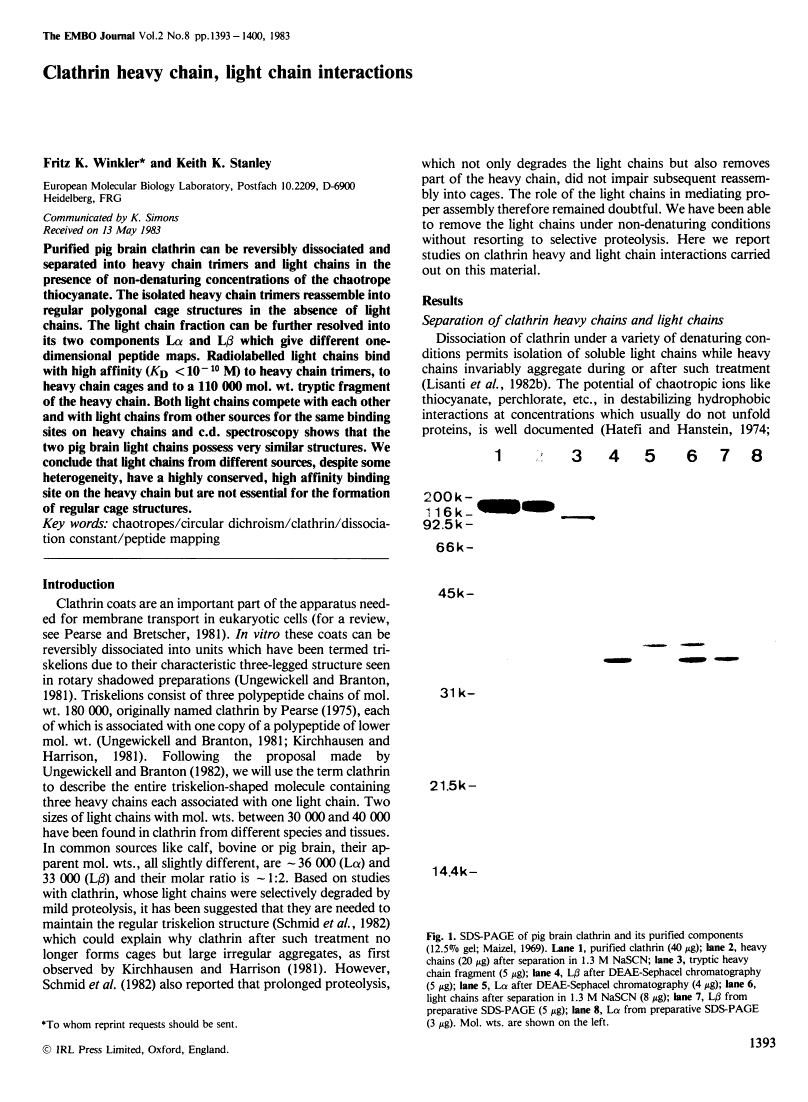
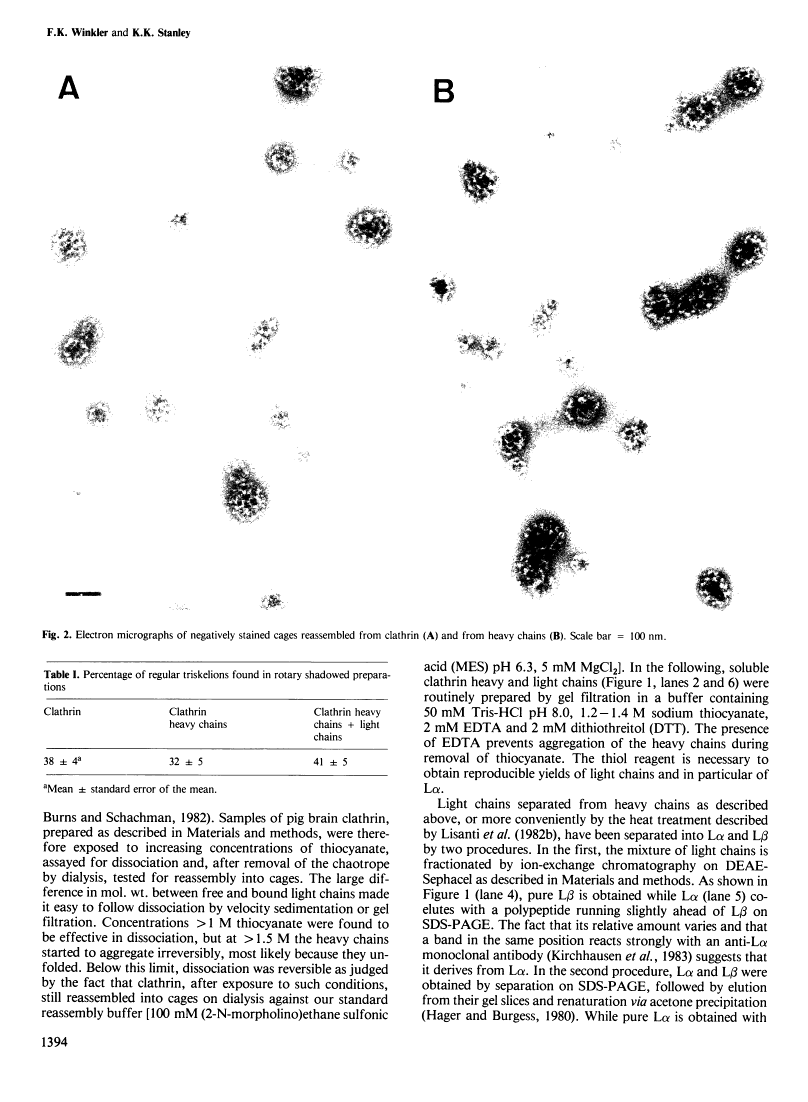
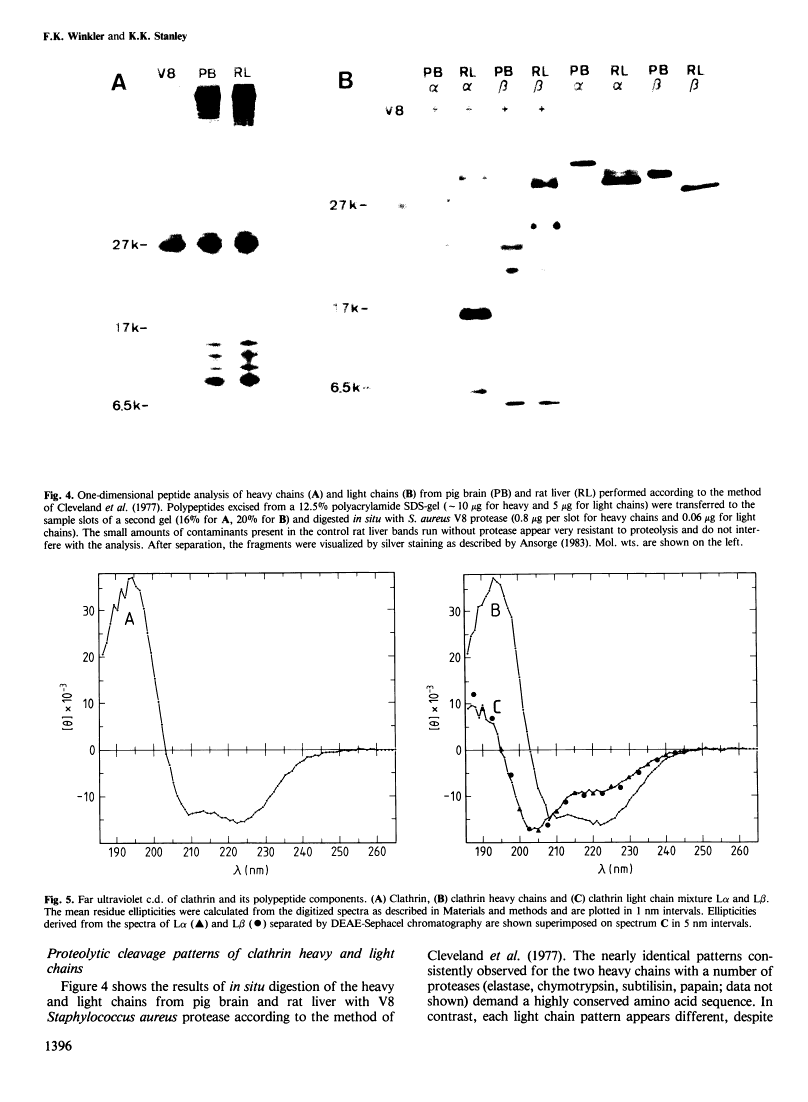
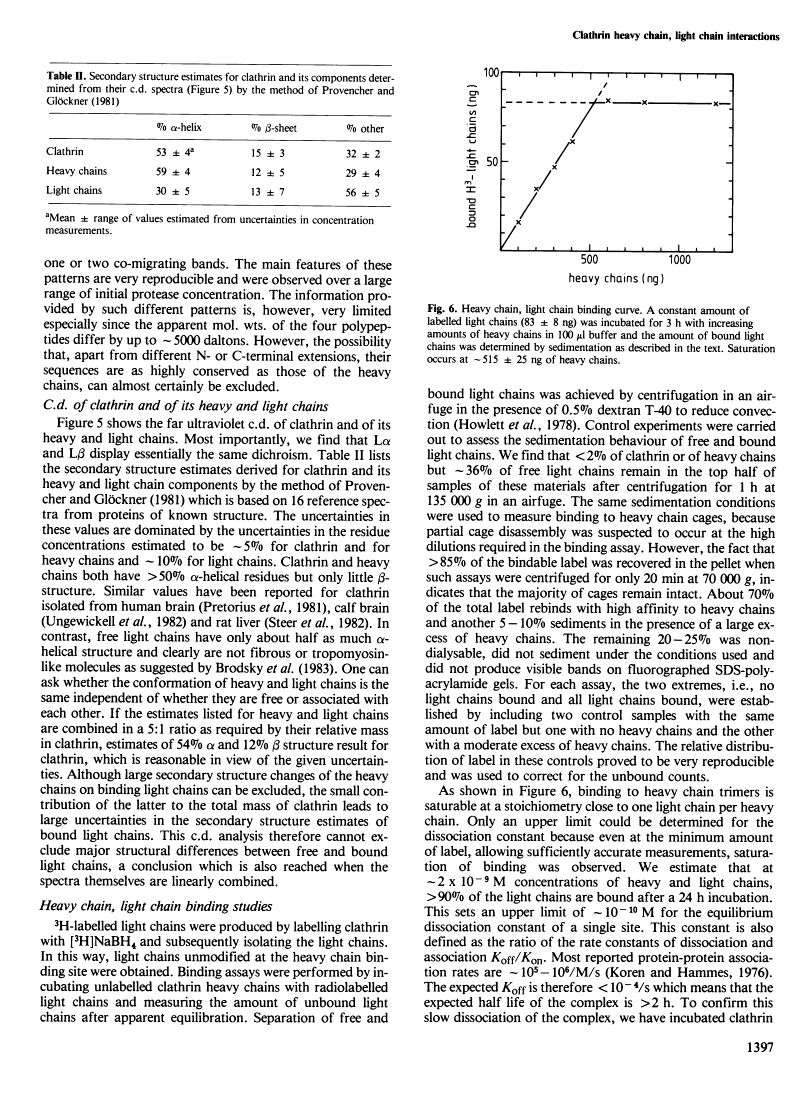
Abstract
Free full text

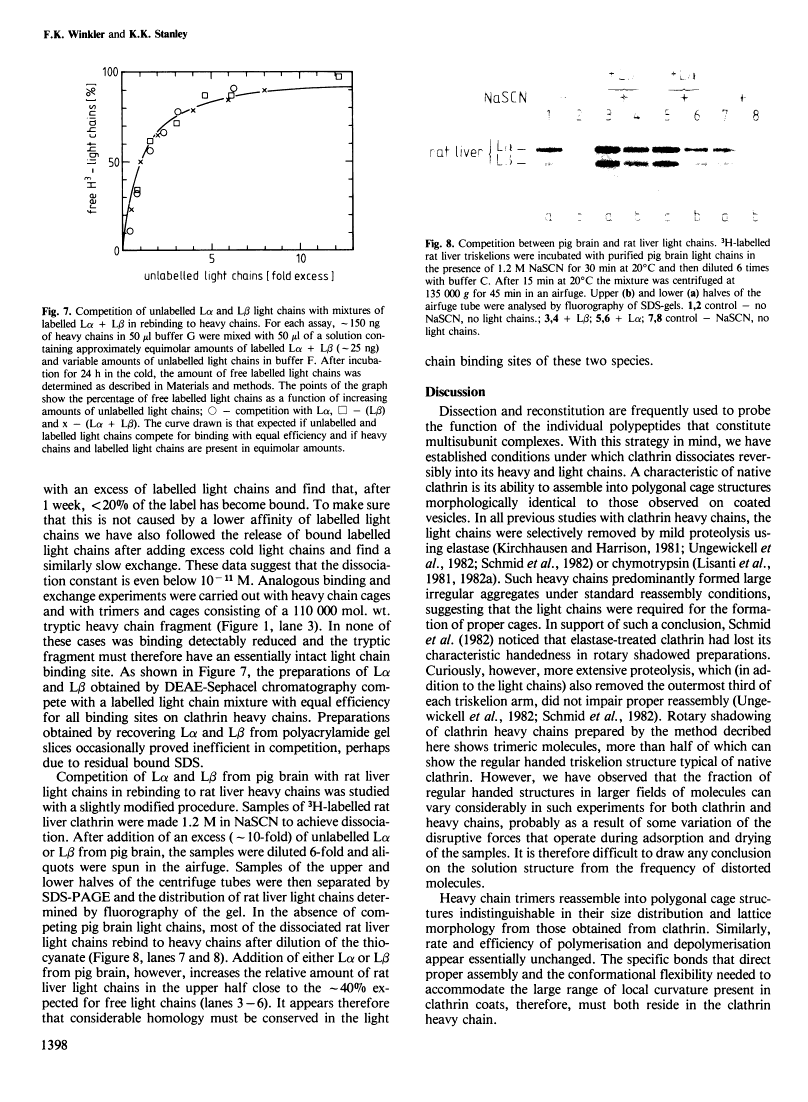
Clathrin heavy chain, light chain interactions.
Abstract
Purified pig brain clathrin can be reversibly dissociated and separated into heavy chain trimers and light chains in the presence of non-denaturing concentrations of the chaotrope thiocyanate. The isolated heavy chain trimers reassemble into regular polygonal cage structures in the absence of light chains. The light chain fraction can be further resolved into its two components L alpha and L beta which give different one-dimensional peptide maps. Radiolabelled light chains bind with high affinity (KD < 10(-10) M) to heavy chain trimers, to heavy chain cages and to a 110,000 mol. wt. tryptic fragment of the heavy chain. Both light chains compete with each other and with light chains from other sources for the same binding sites on heavy chains and c.d. spectroscopy shows that the two pig brain light chains possess very similar structures. We conclude that light chains from different sources, despite some heterogeneity, have a highly conserved, high affinity binding site on the heavy chain but are not essential for the formation of regular cage structures.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (2.7M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brodsky FM, Holmes NJ, Parham P. Tropomyosin-like properties of clathrin light chains allow a rapid, high-yield purification. J Cell Biol. 1983 Mar;96(3):911–914. [Europe PMC free article] [Abstract] [Google Scholar]
