Abstract
Free full text

DNA Damage and Repair Biomarkers of Immunotherapy Response
Abstract
DNA damaging agents are widely used in clinical oncology and exploit deficiencies in tumor DNA repair. Given the expanding role of immune checkpoint blockade as a therapeutic strategy, the interaction of tumor DNA damage with the immune system has recently come into focus, and it is now clear that the tumor DNA repair landscape has an important role in driving response to immune checkpoint blockade. Here, we summarize the mechanisms by which DNA damage and genomic instability have been found to shape the anti-tumor immune response and describe clinical efforts to use DNA repair biomarkers to guide use of immune-directed therapies.
Introduction
DNA Repair Pathway Alterations Are Cancer Drivers
DNA is continually exposed to endogenous and exogenous sources of damage, and the coordinated activity of multiple DNA repair pathways is required to maintain genomic integrity under normal cellular conditions.[1, 2] Failure to repair DNA damage in an accurate and timely manner can result in a variety of genomic aberrations, including point mutations, chromosomal translocations, and gain or loss of chromosomal segments or entire chromosomes.[3] In some cases, these genomic alterations produce changes in cell physiology that drive tumor initiation.[4–6]
In addition to playing a role during the earliest events in tumorigenesis, loss of DNA repair fidelity has important implications for tumor evolution and response to therapy. Common tumor features such as high levels of oxidative stress, replicative stress, and loss or suppression of DNA damage-induced cell cycle checkpoints contribute to an environment that is rich in sources of DNA damage.[7] Further exacerbating the high levels of DNA damage, functional loss of one or more DNA repair pathways is common in tumors, and recent sequencing-based and functional studies have begun to reveal the broad scope of DNA repair pathway deficiencies across cancer.[8, 9] Due to the frequent combination of increased levels of DNA damage and decreased DNA repair capacity, most cancer cells accumulate hundreds to thousands of genomic aberrations that distinguish them from normal (non-cancerous) cells.[10] Although only a minority of these genetic changes may be responsible for driving the tumor phenotype, the overall landscape of DNA alterations provides important information regarding tumor DNA damage exposure and repair capacity, and it can confer the tumor and microenvironment with unique properties that have the potential to be exploited therapeutically.
Tumor DNA Repair Alterations Are Biomarkers and Therapeutic Targets
Tumor DNA repair deficiency has been a therapeutic target in oncology for more than a century, as evidenced by the widespread use of DNA-damaging chemotherapeutic agents and ionizing radiation. Several classes of DNA-damaging chemotherapy agents – including platinum-based agents, alkylating agents, and DNA intercalators – continue to comprise the backbone of many systemic therapy regimens, and radiation (alone or in combination with chemotherapy) is used in a variety of curative approaches. Although the mechanistic underpinnings of increased cancer cell sensitivity to DNA damage remains to be elucidated in many settings, the therapeutic window for DNA-damaging agents is driven by a relative deficiency in DNA repair function in cancer cells relative to normal cells.
In some cases, the nature of the underlying cancer DNA repair deficiency has been characterized, and relevant clinical biomarkers have been developed and are used for both prognostic and predictive purposes. For example, the association between O6-methylguanine DNA methyltransferase (MGMT) promoter methylation and response to temozolomide in glioblastoma multiforme (GBM) is one of the best-characterized associations between a specific DNA repair alteration and response to a DNA-damaging agent (Table 1).[11, 12] Loss of mismatch repair (MMR) function via germline or somatic mutation or gene silencing is a common event in colorectal and endometrial tumors[13, 14], as well as in a smaller percentage of many other tumor types. Loss of MMR function confers the microsatellite instability (MSI) phenotype that is associated with unique clinical features, prognosis, and response to conventional chemotherapy as well as targeted agents (discussed below).[15–17]
Table 1
Association between Deficiencies in Tumor DNA Repair and Immunotherapy Response
| DNA Repair Pathway | Common Tumor Settings | Clinical Diagnostic Criteria | Histopathological Features | Genomic Biomarkers | Clinical ICB Response |
|---|---|---|---|---|---|
| O6-methylguanine–DNA methyltransferase (MGMT) | Glioblastoma multiforme (GBM): ~35% with MGMT promoter methylation (varies widely across clinical contexts) | methylation-specific PCR | MGMT promoter methylation enriched in CpG island methylator phenotype (G-CIMP) subtype | Several clinical trials of temozolomide and ICB are underway in GBM | |
| Mismatch repair (MMR) | Colon: ~20% (varies widely by stage, anatomic location) Endometrial: ~20% (highest in endometrioid subtype) Gastric: 15–20% (highest rates in Western countries) Ovarian: 10% (mostly associated with Lynch syndrome, usually clear cell and endometrioid histology) Prostate, breast, glioma: 0–5% Occassional cases in other tumor types | Germline sequencing (Lynch syndrome) IHC (MSH2, MSH6, MLH1, PMS2) MSI PCR (Bethesda assay) | immune cell infiltrate (↑ CD8+ T cells, Th1 cells) Increased PD-1, PD-L1 staining | ↑ mutational burden, ↑ predicted neoantigens MMR mutational signature(s) ↑ expression of immune-related genes (PD-1, PD-L1, LAG3, CTLA-4) | Improved response to PD-1 blockade (NCT01876511). Several clinical trials of ICB in MMR-deficient tumors are planned or underway. |
| Homologous recombination (HR) | Ovarian: ~50% Breast: 10–40% (varies by subtype) Prostate: 15–25% in metastatic castrate-resistant tumors Other tumor types (pancreatic, gastric, endometrial) | Germline/somatic sequencing of HR genes (BRCA1, BRCA2, PALB2, etc) | Immune cell infiltrate (↑ CD3+ and CD8+ T cells) ↑ PD-1, PD-L1 staining | transcriptomic HR deficiency (HRD) scores: HRD-LOH, HDR-TAI, HDR-LST ↑ mutation burden, ↑ predicted neoantigens HR mutational signature ↑ cytotoxic T cell gene signature | Several clinical trials of ICB in HR-deficient tumors are planned or underway. Whether HR deficiency augments response to ICB remains to be determined. |
