Abstract
Free full text

Human immunodeficiency virus infection is efficiently mediated by a glycolipid-anchored form of CD4.
Abstract
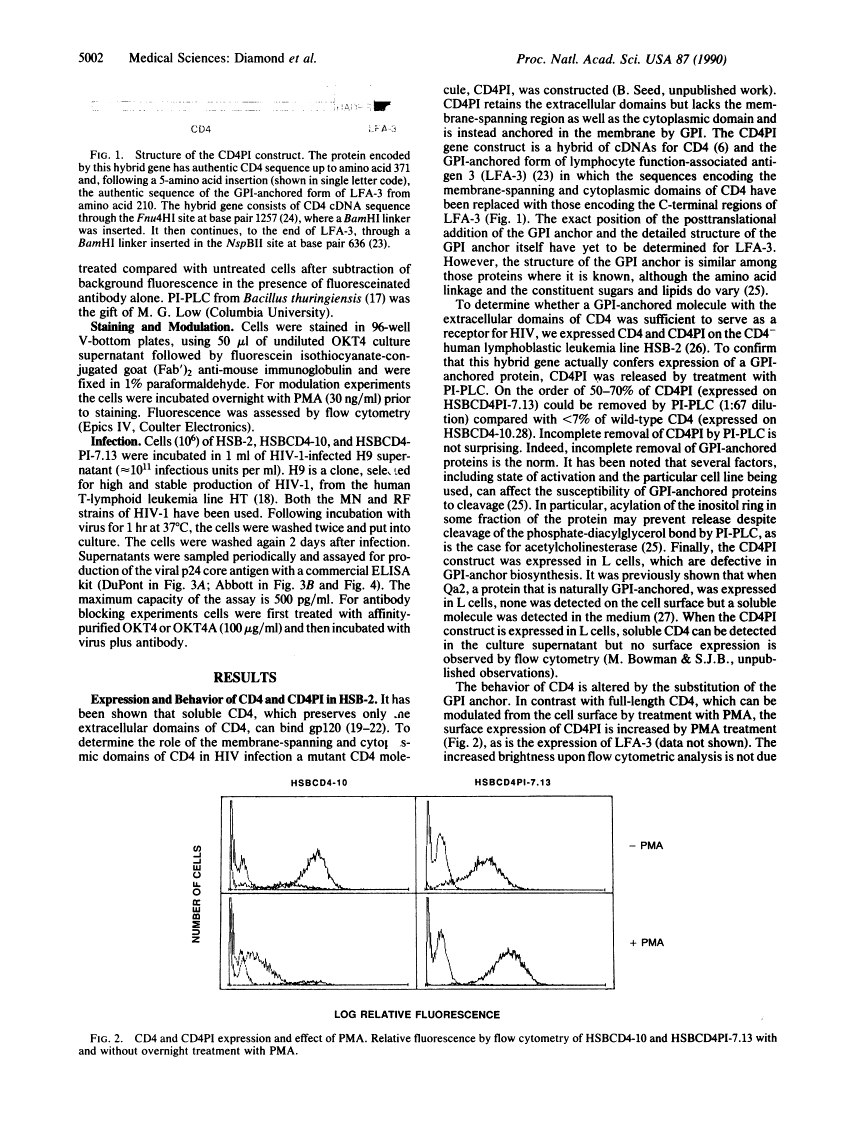
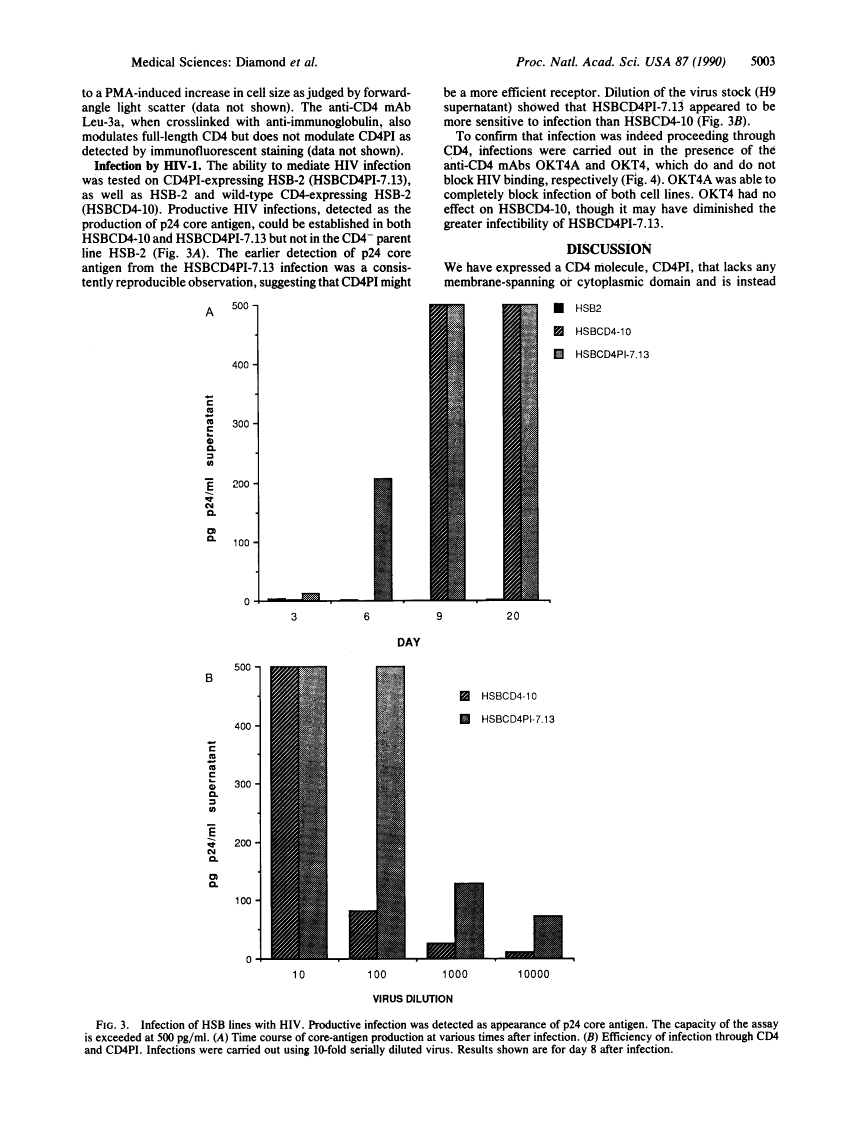
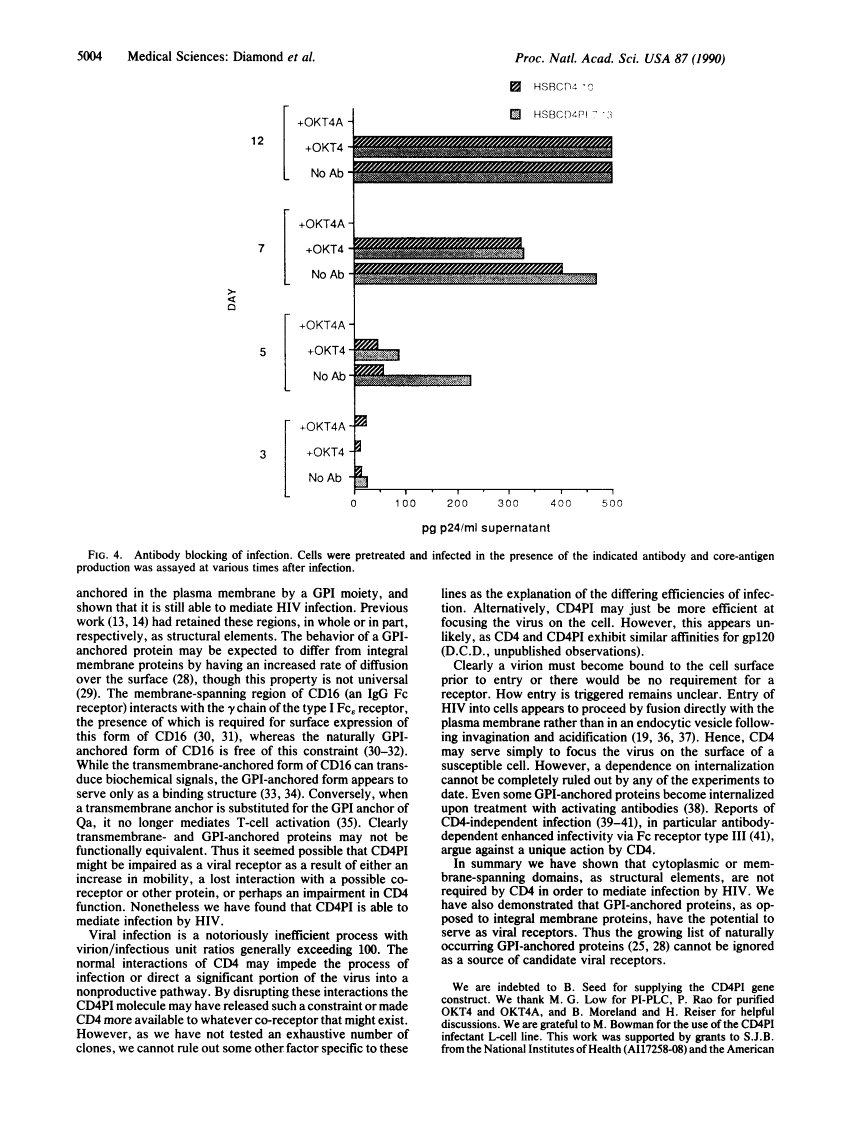
Two broad roles have been revealed for the CD4 molecule. It serves as a receptor for both class II major histocompatibility complex molecules and human immunodeficiency virus (HIV). Upon binding class II major histocompatibility molecules, CD4 functions to enhance T-cell activation. By binding to CD4, HIV gains entry into the cell. We have used a chimeric molecule of CD4 and lymphocyte function-associated antigen 3 (LFA-3), CD4PI, which lacks a membrane-spanning domain and is instead anchored in the membrane by linkage to glycosyl-phosphatidylinositol. To further define the structural attributes of viral receptors, and specifically those of CD4 required for HIV infection, we have expressed CD4PI and CD4 in a human T-cell line, HSB-2. We find that CD4PI is able to mediate infection of these cells by HIV with similar, if not greater efficiency, compared with wild-type CD4. Thus the membrane-spanning region of CD4 is not required for HIV infection, and a lipid-anchored protein can serve as a viral receptor.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.1M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dalgleish AG, Beverley PC, Clapham PR, Crawford DH, Greaves MF, Weiss RA. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. [Abstract] [Google Scholar]
- Klatzmann D, Champagne E, Chamaret S, Gruest J, Guetard D, Hercend T, Gluckman JC, Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. [Abstract] [Google Scholar]
- Maddon PJ, Dalgleish AG, McDougal JS, Clapham PR, Weiss RA, Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. [Abstract] [Google Scholar]
- Doyle C, Strominger JL. Interaction between CD4 and class II MHC molecules mediates cell adhesion. Nature. 1987 Nov 19;330(6145):256–259. [Abstract] [Google Scholar]