- Burns DL, Schachman HK. Assembly of the catalytic trimers of aspartate transcarbamoylase from folded monomers. J Biol Chem. 1982 Aug 10;257(15):8638–8647. [Abstract] [Google Scholar]
- Cleveland DW, Fischer SG, Kirschner MW, Laemmli UK. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [Abstract] [Google Scholar]
- Crowther RA, Pearse BM. Assembly and packing of clathrin into coats. J Cell Biol. 1981 Dec;91(3 Pt 1):790–797. [Europe PMC free article] [Abstract] [Google Scholar]
- Hager DA, Burgess RR. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. [Abstract] [Google Scholar]
- Hatefi Y, Hanstein WG. Destabilization of membranes with chaotropic ions. Methods Enzymol. 1974;31:770–790. [Abstract] [Google Scholar]
- Howlett GJ, Yeh E, Schachman HK. Protein-ligand binding studies with a table-top, air-driven high-speed centrifuge. Arch Biochem Biophys. 1978 Oct;190(2):808–819. [Abstract] [Google Scholar]
- Jaenicke L. A rapid micromethod for the determination of nitrogen and phosphate in biological material. Anal Biochem. 1974 Oct;61(2):623–627. [Abstract] [Google Scholar]
- Kartenbeck J, Schmid E, Müller H, Franke WW. Immunological identification and localization of clathrin and coated vesicles in cultured cells and in tissues. Exp Cell Res. 1981 May;133(1):191–211. [Abstract] [Google Scholar]