| Polymerase (POLE/POLD1) proofreading | Endometrial: 5–10% Colon: 2–5% Rare cases in other tumor types. Germline POLD1 and POLE mutations have been associated with a high risk of multiple colorectal adenomas and carcinomas, whereas germline POLD1 mutations also predispose to endometrial cancers. | None in routine use | Immune cell infiltrate (↑ CD3+ and CD8+ T cells) ↑ PD-1, PD-L1 staining | Somatic point mutation in exonuclease domain of POLE or POLD1 ↑ mutational burden, ↑ predicted neoantigens POLE/POLD1 mutational signatures ↑ cytotoxic T cell gene signature | Case reports of extreme responders POLE mutant tumors are being included with MMR-deficient tumors in several planned or on-going ICB trials. |
| Nucleotide excision repair (NER) | Bladder: 15–20% of primary muscle-invasive tumors | None in routine use | None described to date | Increased mutational burden ERCC2-related mutational signature Somatic mutation in ERCC2 or other NER gene(s) | Not reported |
| Base excision repair (BER) | Biallelic germline mutations in MUTYH are associated with increased risk of colorectal cancer | None in routine use | Prominent immune cell infiltrate (CD3+, CD8+, NK cells, granzyme B) | BER mutational signature Biallelic MUTYH mutations | Not reported |
Cancer predisposition associated with germline mutations in tumor suppressor genes such as BRCA1 and BRCA2 has been appreciated for several decades.[18–20] BRCA1/2 are central players in the homologous recombination (HR) repair pathway, and loss of BRCA1/2 or other genes in the HR pathway, can confer an HR-deficient phenotype in breast, ovarian, prostate, and other tumor types.[21–23] HR-deficient tumors have unique clinical properties, and the recent discovery and clinical implementation of poly(ADP ribose) polymerase (PARP) inhibitors is an example of the potential to employ DNA repair-directed targeted agents in a synthetic lethal approach to target tumors with a specific DNA repair pathway deficiency.[24–27] Small molecule inhibitors of numerous other DNA repair proteins – many of them kinases involved in DNA damage response pathways (such as ATM, ATR, CHEK1, CHEK2, WEE1, and DNA-PKcs) – are now being tested in a variety of DNA repair-deficient and DNA repair-proficient tumor settings as single agents and in combinations with conventional DNA damaging agents.[28–30]
Although DNA damaging chemotherapy and radiation are used in the treatment of hundreds of thousands of patients each year in the United States, few validated biomarkers are available to guide the selection of agent and dose. Whereas the discovery of the DNA repair-associated biomarkers described above (MGMT, MSI, and BRCA1/2) preceded the widespread availability of next-generation sequencing (NGS), recent studies using NGS-based approaches have begun to expand the number of apparent associations between specific DNA repair-deficient states and specific DNA damaging agents. For example, ERCC2 is a DNA helicase that plays a central role in the nucleotide excision repair (NER) pathway responsible for repairing adducts created by DNA damaging agents such as ultraviolet (UV) light and platinum chemotherapies. Somatic missense mutations in ERCC2 are present in up to 20% of primary muscle-invasive bladder cancers (MIBCs), and ERCC2-mutated tumors exhibit improved response to neoadjuvant cisplatin-based chemotherapy regimens compared to wild-type ERCC2 tumors.[31, 32] In addition, deleterious NER mutations have also been identified in ovarian tumors, and have important implications for platinum and PARP inhibitor use in this setting.[33]
It is likely that NGS-based studies will continue to uncover associations between mutation- or expression-based changes in tumor DNA repair pathway function and response to conventional and targeted agents. Moreover, the principles that have been used to identify existing DNA repair biomarkers will likely remain applicable in the search for novel DNA repair biomarkers for emerging therapies, including immunotherapy.
DNA Repair Defects Drive Genomic Instability and Tumor Immunogenicity
Although the coordinated activity of DNA repair pathways swiftly corrects the majority of DNA lesions, delayed or improper repair can lead to changes in the tumor genome that alter the immune balance in the tumor microenvironment. The interaction between the tumor and the host immune system has been appreciated for several decades[34], and therapeutic attempts to activate the host immune system to kill tumor cells have shown some clinical efficacy; for example, use of systemic IL-2 in metastatic melanoma[35] and renal cell carcinoma[36] and intravesicular Bacillus Calmette-Guerin (BCG) in bladder cancer[37].
However, in the past 5 years, the field of cancer immunotherapy has been transformed with the clinical implementation of antibodies against inhibitory signaling molecules on tumor and immune cells.[38, 39] The first immune checkpoint inhibitor that demonstrated a survival benefit was the anti-CTLA-4 antibody ipilumimab in patients with metastatic melanoma.[40, 41] This has been followed by evidence of robust clinical activity of agents targeting the programmed death-1 (PD-1)[42–44] and PD-1 ligand (PD-L1) receptors.[45, 46] Monoclonal antibodies that induce immune checkpoint blockade (ICB) have now been approved in a variety of advanced and upfront disease settings[47–51], and scores of additional trials are underway that are likely to further extend the scope of ICB use.
Despite robust and durable responses to ICB in a subset of tumors, efficacy of ICB varies widely. Even among the tumor types such as melanoma and non-small cell lung cancer for which many of the first ICB trials were conducted, only a subset of patients respond to therapy. As the evidence for ICB use across clinical settings continues to grow, a major unmet need is the identification of reliable biomarkers that predict response to ICB. Numerous lines of evidence now suggest that DNA repair plays an important role in driving sensitivity and response to ICB.
Mutational Load is a Predictor of Response to Immunotherapy
Accumulation of somatic mutations is a hallmark of tumors, but mutational burden varies dramatically both within and among tumor types.[10] The median mutation burden ranges over several orders of magnitude, from approximately 0.1 mutations/megabase (Mb) of the exome in some pediatric tumors to several mutations/Mb in carcinogen-induced tumor types such as melanoma, lung, and bladder. The variation in mutation burden within tumor types is even more dramatic, with the mutation rate commonly varying by >1000X between the most- and least-mutated samples within a specific tumor type. These vast differences in mutation burden reflect significant differences in the balance of DNA damage exposure and DNA repair fidelity among tumors.
