Abstract
Free full text

Abscisic acid (ABA) and key proteins in its perception and signaling pathways are ancient, but their roles have changed through time
ABSTRACT
Homologs of the Arabidopsis core abscisic acid (ABA) signaling component OPEN STOMATA1 (OST1) are best known for their role in closing stomata in angiosperm species. We recently characterized a fern OST1 homolog, GAMETOPHYTES ABA INSENSITIVE ON ANTHERDIOGEN 1 (GAIA1), which is not required for stomatal closure in ferns, consistent with physiologic evidence that shows the stomata of these plants respond passively to changes in leaf water status. Instead, gaia1 mutants reveal a critical role in ABA signaling for spore dormancy and sex determination, in a system regulated by antagonism between ABA and the gibberellin (GA)-derived fern hormone antheridiogen (ACE). ABA and key proteins, including ABA receptors from the PYR/PYL/RCAR family and negative regulators of ABA-signaling from Group A of the type-2C protein phosphatases (PP2Cs), in addition to OST1 homologs, can be found in all terrestrial land plant lineages, ranging from liverworts that lack stomata, to angiosperms. As land plants have evolved and diversified over the past 450 million years, so too have the roles of this important plant hormone and the genes involved in its signaling and perception.
We recently characterized several allelic mutants from the fern Ceratopteris richardii that showed insensitivity to the phytohormone abscisic acid (ABA). We found these mutants to contain defects in the GAMETOPHYTES ABA INSENSITIVE ON ANTHERDIOGEN 1 (GAIA1) gene, which encodes an SNF1-related kinase2 (SnRK2) protein homologous to the Arabidopsis core ABA signaling component OPEN STOMATA1 (OST1).1 OST1 is best known for its role in minimising water loss in Arabidopsis, where OST1 mediates the active/metabolic pathway for stomatal closure in response to ABA synthesized in leaves when water status declines.2,3 OST1 does this by interacting with S-type anion channels (SLACs) within the guard cell membrane to control guard cell turgor and thus the size of the stomatal pore.4 In accordance with this role, ost1 mutants have a severe and characteristic wilted phenotype under low humidity or when desiccated.2 While an OST1-independent pathway for SLAC activation has been identified, involving calcium-dependent protein kinases (CPKs),5,6 the considerable severity of the ost1 mutant stomatal phenotype when compared with the weak/absent stomatal phenotypes of cpk loss of function mutants indicates that OST1 plays a major role in the control of stomatal aperture via SLAC activation.5,7
In contrast, we found that stomatal behavior in C. richardii gaia1 mutants was identical to wild-type plants,1 indicating that, unlike angiosperm OST1 kinases, GAIA1 does not play a critical role in ABA signaling for stomatal closure in the fern C. richardii. Indeed, our findings indicated that both the fern C. richardii, and the lycophyte Selaginella moellendorffii lack what is considered an essential requirement for active ABA-mediated stomatal control: functional, guard-cell specific SnRK2-SLAC pairs.8 These findings are consistent with the results of physiologic studies showing that, in contrast to the dramatic stomatal closure elicited by ABA in seed plants, biologically relevant levels of ABA (within the same order of magnitude of the levels these plants are able to produce endogenously) fail to elicit or maintain stomatal closure in basal vascular plants including lycophytes and ferns. This finding is based on measurements of both stomatal conductance as a measure of leaf gas exchange,9,10 and stomatal aperture,11 which needs to be measured carefully following the same stomata from open to closed to ensure measurements are from live stomata, and using single blind methodology (without knowing the genotype) to avoid unconscious bias.12 Unnaturally high levels of ABA, approximately 1000x higher than endogenous levels, have been found to elicit a small reduction in stomatal aperture in some moss,13 hornwort,14 lycophyte,15 and fern species.16 However, the biologic relevance of