- Keen JH, Willingham MC, Pastan IH. Clathrin-coated vesicles: isolation, dissociation and factor-dependent reassociation of clathrin baskets. Cell. 1979 Feb;16(2):303–312. [Abstract] [Google Scholar]
- Kirchhausen T, Harrison SC. Protein organization in clathrin trimers. Cell. 1981 Mar;23(3):755–761. [Abstract] [Google Scholar]
- Kirchhausen T, Harrison SC, Parham P, Brodsky FM. Location and distribution of the light chains in clathrin trimers. Proc Natl Acad Sci U S A. 1983 May;80(9):2481–2485. [Europe PMC free article] [Abstract] [Google Scholar]
- Koren R, Hammes GG. A kinetic study of protein-protein interactions. Biochemistry. 1976 Mar 9;15(5):1165–1171. [Abstract] [Google Scholar]
- Lisanti MP, Schook W, Moskowitz N, Ores C, Puszkin S. Brain clathrin and clathrin-associated proteins. Biochem J. 1982 Feb 1;201(2):297–304. [Europe PMC free article] [Abstract] [Google Scholar]
- Lisanti MP, Schook W, Moskowitz N, Beckenstein K, Bloom WS, Ores C, Puszkin S. Brain clathrin: studies of its ultrastructural assemblies. Eur J Biochem. 1982 Jan;121(3):617–622. [Abstract] [Google Scholar]
- Lisanti MP, Shapiro LS, Moskowitz N, Hua EL, Puszkin S, Schook W. Isolation and preliminary characterization of clathrin-associated proteins. Eur J Biochem. 1982 Jul;125(2):463–470. [Abstract] [Google Scholar]
- Patzer EJ, Schlossman DM, Rothman JE. Release of clathrin from coated vesicles dependent upon a nucleoside triphosphate and a cytosol fraction. J Cell Biol. 1982 Apr;93(1):230–236. [Europe PMC free article] [Abstract] [Google Scholar]