The relationship between tumor mutational load and response to ICB was first described in metastatic melanoma patients treated with the CTLA-4-blocking antibodies ipilimumab or tremelimumab.[52] Tumor mutational load was significant higher in patients who achieved long-term clinical benefit from anti-CTLA-4 therapy compared to those who had minimal benefit. This association was confirmed in a second study demonstrating non-synonymous mutation burden was significantly higher among patients with disease response and overall survival >1 year.[53]
The link between tumor mutational burden and response to ICB has also been demonstrated for agents targeting the PD-1/PD-L1 axis. In non-small cell lung cancer patients treated with the anti-PD-1 antibody pembroluzimab, higher non-synonymous tumor mutational burden was associated with improved response and longer progression-free survival.[54] The association between mutational burden and ICB response has now been described in several cohorts; however, it is becoming increasingly clear that high mutational burden alone is not sufficient to drive ICB response.
Tumor Mutation Burden Correlates with Predicted Neoantigens and Immune Infiltration
While there is a correlation between tumor mutational burden and likelihood of response to ICB, there is no definitive threshold mutational burden that separates ICB responders from non-responders. Indeed, there are numerous examples of tumors with very few mutations that respond robustly to ICB as well as tumors with many mutations that show no response. Thus, despite the association between tumor mutational burden and ICB response, a critical challenge is identifying which mutations drive antitumor immune response.
Acquired (somatic) mutations in the exome have the potential to manifest as changes at the protein level. Mutant proteins may be processed by the proteasome, and the resultant peptide fragments are bound by MHC class I molecules and presented on the cell surface.[55] Several bioinformatics approaches have been developed to predict tumor neoantigens based on predicted MHC class I binding, T cell receptor binding, and patient HLA type.[56–58] In many clinical studies, including those that first established the relationship between mutational load and ICB response, predicted neoantigen load is closely associated with overall mutational burden and thus also associated with clinical response and survival outcomes.[52–54]
Similar to the principle that one or a small number of genetic alterations in a tumor may be responsible for driving the tumor phenotype, there is a growing appreciation that ICB response may also be driven by host response to a small number of tumor-specific neoantigens. For example, it was recently shown that tumors with high levels of clonal neoantigens have improved responses to ICB and that the loss of clonal neoantigens can be associated with ICB resistance.[59, 60] Conversely, increased neoantigen intratumor heterogeneity characterized by increased number of subclonal mutations has been associated with poor ICB responses in some cases.
The mechanisms underlying the role of neoantigen intratumor heterogeneity as a prognostic and predictive biomarker have not been fully characterized. DNA damaging agents such as cytotoxic chemotherapy and ionizing radiation (discussed below) primarily create subclonal mutations, and patients who receive more cytotoxic therapy are typically those with more aggressive tumors. Thus, in some cases, subclonal mutations may not drive ICB resistance, but rather may simply reflect heavily-treated, refractory disease. In other cases, subclonal mutations may be a surrogate for the ability of the tumor to achieve immune escape, and in yet other contexts, subclonal mutations may actively dampen the anti-tumor immune response by detracting from the host immune response to clonal neoantigens.[61] Conversely, it is even possible that under certain circumstances, subclonal mutations could promote anti-tumor immunity through mechanisms such as epitope spreading.[62]
Finally, evidence to date suggests that the vast majority of predicted neoantigens result from mutations that are unique to a specific tumor and do not typically involve known oncogenes.[55] In addition, it is also worth noting that mutations can also stimulate an immune response through neoantigen-independent mechanisms. For example, mutations can alter the immune environment by inducing changes in gene expression or eliciting an unfolded protein response.[63]
The associations between mutational burden or neoantigen load and ICB response may be explained by the relationships between each of these factors with intratumoral T cell populations. In melanoma and other solid tumors, intratumoral CD8+ T cell infiltration, both before and during treatment, has been associated with response to ICB.[64] CD4+ T cells are also present within tumors and contribute to antitumor immune activation by recognizing MHC class II-bound neoantigens.[65–67] Furthermore, total mutation burden and predicted neoantigens are also associated with T cell cytolytic activity (as defined by transcript levels of granzyme A and perforin), further supporting the notion that neoantigens can drive cytotoxic T cell responses.[68]
In addition to T cell infiltration and cytolytic activity, T cell diversity has been shown to correlate with tumor mutation load and response to ICB.[69] In one example, potential immunogenic somatic mutations were identified on the basis of their co-occurrence with CDR3 sequences of tumor-infiltrating T cells, highlighting the utility of bioinformatics approaches to connect genomic alterations with immune cell infiltrates. Combining tumor immune features with genomic characteristics such as mutational load may yield a stronger prognostic indicator than mutational load alone.[70, 71]
Tumor DNA Repair Deficiencies Impact Response to Immunotherapy
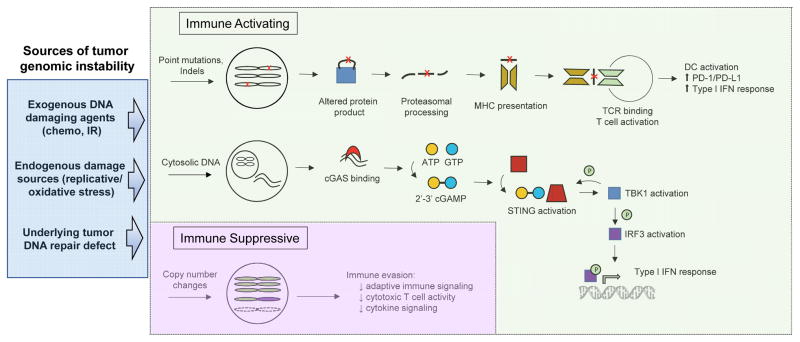
Genomic instability is a hallmark of tumors, and mutations can arise due to increased DNA damage exposure and/or decreased DNA repair capacity. Several reports have now linked a specific DNA damage exposure or a specific DNA repair pathway deficiency with ICB response. For example, tumors with a mutational landscape dominated by C>A transversions, a pattern linked to tobacco exposure[72], were more likely to benefit from ICB, and this genomic smoking signature was more predictive of ICB response than patient-reported smoking history.[54] Moreover, in the same study, several of the patients who achieved durable benefit from ICB had tumors with somatic alterations in genes involved in DNA replication or repair (such as POLE, POLD1, MSH2), suggesting that loss of normal DNA repair fidelity may have contributed to increased mutational burden and ICB response in these tumors (Figure 1).
