Abstract
Free full text

The molecular basis of the specific anti-influenza action of amantadine.
Abstract
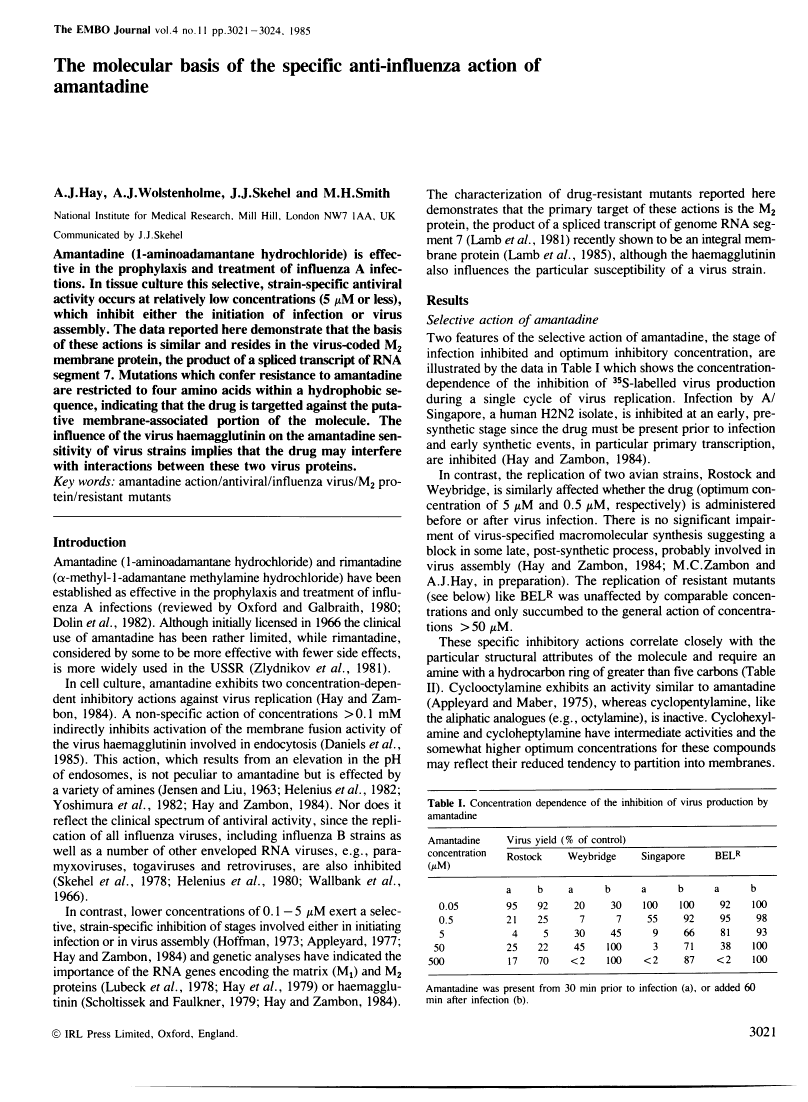
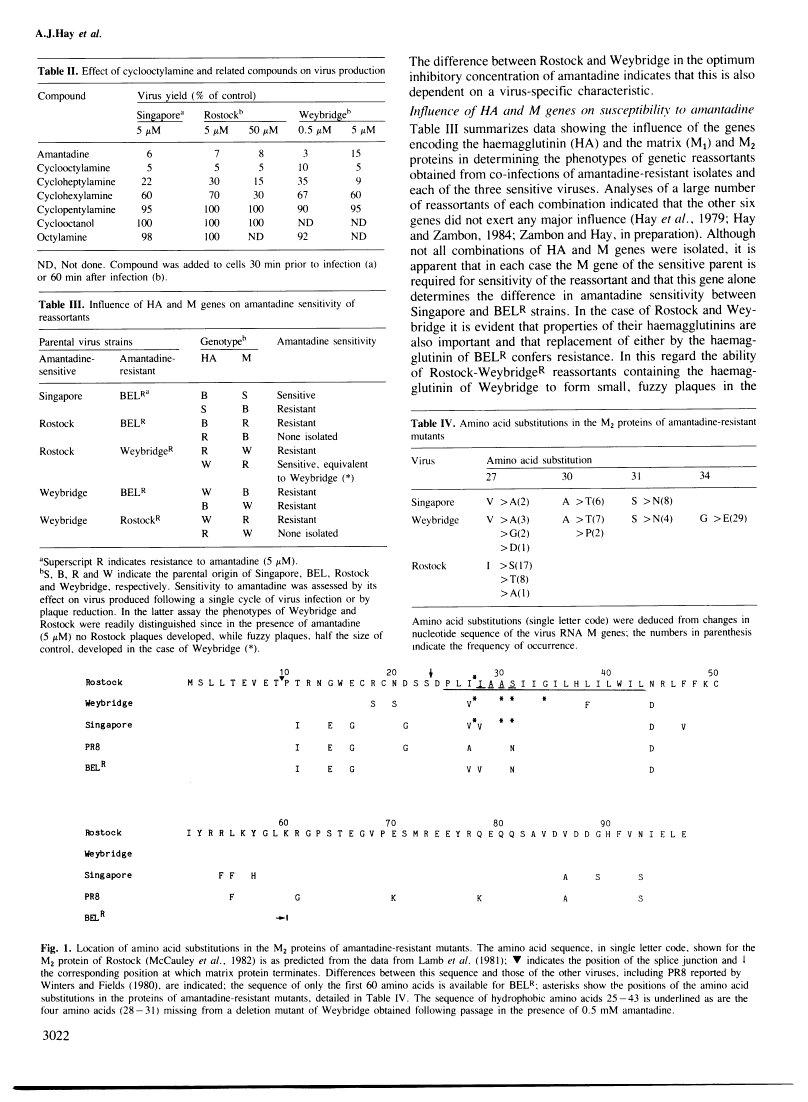
Amantadine (1-aminoadamantane hydrochloride) is effective in the prophylaxis and treatment of influenza A infections. In tissue culture this selective, strain-specific antiviral activity occurs at relatively low concentrations (5 microM or less), which inhibit either the initiation of infection or virus assembly. The data reported here demonstrate that the basis of these actions is similar and resides in the virus-coded M2 membrane protein, the product of a spliced transcript of RNA segment 7. Mutations which confer resistance to amantadine are restricted to four amino acids within a hydrophobic sequence, indicating that the drug is targetted against the putative membrane-associated portion of the molecule. The influence of the virus haemagglutinin on the amantadine sensitivity of virus strains implies that the drug may interfere with interactions between these two virus proteins.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (747K), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard G. Amantadine-resistance as a genetic marker for influenza viruses. J Gen Virol. 1977 Aug;36(2):249–255. [Abstract] [Google Scholar]
- Appleyard G, Maber HB. A plaque assay for the study of influenza virus inhibitors. J Antimicrob Chemother. 1975 Dec;1(4 Suppl):49–53. [Abstract] [Google Scholar]
- Daniels RS, Downie JC, Hay AJ, Knossow M, Skehel JJ, Wang ML, Wiley DC. Fusion mutants of the influenza virus hemagglutinin glycoprotein. Cell. 1985 Feb;40(2):431–439. [Abstract] [Google Scholar]
- Dolin R, Reichman RC, Madore HP, Maynard R, Linton PN, Webber-Jones J. A controlled trial of amantadine and rimantadine in the prophylaxis of influenza A infection. N Engl J Med. 1982 Sep 2;307(10):580–584. [Abstract] [Google Scholar]
- Hay AJ. Studies on the formation of the influenza virus envelope. Virology. 1974 Aug;60(2):398–418. [Abstract] [Google Scholar]
- Hay AJ, Kennedy NC, Skehel JJ, Appleyard G. The matrix protein gene determines amantadine-sensitivity of influenza viruses. J Gen Virol. 1979 Jan;42(1):189–191. [Abstract] [Google Scholar]
- Helenius A, Kartenbeck J, Simons K, Fries E. On the entry of Semliki forest virus into BHK-21 cells. J Cell Biol. 1980 Feb;84(2):404–420. [Europe PMC free article] [Abstract] [Google Scholar]
- Helenius A, Marsh M, White J. Inhibition of Semliki forest virus penetration by lysosomotropic weak bases. J Gen Virol. 1982 Jan;58(Pt 1):47–61. [Abstract] [Google Scholar]