- Bierer BE, Sleckman BP, Ratnofsky SE, Burakoff SJ. The biologic roles of CD2, CD4, and CD8 in T-cell activation. Annu Rev Immunol. 1989;7:579–599. [Abstract] [Google Scholar]
- Sleckman BP, Peterson A, Jones WK, Foran JA, Greenstein JL, Seed B, Burakoff SJ. Expression and function of CD4 in a murine T-cell hybridoma. Nature. 1987 Jul 23;328(6128):351–353. [Abstract] [Google Scholar]
- Gay D, Maddon P, Sekaly R, Talle MA, Godfrey M, Long E, Goldstein G, Chess L, Axel R, Kappler J, et al. Functional interaction between human T-cell protein CD4 and the major histocompatibility complex HLA-DR antigen. Nature. 1987 Aug 13;328(6131):626–629. [Abstract] [Google Scholar]
- Clayton LK, Sieh M, Pious DA, Reinherz EL. Identification of human CD4 residues affecting class II MHC versus HIV-1 gp120 binding. Nature. 1989 Jun 15;339(6225):548–551. [Abstract] [Google Scholar]
- Rosenstein Y, Burakoff SJ, Herrmann SH. HIV-gp120 can block CD4-class II MHC-mediated adhesion. J Immunol. 1990 Jan 15;144(2):526–531. [Abstract] [Google Scholar]
- Diamond DC, Sleckman BP, Gregory T, Lasky LA, Greenstein JL, Burakoff SJ. Inhibition of CD4+ T cell function by the HIV envelope protein, gp120. J Immunol. 1988 Dec 1;141(11):3715–3717. [Abstract] [Google Scholar]
- Gurley RJ, Ikeuchi K, Byrn RA, Anderson K, Groopman JE. CD4+ lymphocyte function with early human immunodeficiency virus infection. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1993–1997. [Europe PMC free article] [Abstract] [Google Scholar]
- Weinhold KJ, Lyerly HK, Stanley SD, Austin AA, Matthews TJ, Bolognesi DP. HIV-1 GP120-mediated immune suppression and lymphocyte destruction in the absence of viral infection. J Immunol. 1989 May 1;142(9):3091–3097. [Abstract] [Google Scholar]
- Bedinger P, Moriarty A, von Borstel RC, 2nd, Donovan NJ, Steimer KS, Littman DR. Internalization of the human immunodeficiency virus does not require the cytoplasmic domain of CD4. Nature. 1988 Jul 14;334(6178):162–165. [Abstract] [Google Scholar]
- Maddon PJ, McDougal JS, Clapham PR, Dalgleish AG, Jamal S, Weiss RA, Axel R. HIV infection does not require endocytosis of its receptor, CD4. Cell. 1988 Sep 9;54(6):865–874. [Abstract] [Google Scholar]