these levels is debatable, especially given the smaller scale of response these levels elicit in basal land plants when compared with the complete stomatal closure elicited by much lower, biologically relevant ABA levels in seed plants.17-19 Furthermore basal vascular plants do not show a strong hysteresis in the recovery of stomatal opening following a period of water deficit, which is characteristic of ABA-mediated stomatal control, resulting from lingering ABA levels and slow rates of ABA catabolism. Instead the stomata of basal vascular plants show ‘passive’ stomatal responses that are directly controlled by leaf water status and plant hydraulics.20,21 Intermediate between the stomatal behaviors of basal vascular plants and angiosperms, gymnosperm species have active ABA-mediated stomatal closure in response to drought, but not in response to more subtle daily changes in air humidity.20 Taken together, these results support a gradualistic model for the evolution of ABA-mediated control of stomatal aperture, which suggests that the most basal vascular plant stomata responded passively to changes in leaf water status, and ‘active’, ABA-driven mechanisms for stomatal responses to water status evolved after the divergence of seed plants, culminating in the complex, and highly sensitive ABA-mediated responses observed in modern angiosperms.22
Instead of a role in stomatal responses, we found the SnRK2-ABA signaling pathway involving GAIA1 in C. richardii played important roles in spore dormancy and in sex determination in fern gametophytes, in a system regulated by antagonism between ABA and the gibberellin (GA)-derived fern hormone antheridiogen (ACE).1 In C. richardii, the pathway for sex determination has been elucidated from the epistatic interactions of more than 100 mutants.23-26 This pathway includes an indirect negative feedback loop between 2 major classes of sex-regulating loci: the TRANSFORMER loci (TRAs; necessary for female traits) and the FEMINIZATION1 locus (FEM1; required for male traits; named from mutant phenotype). ACE determines whether FEM or TRAs are activated in the gametophyte, by activating the TRA-repressing HERMAPHRODITIC loci (HERs). We can now update this model to include GAIA1, which links the ABA and ACE signaling pathways, and represses the ACE response (Fig. 1).1 In Arabidopsis, the OST1 subgroup SnRK2s act at multiple points within a comparable GA signaling pathway (Fig. 1), with GA biosynthesis, receptor and signaling genes upregulated in the ost1 snrk2–2 snrk2–3 triple mutant.27 Although the precise mechanism for GAIA1 regulation of sex determination in C. richardii is not yet clear, it is possible that it acts at multiple points in this pathway, in a similar manner.

The hypothesized GA signaling pathway in Arabidopsis and the sex determination pathway in the fern Ceratopteris richardii including the involvement of ABA. Arrows indicate activating events, while bars represent repressive events. Dashed lines indicate putative effects. Adapted from ref. 37.
Roles have also been identified for ABA and GAs/GA precursors in spore dormancy in mosses, seed dormancy in seed plants, and sex determination in angiosperms.28-30 Furthermore, there is clear evidence for an ancient role for ABA in desiccation tolerance, through the upregulation of proteins that function in osmoregulation,31 separate from the role of ABA described in modern seed plants in the prevention of desiccation via stomatal closure. Accordingly, and despite a recent report to the contrary,32 ABA receptors belonging to the PYR/PYL/RCAR receptor family are found in all lineages of land plants from bryophytes (including liverworts, which lack stomata) to angiosperms (Fig. 2). Phylogenetic analysis shows that this family diverged into 2 major clades in the vascular plants, indicating a duplication event after separation from the bryophyte lineages. Clade II includes 2 subclades of genes in fern, gymnosperm and angiosperm models (previously characterized as clades II and III in Arabidopsis),33 indicating a second duplication occurred in this clade after lycophyte divergence.