- Pearse BM. Coated vesicles from pig brain: purification and biochemical characterization. J Mol Biol. 1975 Sep 5;97(1):93–98. [Abstract] [Google Scholar]
- Pearse BM. Clathrin: a unique protein associated with intracellular transfer of membrane by coated vesicles. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1255–1259. [Europe PMC free article] [Abstract] [Google Scholar]
- Pearse BM, Bretscher MS. Membrane recycling by coated vesicles. Annu Rev Biochem. 1981;50:85–101. [Abstract] [Google Scholar]
- Pretorius HT, Nandi PK, Lippoldt RE, Johnson ML, Keen JH, Pastan I, Edelhoch H. Molecular characterization of human clathrin. Biochemistry. 1981 May 12;20(10):2777–2782. [Abstract] [Google Scholar]
- Provencher SW, Glöckner J. Estimation of globular protein secondary structure from circular dichroism. Biochemistry. 1981 Jan 6;20(1):33–37. [Abstract] [Google Scholar]
- Schmid SL, Matsumoto AK, Rothman JE. A domain of clathrin that forms coats. Proc Natl Acad Sci U S A. 1982 Jan;79(1):91–95. [Europe PMC free article] [Abstract] [Google Scholar]
- Sivaramakrishnan M, Burke M. The free heavy chain of vertebrate skeletal myosin subfragment 1 shows full enzymatic activity. J Biol Chem. 1982 Jan 25;257(2):1102–1105. [Abstract] [Google Scholar]
- Steer CJ, Klausner RD, Blumenthal R. Interaction of liver clathrin coat protein with lipid model membranes. J Biol Chem. 1982 Jul 25;257(14):8533–8540. [Abstract] [Google Scholar]