- Sleckman BP, Bigby M, Greenstein JL, Burakoff SJ, Sy MS. Requirements for modulation of the CD4 molecule in response to phorbol myristate acetate. Role of the cytoplasmic domain. J Immunol. 1989 Mar 1;142(5):1457–1462. [Abstract] [Google Scholar]
- Ratnofsky SE, Peterson A, Greenstein JL, Burakoff SJ. Expression and function of CD8 in a murine T cell hybridoma. J Exp Med. 1987 Dec 1;166(6):1747–1757. [Europe PMC free article] [Abstract] [Google Scholar]
- Low MG, Stiernberg J, Waneck GL, Flavell RA, Kincade PW. Cell-specific heterogeneity in sensitivity of phosphatidylinositol-anchored membrane antigens to release by phospholipase C. J Immunol Methods. 1988 Oct 4;113(1):101–111. [Abstract] [Google Scholar]
- Popovic M, Sarngadharan MG, Read E, Gallo RC. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. [Abstract] [Google Scholar]
- Smith DH, Byrn RA, Marsters SA, Gregory T, Groopman JE, Capon DJ. Blocking of HIV-1 infectivity by a soluble, secreted form of the CD4 antigen. Science. 1987 Dec 18;238(4834):1704–1707. [Abstract] [Google Scholar]
- Hussey RE, Richardson NE, Kowalski M, Brown NR, Chang HC, Siliciano RF, Dorfman T, Walker B, Sodroski J, Reinherz EL. A soluble CD4 protein selectively inhibits HIV replication and syncytium formation. Nature. 1988 Jan 7;331(6151):78–81. [Abstract] [Google Scholar]
- Deen KC, McDougal JS, Inacker R, Folena-Wasserman G, Arthos J, Rosenberg J, Maddon PJ, Axel R, Sweet RW. A soluble form of CD4 (T4) protein inhibits AIDS virus infection. Nature. 1988 Jan 7;331(6151):82–84. [Abstract] [Google Scholar]
- Traunecker A, Lüke W, Karjalainen K. Soluble CD4 molecules neutralize human immunodeficiency virus type 1. Nature. 1988 Jan 7;331(6151):84–86. [Abstract] [Google Scholar]
- Seed B. An LFA-3 cDNA encodes a phospholipid-linked membrane protein homologous to its receptor CD2. Nature. 329(6142):840–842. [Abstract] [Google Scholar]
- Maddon PJ, Littman DR, Godfrey M, Maddon DE, Chess L, Axel R. The isolation and nucleotide sequence of a cDNA encoding the T cell surface protein T4: a new member of the immunoglobulin gene family. Cell. 1985 Aug;42(1):93–104. [Abstract] [Google Scholar]