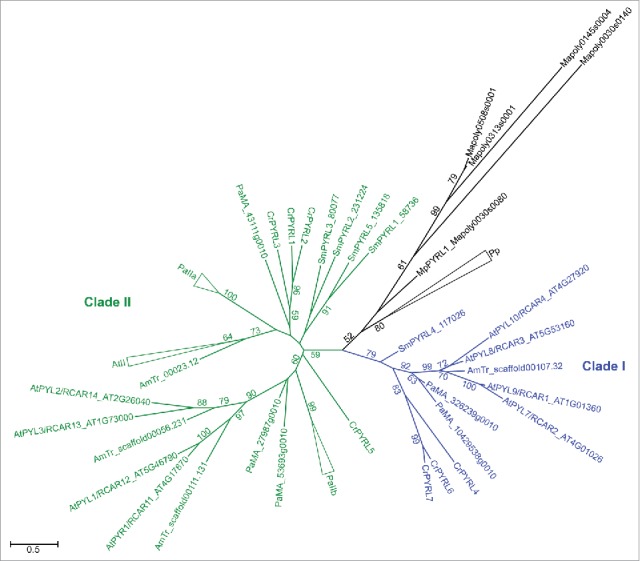
The land plant PYR/PYL/RCAR receptor family. The maximum likelihood phylogenetic tree of PYR/RCAR-like (PYRL) genes was generated using PhyML 3.0 with Smart Model Selection38 from a MAFFT alignment of full length predicted protein sequences for genes identified by reciprocal BLAST search in available resources for representative angiosperm (At, Arabidopsis thaliana, TAIR10; AmTr, Amborella trichopoda, v1.0), gymnosperm (Pa, Picea abies, v1.0, http://congenie.org/), fern (Cr, Ceratopteris richardii, unpublished transcriptome,1 Supplemental Text), lycophyte (Sm, Selaginella moellendorffii, v1.0), moss (Pp, Physcomitrella patens, v3.3), and liverwort (Mp/Mapoly, Marchantia polymorpha, v3.1) species at Phytozome (http://phytozome.jgi.doe.gov/) unless otherwise indicated. Vascular plant clades are indicated. Large, single-species sub-clades have been collapsed for figure clarity as follows: AtII (AtPYL4/RCAR10_AT2G38310, AtPYL5/RCAR8_AT5G05440, AtPYL6/RCAR9_AT2G40330, AtPYL13/RCAR7_AT4G18620, AtPYL11/RCAR5_AT5G45860, AtPYL12/RCAR6_AT5G45870); PaIIa (MA_7378814 g0010, MA_74427 g0010, MA_9600951 g0010, MA_10177719 g0010, MA_14451 g0010, MA_52202 g0010, MA_10427386 g0010, MA_554564 g0010, MA_15750 g0010, MA_407237 g0010); PaIIb (MA_10117348 g0010, MA_9939588 g0010, MA_99662 g0010, MA_181781 g0010, MA_18800 g0010, MA_10302927 g0010, MA_10355251 g0010, MA_10388164 g0010); Pp (PpPYRL1_Pp3c26_15240, PpPYRL2_Pp3c13_7110, PpPYRL3_Pp3c7_26290; PpPYRL4_Pp3c9_19760, PpPYRL5_Pp3c3_660, PpPYRL6_Pp3c7_4410, PpPYRL7_Pp3c15_19950, PpPYRL8_Pp3c20_7480). Bootstrap values from 1000 replicates are shown as percentages for clades with >50% support. The scale bar indicates amino acid changes.
While the PYR/PYL/RCAR receptor family is land plant-specific,34 other ABA-signaling components, including the SnRKs and type-2C protein phosphatases (PP2Cs), belong to older protein families that were present in an early eukaryote ancestor. Genes within Group A of the PP2C family, which includes the key Arabidopsis ABA-signaling gene ABA-INSENSITIVE 1 (ABI1), are found in all land plants (Fig. 3). While these genes evolved a role as negative regulators of ABA-signaling at an early stage during plant evolution,35 the nature of this role has changed over time. Specifically, while ABI1 phosphatases physically prevent SnRK2 function in the absence of ABA in angiosperms, orthologous phosphatases in bryophytes act downstream of SnRK2 kinases to control an ancient desiccation tolerance pathway.36

Land plant Group A PP2C phosphatases. The maximum likelihood phylogenetic tree was generated using PhyML 3.0 with Smart Model Selection38 from a MAFFT alignment of full length predicted protein sequences for genes identified by reciprocal BLAST search in available resources for representative species for each land plant lineage, as described for Figure 2. Vascular plant clades are indicated. Abbreviations as follows: At, Arabidopsis thaliana; AmTr, Amborella trichopoda; Cr, Ceratopteris richardii; Mp, Marchantia polymorpha; Pa, Picea abies; Pp, Physcomitrella patens; Sm, Selaginella moellendorffii (accessions indicated where full length sequence was obtained from GenBank). Bootstrap values from 1000 replicates are shown as percentages for clades with >50% support. The scale bar indicates amino acid changes.