- Tack BF, Dean J, Eilat D, Lorenz PE, Schechter AN. Tritium labeling of proteins to high specific radioactivity by reduction methylation. J Biol Chem. 1980 Sep 25;255(18):8842–8847. [Abstract] [Google Scholar]
- Ungewickell E, Branton D. Assembly units of clathrin coats. Nature. 1981 Jan 29;289(5796):420–422. [Abstract] [Google Scholar]
- Ungewickell E, Unanue ER, Branton D. Functional and structural studies on clathrin triskelions and baskets. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):723–731. [Abstract] [Google Scholar]
- Van Jaarsveld PP, Nandi PK, Lippoldt RE, Saroff H, Edelhoch H. Polymerization of clathrin protomers into basket structures. Biochemistry. 1981 Jul 7;20(14):4129–4135. [Abstract] [Google Scholar]
Associated Data
Articles from The EMBO Journal are provided here courtesy of Nature Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1002/j.1460-2075.1983.tb01597.x
Read article for free, from open access legal sources, via Unpaywall:
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1002/j.1460-2075.1983.tb01597.x
Citations & impact
Impact metrics
Citations of article over time
Article citations
Clathrin Light Chains: Not to Be Taken so Lightly.
Front Cell Dev Biol, 9:774587, 14 Dec 2021
Cited by: 8 articles | PMID: 34970544 | PMCID: PMC8712872
Review Free full text in Europe PMC
Clathrin light chain diversity regulates membrane deformation in vitro and synaptic vesicle formation in vivo.