- Low MG. Glycosyl-phosphatidylinositol: a versatile anchor for cell surface proteins. FASEB J. 1989 Mar;3(5):1600–1608. [Abstract] [Google Scholar]
- Adams RA, Flowers A, Davis BJ. Direct implantation and serial transplantation of human acute lymphoblastic leukemia in hamsters, SB-2. Cancer Res. 1968 Jun;28(6):1121–1125. [Abstract] [Google Scholar]
- Stroynowski I, Soloski M, Low MG, Hood L. A single gene encodes soluble and membrane-bound forms of the major histocompatibility Qa-2 antigen: anchoring of the product by a phospholipid tail. Cell. 1987 Aug 28;50(5):759–768. [Abstract] [Google Scholar]
- Low MG, Saltiel AR. Structural and functional roles of glycosyl-phosphatidylinositol in membranes. Science. 1988 Jan 15;239(4837):268–275. [Abstract] [Google Scholar]
- Phelps BM, Primakoff P, Koppel DE, Low MG, Myles DG. Restricted lateral diffusion of PH-20, a PI-anchored sperm membrane protein. Science. 1988 Jun 24;240(4860):1780–1782. [Abstract] [Google Scholar]
- Hibbs ML, Selvaraj P, Carpén O, Springer TA, Kuster H, Jouvin MH, Kinet JP. Mechanisms for regulating expression of membrane isoforms of Fc gamma RIII (CD16). Science. 1989 Dec 22;246(4937):1608–1611. [Abstract] [Google Scholar]
- Kurosaki T, Ravetch JV. A single amino acid in the glycosyl phosphatidylinositol attachment domain determines the membrane topology of Fc gamma RIII. Nature. 1989 Dec 14;342(6251):805–807. [Abstract] [Google Scholar]
- Lanier LL, Cwirla S, Yu G, Testi R, Phillips JH. Membrane anchoring of a human IgG Fc receptor (CD16) determined by a single amino acid. Science. 1989 Dec 22;246(4937):1611–1613. [Abstract] [Google Scholar]
- Lanier LL, Ruitenberg JJ, Phillips JH. Functional and biochemical analysis of CD16 antigen on natural killer cells and granulocytes. J Immunol. 1988 Nov 15;141(10):3478–3485. [Abstract] [Google Scholar]
- Selvaraj P, Carpén O, Hibbs ML, Springer TA. Natural killer cell and granulocyte Fc gamma receptor III (CD16) differ in membrane anchor and signal transduction. J Immunol. 1989 Nov 15;143(10):3283–3288. [Abstract] [Google Scholar]