Additional studies have also shown a similar correlation among alterations in specific DNA repair genes, mutation burden, and ICB response. In a cohort of 38 patients with metastatic melanoma treated with pembroluzimab or nivolumab, 6 of 21 ICB responders harbored a predicted deleterious mutation in BRCA2 versus only 1 of 17 non-responders.[73] Supporting this, BRCA2-mutated melanomas had significantly higher mutational burdens than BRCA2-wild type tumors. Among ovarian tumors in the TCGA dataset, tumors with BRCA1/2 alterations had significantly higher predicted neoantigen levels than HR-proficient tumors.[74] Likewise, wild type BRCA1/2 ovarian tumors with another genomic event predicted to result in HR loss (such as mutations in RAD51, ATM, ATR; PTEN deletion; or BRCA1 promoter hypermethylation) had higher predicted neoantigen levels than tumors predicted to be HR proficient. BRCA1/2-mutated ovarian tumors also had higher levels of CD3+ and CD8+ tumor-infiltrating lymphocyotes as well as higher immunohistochemical levels of PD-1 and PD-L1 compared to HR-proficient tumors.[75, 76] Despite this compelling preclinical data, early data from a clinical trial of avelumab (an anti-PD-L1 agent) have not shown improved response among BRCA1/2-mutated ovarian tumors, although the numbers are small and the results are preliminary.[77]
Patients with biallelic germline mutations in the base excision repair (BER) gene MUTYH are at increased risk of developing colorectal cancer (CRC). Histologically, MUTYH-associated tumors show dense lymphocytic infiltration[78], and tumor sequencing reveals a distinct pattern of C>A transversions.[79, 80] These findings provide another example of a link between a specific DNA repair deficiency and tumor immune properties, and raise the possibility that ICB may be a useful treatment strategy in MUTYH-associated CRC.
Tumors with somatic point mutations in the exonuclease (‘proofreading’) domain of polymerase epsilon (POLE) or polymerase delta (POLD1), the two polymerases responsible for the majority of nuclear DNA replication, have some of the highest mutational burdens identified to date.[13, 81] These ‘ultra-mutated’ tumors are predicted to express many neoantigens, and analysis of endometrial tumors with POLE exonuclease domain mutations has indeed shown high levels of TIL infiltration and PD-1/PD-L1 expression (Table 1).[82, 83] Perhaps driven by genomic instability and increased immunogenicity, improved survival has been reported for POLE-mutated tumors in the non-ICB setting[13, 84], and recent case reports also describe dramatic responses to ICB.[85, 86]
The most robust current evidence for the association between DNA repair deficiency and ICB activity is in tumors with loss of mismatch repair (MMR) function.[87] Initial evidence for an interaction among MMR deficiency, the immune microenvironment, and clinical outcomes came from immunohistochemical and genomic studies that showed colorectal tumors with an activated immune microenvironment had improved prognosis and frequently harbored defects in the MMR pathway.[88, 89] Among these MMR deficient tumors, the activated immune environment was counter-balanced by upregulation of immune checkpoints including PD-1, PD-L1, and CTLA-4.[90] Thus, these tumors appear to avoid host immune-mediated elimination through activation of immune checkpoints, raising the possibility that checkpoint blockade may represent an effective treatment strategy for MMR deficient tumors.
The first clinical evidence for activity of ICB in MMR deficient tumors came from a study conducted primarily in patients with colorectal cancer (CRC).[91] Interestingly, the earliest trials of ICB did not reveal significant activity in CRC.[42, 43] However, a patient who did respond was later noted to have a tumor with microsatellite instability (MSI), a marker of MMR deficiency.[92] Based on this finding and the known association among MMR deficiency, high somatic point mutation burden, and prominent T cell infiltrate, a small phase II trial was initiated to test the activity of pembroluzimab in three cohorts of patients with treatment-refractory disease: (1) mismatch repair deficient (MMRd) CRC, (2) MMR-proficient CRC, and (3) MMRd non-CRC tumors.[91] Both MMRd CRC and MMRd non-CRC cohorts included patients with inherited germline MMR deficiency (Lynch syndrome) as well as patients with sporadic MMRd tumors. Among patients with MMRd tumors, the immune-related objective response rate was 40% and 71% for patients with CRC and non-CRC MMRd tumors, respectively, versus 0% in patients with MMR-proficient CRC. Within the MMRd CRC cohort, patients with Lynch syndrome had lower response rates than patients with sporadic MMRd CRC (3/11 vs 6/6), raising the possibility that higher background mutational activity in patients with a germline DNA repair deficiency such as Lynch syndrome may shape the immune system, resulting in a more immune tolerant microenvironment, reduced immunosuppressive signaling, and decreased sensitivity to ICB.
Both the somatic mutation burden and the number of predicted neoantigens was higher in MMRd tumors compared to MMR proficient tumors, with an average of 1782 versus 73 mutations and 578 versus 21 predicted neoantigens, respectively. Similarly, the density of CD8+ lymphoid cells and the fraction of PD-L1-positive cells was higher in MMRd tumors compared to MMR proficient tumors. Together, the results of this trial provide compelling evidence that MMR deficiency is a predictive biomarker for ICB response, and have led to a number of planned and on-going trials for MSI tumors (Figure 2). For example, a trial of avelumab (anti-PD-L1) in MSI-H or POLE-mutated metastatic endometrial cancer will be opening soon at our institution (NCT02912572).