- Lamb RA, Lai CJ, Choppin PW. Sequences of mRNAs derived from genome RNA segment 7 of influenza virus: colinear and interrupted mRNAs code for overlapping proteins. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4170–4174. [Europe PMC free article] [Abstract] [Google Scholar]
- Lamb RA, Zebedee SL, Richardson CD. Influenza virus M2 protein is an integral membrane protein expressed on the infected-cell surface. Cell. 1985 Mar;40(3):627–633. [Abstract] [Google Scholar]
- Lubeck MD, Schulman JL, Palese P. Susceptibility of influenza A viruses to amantadine is influenced by the gene coding for M protein. J Virol. 1978 Dec;28(3):710–716. [Europe PMC free article] [Abstract] [Google Scholar]
- McCauley JW, Mahy BW, Inglis SC. Nucleotide sequence of fowl plague virus RNA segment 7. J Gen Virol. 1982 Jan;58(Pt 1):211–215. [Abstract] [Google Scholar]
- Ortín J, Martínez C, del Río L, Dávila M, López-Galíndez C, Villanueva N, Domingo E. Evolution of the nucleotide sequence of influenza virus RNA segment 7 during drift of the H3N2 subtype. Gene. 1983 Aug;23(2):233–239. [Abstract] [Google Scholar]
- Oxford JS, Galbraith A. Antiviral activity of amantadine: a review of laboratory and clinical data. Pharmacol Ther. 1980;11(1):181–262. [Abstract] [Google Scholar]
- Patel TP, Millican TA, Bose CC, Titmas RC, Mock GA, Eaton MA. Improvements to solid phase phosphotriester synthesis of deoxyoligonucleotides. Nucleic Acids Res. 1982 Sep 25;10(18):5605–5620. [Europe PMC free article] [Abstract] [Google Scholar]
- Porter AG, Barber C, Carey NH, Hallewell RA, Threlfall G, Emtage JS. Complete nucleotide sequence of an influenza virus haemagglutinin gene from cloned DNA. Nature. 1979 Nov 29;282(5738):471–477. [Abstract] [Google Scholar]
- PORTERFIELD JS. A simple plaque-inhibition test for the study of arthropod-borne viruses. Bull World Health Organ. 1960;22:373–380. [Europe PMC free article] [Abstract] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. [Europe PMC free article] [Abstract] [Google Scholar]
- Schiffer M, Edmundson AB. Use of helical wheels to represent the structures of proteins and to identify segments with helical potential. Biophys J. 1967 Mar;7(2):121–135. [Europe PMC free article] [Abstract] [Google Scholar]
- Skehel JJ, Hay AJ, Armstrong JA. On the mechanism of inhibition of influenza virus replication by amantadine hydrochloride. J Gen Virol. 1978 Jan;38(1):97–110. [Abstract] [Google Scholar]
- Scholtissek C, Faulkner GP. Amantadine-resistant and -sensitive influenza A strains and recombinants. J Gen Virol. 1979 Sep;44(3):807–815. [Abstract] [Google Scholar]
- Wallbank AM, Matter RE, Klinikowski NG. 1-Adamantanamine hydrochloride: inhibition of Rous and Esh Sarcoma viruses in cell culture. Science. 1966 Jun 24;152(3730):1760–1761. [Abstract] [Google Scholar]