- Robinson PJ, Millrain M, Antoniou J, Simpson E, Mellor AL. A glycophospholipid anchor is required for Qa-2-mediated T cell activation. Nature. 1989 Nov 2;342(6245):85–87. [Abstract] [Google Scholar]
- Stein BS, Gowda SD, Lifson JD, Penhallow RC, Bensch KG, Engleman EG. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell. 1987 Jun 5;49(5):659–668. [Abstract] [Google Scholar]
- McClure MO, Marsh M, Weiss RA. Human immunodeficiency virus infection of CD4-bearing cells occurs by a pH-independent mechanism. EMBO J. 1988 Feb;7(2):513–518. [Europe PMC free article] [Abstract] [Google Scholar]
- Bamezai A, Goldmacher V, Reiser H, Rock KL. Internalization of phosphatidylinositol-anchored lymphocyte proteins. I. Documentation and potential significance for T cell stimulation. J Immunol. 1989 Nov 15;143(10):3107–3116. [Abstract] [Google Scholar]
- Clapham PR, Weber JN, Whitby D, McIntosh K, Dalgleish AG, Maddon PJ, Deen KC, Sweet RW, Weiss RA. Soluble CD4 blocks the infectivity of diverse strains of HIV and SIV for T cells and monocytes but not for brain and muscle cells. Nature. 1989 Jan 26;337(6205):368–370. [Abstract] [Google Scholar]
- Harouse JM, Kunsch C, Hartle HT, Laughlin MA, Hoxie JA, Wigdahl B, Gonzalez-Scarano F. CD4-independent infection of human neural cells by human immunodeficiency virus type 1. J Virol. 1989 Jun;63(6):2527–2533. [Europe PMC free article] [Abstract] [Google Scholar]
- Homsy J, Meyer M, Tateno M, Clarkson S, Levy JA. The Fc and not CD4 receptor mediates antibody enhancement of HIV infection in human cells. Science. 1989 Jun 16;244(4910):1357–1360. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.87.13.5001
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc54249?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.87.13.5001
Article citations
Transmembrane segments of complement receptor 3 do not participate in cytotoxic activities but determine receptor structure required for action of Bordetella adenylate cyclase toxin.