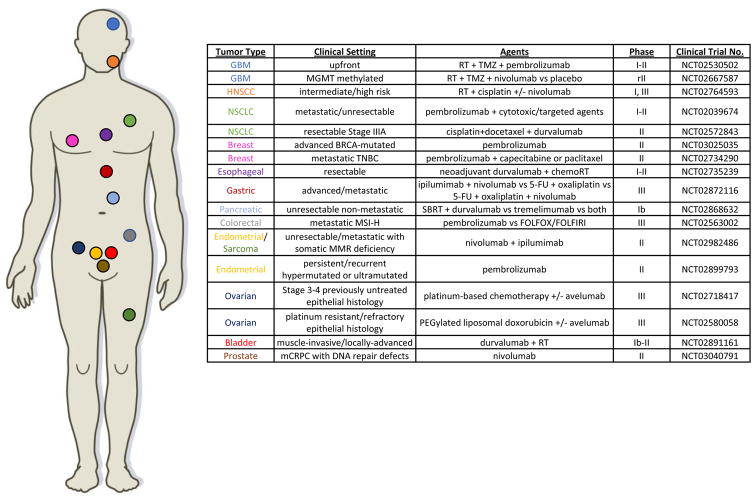
Representative Clinical Trials of ICB Agents in Combination with DNA Damaging Agents or in DNA Repair-Deficient Settings
Given its important prognostic implications, many institutions have implemented routine MSI testing (using IHC and/or PCR-based assays) for all patients with newly diagnosed colorectal and endometrial tumors. In addition, increased use of targeted sequencing panels – which can be used to estimate mutational load, infer MSI status, and predict clinical benefit to PD-1 blockade – are now becoming standard at many cancer centers.[93, 94]
DNA Repair Factors Beyond Mutational Load Can Also Impact Anti-Tumor Immunity
The STING Pathway is Activated by DNA Damaging Agents
An emerging body of data supports a role for non-neoantigen-based mechanisms of tumor cell recognition and targeting by the host immune system. The DNA damage response (DDR) is directly linked to innate immunity, as cells are adept at sensing damaged and foreign DNA.[95] The STING (Stimulator of Interferon Genes) pathway was originally characterized as a mechanism by which cells sense DNA viruses, but is also activated in the setting of microbial infections and certain autoimmune inflammatory conditions. Now, several lines of evidence suggest that the STING pathway also plays a role in tumor detection.[96]
The STING pathway is activated when cGAMP synthase (cGAS) interacts with cytosolic DNA and catalyzes the synthesis of cGAMP, a cyclic dinucleotide that acts as a second messenger to activate STING (Figure 1).[97] Upon activation, STING undergoes a conformation change that results in its shuttling from the endoplasmic reticulum to perinuclear endosomes, where it activates and is phosphorylated by TBK1. TBK1 also phosphorylates interferon regulatory factor 3 (IRF3), which translocates to the nucleus to drive transcription of type I interferon (IFN) genes, including IFNβ.[98]
The host STING pathway appears to be the primary innate immune sensing pathway for detection of tumors, and STING pathway activation within antigen presenting cells (APCs) in the tumor microenvironment drives T cell priming against tumor-associated antigens. This is supported by data demonstrating that animals deficient in STING or IRF3 have a defect in T cell priming and fail to reject immunogenic tumors.[99] While the mechanism has not yet been fully elucidated, current evidence supports a model in which dendritic cells (DCs) engulf dying tumor cells, sense free tumor DNA, and subsequently upregulate type I IFN signaling pathways to activate T cells.[100, 101]
Alterations in the DNA damage response, mediated by either exposure to cytotoxic agents or loss of normal DNA repair capacity, may contribute to STING pathway-mediated anti-tumor immunity. A recent study compared immune activity in DNA damage response-deficient (DDRD) breast tumors to non-DDRD tumors. DDRD tumors were defined by a 44-gene expression signature associated with loss of the S-phase-specific DNA damage response and improved response to DNA-damaging chemotherapy.[102] In the study, DDRD tumors had increased IFN-related gene expression compared to non-DDRD tumors as well as increased levels of CD4+ and CD8+ T cells in the tumor and stroma[103]. Subsequent cell-based assays demonstrated that expression of the chemokines CXCL10 and CCL5, which play a key role in CD4+ and CD8+ T cell chemotaxis, increased following siRNA-mediated depletion of individual DNA repair genes (such as BRCA1, BRCA2, and FANCD2). Increased IRF3 and TBK1 phosphorylation were observed in BRCA1/2-deficient compared to BRCA1/2-corrected cell lysates, and conditioned media from BRCA1/2-mutant or BRCA1/2-depleted cells led to increased migration of peripheral lymphocytes. Similar STING-mediated upregulation of IFN signaling has been observed in cells from Ataxia-Telangiectasia (AT) patients and Atm−/− mice.[104]
In addition to being upregulated in the setting of DNA repair deficiency, the STING pathway is activated following exposure to DNA damaging agents. Chemotherapy-induced genotoxic stress drove a type I IFN response across a panel of breast cancer cell lines, and STING pathway silencing abrogated this response.[105] Damage-induced IFN response has also been observed following exposure to other clinically relevant agents, including etoposide, camptothecin, mitomycin C, and adriamycin.[106] Interestingly, STING activation also occurs following Cre-mediated DNA cutting[107], raising the possibility that in vivo gene-editing techniques that generate targeted DNA lesions, such as Cre/loxP, CRISPR/Cas9, and TALEN systems, may be accompanied by activation of the innate immune system and an immune response against the edited cell.
The STING pathway relies on activation by cytosolic DNA, and increased levels of cytosolic DNA are present in BRCA1/2- or ATM-deficient cell lines compared to their wild type counterparts.[103, 104] DNA-damaging agents such as cisplatin and etoposide can also increase cytosolic DNA levels in the absence of known DNA repair defects.[108] However, the mechanism by which this free DNA arises is currently not understood. It is possible that re-establishing DNA replication at stalled or damaged forks during S phase and/or gap synthesis mediated by DNA damage tolerance pathways during G1 phase may liberate free DNA, and there is evidence that canonical DNA repair proteins such as Mre11 play a role in cytosolic DNA processing and cGAS activation.[109]
Not all activities of the STING pathway exert an anti-tumor effect. The STING pathway plays an important role in promoting inflammation-induced tumorigenesis through augmentation of inflammatory cytokine signaling, and STING−/− mice are resistant to inflammation-induced skin cancers.[108] STING pathway activation also drives PD-L1 expression on cancer cells and infiltrating lymphocytes following DNA damage, thereby dampening immune-mediated tumor killing despite increased numbers of CD4+ and CD8+ T cells.[110, 111] This observation provides a potential biological rationale for combining a STING-inducing DNA damaging agent (such as cisplatin) with anti-PD-1/PD-L1 therapy (discussed below).
