C-JY, DSC, YL, DK, HK, LHO, RTTP and DPB interpreted the data. RG developed the figures. All authors participated in the preparation and review of the manuscript.
Conflicts of Interest:
DSC, RG, YL, DK, HK, and LHO are or have been employed by GlaxoSmithKline and hold stock ownership. RL is a consultant for GlaxoSmithKline. TY, WS, YC, C-JY, WL, P-JC, TS, AC, KER, RTTP and DPB report no conflicts of interest.
References
Full text links
Read article at publisher's site: https://doi.org/10.1158/1078-0432.ccr-16-1789
Read article for free, from open access legal sources, via Unpaywall:
https://clincancerres.aacrjournals.org/content/clincanres/23/10/2405.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Phase I-IV Drug Trials on Hepatocellular Carcinoma in Asian Populations: A Systematic Review of Ten Years of Studies.
Int J Mol Sci, 25(17):9286, 27 Aug 2024
Cited by: 0 articles | PMID: 39273237 | PMCID: PMC11395253
Review Free full text in Europe PMC
Therapeutic advances of targeting receptor tyrosine kinases in cancer.
Signal Transduct Target Ther, 9(1):201, 14 Aug 2024
Cited by: 1 article | PMID: 39138146 | PMCID: PMC11323831
Review Free full text in Europe PMC
MERTK Inhibition as a Targeted Novel Cancer Therapy.
Int J Mol Sci, 25(14):7660, 12 Jul 2024
Cited by: 0 articles | PMID: 39062902 | PMCID: PMC11277220
Review Free full text in Europe PMC
Development of nanoparticles incorporated with quercetin and ACE2-membrane as a novel therapy for COVID-19.
J Nanobiotechnology, 22(1):169, 12 Apr 2024
Cited by: 1 article | PMID: 38609998 | PMCID: PMC11015574
Foretinib Is Effective in Acute Myeloid Leukemia by Inhibiting FLT3 and Overcoming Secondary Mutations That Drive Resistance to Quizartinib and Gilteritinib.
Cancer Res, 84(6):905-918, 01 Mar 2024
Cited by: 1 article | PMID: 38231480 | PMCID: PMC10940854
Go to all (30) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT00920192
SNPs (2)
- (1 citation) dbSNP - rs2307424
- (1 citation) dbSNP - rs2307418
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A Phase 1 dose-escalation study of the safety and pharmacokinetics of once-daily oral foretinib, a multi-kinase inhibitor, in patients with solid tumors.
Invest New Drugs, 31(3):742-750, 06 Oct 2012
Cited by: 29 articles | PMID: 23054208
A phase-I study of lapatinib in combination with foretinib, a c-MET, AXL and vascular endothelial growth factor receptor inhibitor, in human epidermal growth factor receptor 2 (HER-2)-positive metastatic breast cancer.
Breast Cancer Res, 19(1):54, 02 May 2017
Cited by: 15 articles | PMID: 28464908 | PMCID: PMC5414192
Phase II and biomarker study of the dual MET/VEGFR2 inhibitor foretinib in patients with papillary renal cell carcinoma.
J Clin Oncol, 31(2):181-186, 03 Dec 2012
Cited by: 240 articles | PMID: 23213094 | PMCID: PMC3532390
Selective Inhibitor of the c-Met Receptor Tyrosine Kinase in Advanced Hepatocellular Carcinoma: No Beneficial Effect With the Use of Tivantinib?
Front Immunol, 12:731527, 02 Nov 2021
Cited by: 6 articles | PMID: 34804015 | PMCID: PMC8600564
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Intramural NIH HHS (1)
Grant ID: Z01 BC011124-01