Pathog Dis, 74(3):ftw008, 21 Jan 2016
Cited by: 9 articles | PMID: 26802078
Glycosyl-phosphatidylinositol (GPI)-anchored membrane association of the porcine reproductive and respiratory syndrome virus GP4 glycoprotein and its co-localization with CD163 in lipid rafts.
Virology, 424(1):18-32, 04 Jan 2012
Cited by: 19 articles | PMID: 22222209 | PMCID: PMC7111931
The erythrocyte viral trap: transgenic expression of viral receptor on erythrocytes attenuates coxsackievirus B infection.
Proc Natl Acad Sci U S A, 102(36):12897-12902, 25 Aug 2005
Cited by: 31 articles | PMID: 16123123 | PMCID: PMC1200307
Fusion activity of lipid-anchored envelope glycoproteins of herpes simplex virus type 1.
Virology, 324(1):213-228, 01 Jun 2004
Cited by: 32 articles | PMID: 15183068
Cancer vaccine development: protein transfer of membrane-anchored cytokines and immunostimulatory molecules.
Immunol Res, 29(1-3):231-240, 01 Jan 2004
Cited by: 11 articles | PMID: 15181285
Review
Go to all (25) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Glycosylphosphatidylinositol-anchored CD4/Thy-1 chimeric molecules serve as human immunodeficiency virus receptors in human, but not mouse, cells and are modulated by gangliosides.
J Virol, 65(1):440-444, 01 Jan 1991
Cited by: 17 articles | PMID: 1670644 | PMCID: PMC240534
Glycolipid-anchored form of CD4 increases intercellular adhesion but is unable to enhance T cell activation.
J Immunol, 147(2):428-431, 01 Jul 1991
Cited by: 11 articles | PMID: 1677022
Combined intra- and extracellular immunization against human immunodeficiency virus type 1 infection with a human anti-gp120 antibody.
Proc Natl Acad Sci U S A, 91(13):5932-5936, 01 Jun 1994
Cited by: 44 articles | PMID: 8016092 | PMCID: PMC44111
Structural and functional roles of glycosyl-phosphatidylinositol in membranes.
Science, 239(4837):268-275, 01 Jan 1988
Cited by: 480 articles | PMID: 3276003
Review
Funding
Funders who supported this work.
NIAID NIH HHS (2)
Grant ID: AI17258-08
Grant ID: 1F32 AI07843-01
NIGMS NIH HHS (2)
Grant ID: 1T32 GM07753-08
Grant ID: T32 GM007753