Together, these findings are consistent with a model in which a shift in the balance of DNA damage and repair driven by either exposure to exogenous DNA damaging agents or loss of a DNA repair pathway can stimulate a STING-mediated innate immune response. Given the potent anti-tumor immune response driven by STING, direct STING activation represents an attractive therapeutic strategy, and several cyclic dinucleotide mimetics that activate STING have demonstrated activity in pre-clinical studies.[101, 112] Given that STING signaling activates type I IFN signaling, tumors that lack baseline type I IFN signaling may be ideal targets for treatment with a STING agonist, and STING agonists have been shown to promote IFN signaling and extend survival in two AML mouse models.[113]
Tumor Aneuploidy is Associated with Immune Evasion
In addition to driving increased point mutational burden, genomic instability can also result in gain or loss of chromosomal segments or entire chromosomes. Aneuploidy is a common feature of tumors, and recent studies investigating the role of somatic copy number alterations (SCNAs) on tumor properties have revealed an apparent link between SCNAs and immune suppression (Figure 1). Analyzing more than 5000 TCGA tumors across 12 tumor types, Davoli et al noted a correlation between high levels of SCNAs (gain or loss) and reduced expression of cytotoxic immune cell markers.[114] Although there was a positive correlation between SCNAs and tumor mutation burden in most tumor types, and although tumor mutational burden has been associated with immune activation in several tumor contexts (as discussed above), increased SCNAs correlated with reduced immune activation in all tumors types except brain tumors. The association between SCNAs and reduced immune activation was driven primarily by arm-level and whole-chromosome gain or loss rather than by gain or loss of focal chromosomal segments, suggesting that global gene dosage effects rather than expression changes in single genes may be responsible for altering the immune landscape.
Analysis of clinical cohorts receiving ICB agents have revealed similar trends. In metastatic melanoma patients treated with anti-CTLA-4 therapy followed by anti-PD-1 therapy, the burden of copy number loss was higher in pre-treatment biopsies from patients who did not respond to either anti-CTLA-4 or anti-PD-1 therapy than in anti-CTLA-4 responders.[115] Similarly, an association between increased SCNAs and worse outcomes was demonstrated in two melanoma cohorts treated with anti-CTLA-4 therapy.[114, 115] Moreover, combining SCNA status with tumor mutational burden provided better predictive power than either factor alone.
In these analyses, the effect of SCNA burden on survival appeared to be more prominent in patients receiving anti-CTLA-4 therapy than in separate cohorts of patients not treated with ICB. However, given that aneuploidy is associated with worse prognosis in many tumor settings (independent of treatment), similar analyses in randomized trials of ICB will help to clarify the extent to which the lack of ICB response contributes to worse outcomes in the high SCNA setting. In addition, further defining the role of factors such as the tumor-stroma ratio and aneuploidy characteristics (i.e., extent of chromosomal gain/loss) will be required to more fully understand the link between aneuploidy and the anti-tumor immune response.
Taken together, these data reveal a relationship between another manifestation of tumor genomic instability – aneuploidy – and response to immune checkpoint blockade. When combined with previous studies that show a positive correlation between point mutation burden and ICB response, tumors that would be predicted to have the best response to ICB are those with high mutation burden but few SCNAs. POLE-mutated tumors are an extreme example of this genomic context, as they have extremely high point mutation burdens but are typically near-diploid, and several impressive responses of POLE-mutated tumors to ICB have been reported (discussed above).
Interactions between the Immune System and DNA Damage/Repair Can Mediate Resistance
As clinical implementation of ICB therapy continues to expand and collective experience grows, examples of acquired resistance to ICB have emerged. Numerous mechanisms have been described and are reviewed in detail elsewhere.[38, 116] Examples include upregulation of inhibitory signaling through indoleamine 2,3-dioxygenase (IDO), PD-L1, or regulatory T cells (Tregs)[110, 117]; stabilization of PD-L1 through disruption of the 3′-UTR[118]; and upregulation of alternative immune checkpoints such as TIM-3.[119]
In addition to acquired resistance to ICB, some tumors display intrinsic resistance. Clinically, this has been evident since the earliest trials of ICB agents, as only a subset of patients respond to primary ICB. Several recent studies have attempted to identify genomic features that mediate innate resistance to ICB. In melanoma patients, innate resistance to PD-1 blockade has been associated with a transcriptional signature characterized by upregulation of genes involved in mesenchymal transition and extracellular matrix remodeling.[73] WNT/β-catenin pathway activity has also been associated with the absence of a T cell gene expression signature and lack of response to anti-CTLA-4/anti-PD-L1 therapy in melanoma[120], and CTNNB1 mutations (which can drive WNT/β-catenin pathway activity) were associated with lower neoantigen load among copy-number low/endometrioid endometrial tumors.[121] Loss of PTEN has also been associated with decreased T cell infiltration and worse outcomes for melanoma patients treated with anti-PD-1 therapy.[122]
The role of the immune microenvironment in mediating resistance to DNA-damaging agents has recently come into focus. Fibroblasts are a major cellular component of the tumor microenvironment, and a recent study using mouse models of high-grade epithelial ovarian cancer (HG-EOC) revealed that fibroblasts mediate cisplatin resistance through release of thiols such as glutathione and cysteine, and that this protective effect is countered by CD8+ T cell-mediated IFNγ signaling.[123] In a clinical cohort of HG-EOC patients, stromal fibroblasts were negatively associated with chemotherapy response and patient survival, while CD8+ T cell levels were associated with improved treatment response and overall survival. Thus, an additional mechanism through which ICB may promote tumor cell killing is by overcoming protective effects mediated by stromal cells.
Reliable DNA Repair Biomarkers Are Needed to Identify Past and Present DNA Repair Deficiency in Tumors
Whereas resistance to DNA repair-based therapies is often driven by restoration of DNA repair pathway function (such as restoration of HR in tumors with acquired PARP inhibitor resistance), reversal of an underlying tumor DNA repair defect has not been described as a mechanism of resistance to ICB. Although a DNA repair deficiency may contribute to ICB sensitivity through generation of tumor-specific neoantigens, correcting an underlying DNA repair defect would not reverse the hundreds or thousands of somatic alterations that had accumulated across the lifetime of a cancer cell.
This principle highlights one of the fundamental limitations of using features such as tumor mutational burden or mutational signatures to define the DNA repair status of a tumor: mutational burdens (and mutational signatures) represent a ‘historical’ record of DNA damage and repair events in a cell, but do not reflect the current DNA repair functional status. Therefore, developing and validating assays that provide information regarding real-time DNA repair function (such as expression-based or functional tests) remains an important challenge.