Proc Natl Acad Sci U S A, 117(38):23527-23538, 09 Sep 2020
Cited by: 14 articles | PMID: 32907943 | PMCID: PMC7519287
The AP2 adaptor enhances clathrin coat stiffness.
FEBS J, 286(20):4074-4085, 03 Jul 2019
Cited by: 8 articles | PMID: 31199077 | PMCID: PMC6852553
Cryo-EM of multiple cage architectures reveals a universal mode of clathrin self-assembly.
Nat Struct Mol Biol, 26(10):890-898, 03 Oct 2019
Cited by: 25 articles | PMID: 31582853 | PMCID: PMC7100586
Stoichiometric balance of protein copy numbers is measurable and functionally significant in a protein-protein interaction network for yeast endocytosis.
PLoS Comput Biol, 14(3):e1006022, 08 Mar 2018
Cited by: 9 articles | PMID: 29518071 | PMCID: PMC5860782
Go to all (67) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Biochemical and immunological studies on clathrin light chains and their binding sites on clathrin triskelions.
EMBO J, 2(8):1401-1408, 01 Jan 1983
Cited by: 54 articles | PMID: 10872337 | PMCID: PMC555289
Bovine brain clathrin light chains impede heavy chain assembly in vitro.
J Biol Chem, 266(19):12710-12714, 01 Jul 1991
Cited by: 48 articles | PMID: 2061336
Analysis of clathrin light chain-heavy chain interactions using truncated mutants of rat liver light chain LCB3.
J Biol Chem, 265(7):3661-3668, 01 Mar 1990
Cited by: 21 articles | PMID: 2406259
The occurrence of disulphide bonds in purified clathrin light chains.
Biochem J, 257(3):775-781, 01 Feb 1989
Cited by: 9 articles | PMID: 2930486 | PMCID: PMC1135655