- Winter G, Fields S. Cloning of influenza cDNA ino M13: the sequence of the RNA segment encoding the A/PR/8/34 matrix protein. Nucleic Acids Res. 1980 May 10;8(9):1965–1974. [Europe PMC free article] [Abstract] [Google Scholar]
- Yoshimura A, Kuroda K, Kawasaki K, Yamashina S, Maeda T, Ohnishi S. Infectious cell entry mechanism of influenza virus. J Virol. 1982 Jul;43(1):284–293. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from The EMBO Journal are provided here courtesy of Nature Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1002/j.1460-2075.1985.tb04038.x
Read article for free, from open access legal sources, via Unpaywall:
https://www.onlinelibrary.wiley.com/doi/pdfdirect/10.1002/j.1460-2075.1985.tb04038.x
Citations & impact
Impact metrics
Article citations
Inhibition Effects and Mechanisms of Marine Compound Mycophenolic Acid Methyl Ester against Influenza A Virus.
Mar Drugs, 22(5):190, 23 Apr 2024
Cited by: 0 articles | PMID: 38786581 | PMCID: PMC11122424
Antiviral Chemotherapy in Avian Medicine-A Review.
Viruses, 16(4):593, 12 Apr 2024
Cited by: 1 article | PMID: 38675934 | PMCID: PMC11054683
Review Free full text in Europe PMC
The Potential of Cyclodextrins as Inhibitors for the BM2 Protein: An In Silico Investigation.
Molecules, 29(3):620, 28 Jan 2024
Cited by: 0 articles | PMID: 38338365 | PMCID: PMC10856705
COVID-19 ORF3a Viroporin-Influenced Common and Unique Cellular Signaling Cascades in Lung, Heart, and the Brain Choroid Plexus Organoids with Additional Enriched MicroRNA Network Analyses for Lung and the Brain Tissues.
ACS Omega, 8(48):45817-45833, 17 Nov 2023
Cited by: 0 articles | PMID: 38075756 | PMCID: PMC10701872
Rapid Determination of the Topology of Oligomeric α-Helical Membrane Proteins by Water- and Lipid-Edited Methyl NMR.
J Phys Chem B, 127(34):7518-7530, 22 Aug 2023
Cited by: 2 articles | PMID: 37606918 | PMCID: PMC10893779
Go to all (470) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Molecular basis of resistance of influenza A viruses to amantadine.
J Antimicrob Chemother, 18 Suppl B:19-29, 01 Oct 1986
Cited by: 91 articles | PMID: 3793659
Amantadine resistance among hemagglutinin subtype 5 strains of avian influenza virus.
Avian Dis, 35(1):31-39, 01 Jan 1991
Cited by: 8 articles | PMID: 1827579
Biologic potential of amantadine-resistant influenza A virus in an avian model.
J Infect Dis, 159(6):1050-1056, 01 Jun 1989
Cited by: 74 articles | PMID: 2723453
Emergence of amantadine-resistant influenza A viruses: epidemiological study.
J Infect Chemother, 9(3):195-200, 01 Sep 2003
Cited by: 120 articles | PMID: 14513385
Review