Although a clear example has not yet been described, it is plausible that a specific somatic mutation responsible for generating a neoantigen that elicits a strong host immune response could be reversed or silenced as a mechanism to decrease tumor immunogenicity (if the mutation is not a cancer driver). Along these lines, deletion of large chromosomal segments has been described as a mechanism to eliminate clonal neoantigens.[60] Perhaps the clearest examples of coding mutations leading to clinical ICB resistance are truncating mutations in JAK1 or JAK2 that result in abrogation of IFNγ-mediated signaling and a truncating mutation in beta-2-microglobulin (B2M) that results in loss of cancer cell MHC class I expression.[124] It is likely that novel mechanisms of ICB resistance involving gene mutation(s) will continue to emerge as ICB experience grows.
Combining DNA Damage and Repair-based Therapies with Immunotherapy
DNA-damaging chemotherapy
Traditionally, most conventional chemotherapies, including direct DNA-damaging agents, have been considered to be immunosuppressive, and lymphopenia remains one of the most common dose-limiting toxicities of cytotoxic chemotherapy. However, an increasing body of empiric and experimental evidence now suggests that some chemotherapies, delivered at standard doses, can promote immunogenic tumor cell death and shape the tumor microenvironment to promote anti-tumor immunity.
The first mechanism by which chemotherapy has been shown to activate the host immune system is through induction of immunogenic cell death pathways.[125] Unlike apoptosis, which is typically considered to be non-immunogenic, chemotherapy can result in cell killing accompanied by release of tumor cell antigens. Chemotherapy-induced cellular stress promotes surface expression and secretion of danger-associated molecular patterns (DAMPs), which increase the cell’s immunogenicity.[100] In addition to cytosolic DNA, a potent DAMP, a variety of cellular proteins have also been found to act as DAMPs, including High Mobility Group Box 1 (HMGB1), calreticulin, hyaluronan, and heat-shock proteins.[125] Released DAMPs bind receptors on cancer and stromal cells and elicit a host immune response that resembles the response to pathogens. DAMP activation promotes secretion of type I IFN and other chemokines, which are required by DCs to activate tumor-specific CD8+ T cells.[126] The importance of DAMP-mediated immune activation has been demonstrated in studies linking polymorphisms in DAMP receptors such as toll-like receptor 4 (TLR4) and the purinergic receptor P2FX7 with decreased response to DNA-damaging agents and worse prognosis in several tumor types.[127, 128]
Numerous chemotherapy classes have been shown to induce immunogenic cell death.[129] For example, increased numbers of TILs were observed following neoadjuvant paclitaxel in a cohort of breast cancer patients, and the extent of TIL response correlated with clinical response.[130] Anthracyclines activate expression of Toll-like receptor-3 (TLR3) and type I IFN secretion, resulting in immunogenic tumor cell death[131], and a type I IFN gene signature predicted response to anthracycline therapy in several cohorts of breast cancer patients.[132] Recently, anthracycline-induced antitumor immunity was shown to require formyl peptide receptor 1 (FPR1) to mediate the interaction between dying cancer cells and host T cells.[133] Elegant work in mice has shown that autophagy is required for immune cell recruitment following chemotherapy and that suppression of autophagy inhibits release of the inflammasome inducer ATP from dying tumor cells.[134] DNA-damaging chemotherapy can also increase expression of MHC class I and cancer-testis antigens as well as decrease expression of inhibitory mediators such as PD-L1 from the cancer cell surface.[135, 136]
A second mechanism by which DNA-damaging chemotherapies can elicit antitumor immunity is through effects on the tumor microenvironment, including immune regulatory cell activity and the tumor vasculature. Numerous feedback mechanisms exist to curtail the host immune response, and chemotherapies have been shown to downregulate these inhibitory signals in several settings. For instance, drugs such as gemcitabine, cyclophosphamide, paclitaxel, fludarabine, and 5-fluorouracil have each been shown to suppress Treg or myeloid-derived suppressor cell (MDSC) function in experimental models.[137–140] Immune activation can also be achieved through upregulation of DC function, and chemotherapy agents such as cyclophosphamide have been shown to increase the number and activity of DCs.[141, 142] In many cases, these effects appear to be dependent on the dose and timing of chemotherapy administration, and additional studies are needed to determine if they are active at clinically-relevant doses and schedules.
Given the interplay between DNA damaging agents and the tumor immune response, it is perhaps not surprising that several lines of evidence now suggest cytotoxic chemotherapy can sensitize tumors to ICB. For example, pre-treatment with oxaliplatin and cyclophosphamide was sufficient to induce sensitivity to host T cell immunity in a lung adenocarcinoma mouse model.[143] Similarly, decitabine enhanced lymphocyte function and synergized with CTLA-4 blockade in ovarian cancer.[144] Furthermore, the combination of gemcitabine and CTLA-4 blockade induced robust antitumor immune responses in two non-immunogenic lung cancer models[145], and CTLA-4 blockade reduced tumor cell repopulation between cisplatin cycles in a murine mesothelioma model.[146] Reciprocally, knockdown of IDO, a negative immune regulator, sensitizes cells to gemcitabine and methoxyamine, a novel inhibitor of base excision repair (BER).[147]
Clinical attempts to combine conventional chemotherapy with immune-modulating agents date back more than 20 years. Several ‘biochemotherapy’ approaches involving concurrent delivery of cytototoxic chemotherapy with immune-stimulating agents such as IL-2 and/or IFNα have been investigated, primarily in metastatic melanoma. While some combinations appeared to be active and several trials showed an improvement in some disease-related endpoints, the lack of overall survival benefit and concerns regarding toxicity limited widespread clinical incorporation of these approaches.[148, 149]
The first clinical trial comparing ICB plus chemotherapy to chemotherapy alone was reported in 2011 and included patients with metastatic melanoma randomized to dacarbazine with or without ipilumimab.[41] The addition of ipilumimab led to an improvement in median overall survival of approximately 2 months; however, it did not appear that the combination provided a benefit compared to ipilumimab alone and may have contributed to an increase in observed hepatic toxicity.