In conclusion, the results from both physiologic and genetic studies highlight the important role that the plant hormone ABA has played in plant development throughout land plant evolution. Importantly, these findings show that the roles of ABA itself, in addition to key components of the ABA perception and signaling pathways, have also evolved and diversified through time.
Funding details
This work was supported by the Australian Research Council under Grants DE140100946 (S.M.) and DP140100666 (T.B.); and the National Science Foundation under Grant IOS1258091 (J.B.).
References
Articles from Plant Signaling & Behavior are provided here courtesy of Taylor & Francis
Full text links
Read article at publisher's site: https://doi.org/10.1080/15592324.2017.1365210
Read article for free, from open access legal sources, via Unpaywall:
https://www.tandfonline.com/doi/pdf/10.1080/15592324.2017.1365210?needAccess=true
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Identification and Characterization of the <i>AREB/ABF</i> Gene Family in Three Orchid Species and Functional Analysis of <i>DcaABI5</i> in <i>Arabidopsis</i>.
Plants (Basel), 13(6):774, 08 Mar 2024
Cited by: 0 articles | PMID: 38592811 | PMCID: PMC10974128
Physiological Mechanism through Which Al Toxicity Inhibits Peanut Root Growth.
Plants (Basel), 13(2):325, 22 Jan 2024
Cited by: 3 articles | PMID: 38276782 | PMCID: PMC10820445
Transcriptome Sequencing Analysis of Root in Soybean Responding to Mn Poisoning.
Int J Mol Sci, 24(16):12727, 12 Aug 2023
Cited by: 5 articles | PMID: 37628908 | PMCID: PMC10454639
GhWRKY1-like, a WRKY transcription factor, mediates drought tolerance in Arabidopsis via modulating ABA biosynthesis.
BMC Plant Biol, 21(1):458, 08 Oct 2021
Cited by: 23 articles | PMID: 34625048 | PMCID: PMC8501554
Stomatal morphology and physiology explain varied sensitivity to abscisic acid across vascular plant lineages.
Plant Physiol, 186(1):782-797, 01 May 2021
Cited by: 11 articles | PMID: 33620497
Go to all (15) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
PYR/PYL/RCAR family members are major in-vivo ABI1 protein phosphatase 2C-interacting proteins in Arabidopsis.
Plant J, 61(2):290-299, 26 Oct 2009
Cited by: 278 articles | PMID: 19874541 | PMCID: PMC2807913
A link between magnesium-chelatase H subunit and sucrose nonfermenting 1 (SNF1)-related protein kinase SnRK2.6/OST1 in Arabidopsis guard cell signalling in response to abscisic acid.
J Exp Bot, 66(20):6355-6369, 13 Jul 2015
Cited by: 12 articles | PMID: 26175350 | PMCID: PMC4588886
PYR/RCAR receptors contribute to ozone-, reduced air humidity-, darkness-, and CO2-induced stomatal regulation.
Plant Physiol, 162(3):1652-1668, 23 May 2013
Cited by: 103 articles | PMID: 23703845
A brand new START: abscisic acid perception and transduction in the guard cell.
Sci Signal, 4(201):re4, 29 Nov 2011
Cited by: 81 articles | PMID: 22126965
Review