Similar combination trials of chemotherapy and CTLA-4 blockade have been reported in lung cancer. In two studies, the addition of ipilumimab to carboplatin and paclitaxel using a phase dosing schedule (ipilumimab delivered with cycles 3–6 of carboplatin/paclitaxel) resulted in improved immune-related PFS whereas the addition of ipilumimab using a concurrent schedule (delivered with cycles 1–4 of carboplatin/paclitaxel) was not significantly better than carboplatin/paclitaxel alone.[150, 151]
Numerous combination chemotherapy-immunotherapy trials are now underway and are testing a variety of agents in different dosing and timing regimens (Figure 2). Given the complex interaction between DNA-damaging agents and immune function, it is likely that the optimal timing of DNA-damaging chemotherapy with immune checkpoint blockade (or other immune-directed agents) will vary across disease settings. For example, ICB prior to chemotherapy may improve response by reducing the impact of chemotherapy-induced PD-L1 upregulation in some settings, whereas in other instances, DNA damage-induced tumor immunogenicity may be required to drive subsequent ICB response.
Ionizing radiation
The interaction between the immune system and ionizing radiation has been appreciated for many decades. Therapeutic radiation creates numerous types of DNA damage, including double-strand breaks (DSBs).[152] Historically, radiation was associated with immunosuppression due to the exquisite sensitivity of lymphocytes to DNA damage-induced apoptosis.[153] However, technical improvements in radiation delivery now allow high doses of radiation to be delivered to tumors while avoiding excessive bone marrow exposure, and recently, focus has turned to the potential therapeutic interactions between radiation and the immune system.
The immune system can impact radiation-associated tumor control both locally (within the radiation field) and distantly (at unirradiated tumor sites). Radiation has been shown to have numerous immunostimulatory effects on the local tumor environment[154], including a dose-dependent upregulation of MHC class I [155, 156] and co-stimulatory molecules such as CD86 and CD70 on dendritic cells.[157] Radiation activates chemokine release that stimulates DCs[158], promotes cross-presentation of tumor antigens[159], attracts TILs[160], and enhances TIL extravasation via upregulation of cell adhesion molecules.[161] Additionally, radiotherapy stimulates release of DAMPs such as HMGB1[162] and increases FAS expression to promote caspase-induced apoptosis.[163, 164]
Case reports of systemic responses following focal radiation – the so-called ‘abscopal effect’ – date back more than half a century.[165, 166] However, despite intense interest, the molecular underpinnings of the abscopal effect remain incompletely understood. Some of the earliest experimental evidence for the role of the immune system in mediating a radiation-induced abscopal effect came from experiments performed in mice bearing bilateral syngeneic tumors. Treatment with Flt3-Ligand (Flt3-L) increased the DC population and promoted regression of an unirradiated tumor following radiation of the contralateral tumor.[167] One of the first attempts to harness the immune system to increase the efficacy of radiation in a clinical setting involved treatment with granulocyte-macrophage colony-stimulating factor (GM-CSF) and local radiation to a metastatic site of disease, and abscopal responses were observed in approximately 25% of patients.[168]
An abscopal effect has also been observed in patients receiving localized radiation and immune checkpoint blockade. For example, a patient with metastatic melanoma receiving anti-CTLA-4 therapy had marked regression of multiple metastatic lesions following hypofractionated radiation to a symptomatic paraspinal mass.[169] Post-radiation antibody titers against the cancer-testis antigen NY-ESO-1 increased 30-fold relative to pre-radiation levels, and changes in immune cell profiles were consistent with radiation-induced T cell activation. Numerous additional examples of shrinkage of non-irradiated lesions in patients receiving combined radiation and anti-CTLA-4 therapy have now been reported.[170, 171]
Several studies have now begun to unravel the mechanisms by which ICB increases radiation sensitivity and improves radiotherapy-mediated tumor control. Radiation increases PD-L1 expression on tumor cells, and this inhibitory effect can be overcome with PD-L1 blockade[172, 173]. An elegant study showed that radiation, anti-CTLA4, and anti-PD-1/PD-L1 agents function in non-redundant ways to activate the immune system.[174] Data from melanoma patients and preclinical melanoma models suggest that while radiation diversifies the T cell repertoire and anti-CTLA-4 therapy inhibits Tregs, anti-PD-L1 therapy is necessary to overcome resistance driven by increased PD-L1 expression on tumor cells.
Despite the plethora of mechanisms by which radiotherapy appears to stimulate the immune system, conventionally fractionated radiotherapy alone does not typically produce an abscopal effect, suggesting that the immunostimulatory effects of radiotherapy that have been extensively characterized in preclinical systems are insufficient to overcome the immunosuppressive microenvironment in most human tumors. In addition to upregulation of PD-L1 on tumor cells, radiation can activate Tregs in some settings[175, 176] and dampen the immune response via TGFβ-mediated signaling.[177] Interestingly, in a mouse model of lung cancer, PD-1 blockade synergized with radiation in the upfront setting but had no therapeutic benefit (and instead induced T cell inhibitory markers) in tumors that had relapsed after radiation.[178]
Numerous clinical trials are now underway that combine radiation with ICB in both the upfront and metastatic settings.[179] The optimal radiation dose, timing, and fractionation for maximizing the therapeutic interaction with ICB is currently not known, but it is likely that a one-size-fits-all approach will not apply. Given that cellular effects of radiation-induced DNA damage are strongly dose- and cell context-dependent, it is reasonable to assume that the downstream immunologic effects of DNA damage vary depending on the tumor DNA repair landscape, which in turn varies widely across histology, molecular subtype, and clinical context.[180]
DNA Repair Targeted Therapies
Finally, there is emerging evidence that combining ICB with DNA repair targeted agents such as PARP inhibitors may be a useful therapeutic strategy. Administration of BMN 673, a PARP1 inhibitor, increased intratumoral CD8+ T cells and drove production of IFNγ and TNFα in syngeneic BRCA1-deficient murine ovarian tumors[181], and adding CTLA-4 blockade to PARP inhibition further increased IFNγ production and T cell activation and extended survival compared to PARP inhibition alone.[182] However, PARP inhibition may also induce immunosuppressive effects such as upregulation of PD-L1[183], providing further rationale for combining ICB with PARP inhibition.
Further work is needed to uncover the mechanisms by which PARP inhibitors or other DNA repair-directed agents (such as ATM, ATR, or DNA-PKcs inhibitors) modulate the tumor immune environment and impact sensitivity to ICB. However, it is reasonable to hypothesize that genomic instability induced by disruption of normal DNA repair pathway function could result in increased tumor mutational burden, neoantigens, and/or STING pathway activation, all of which could contribute to heightened ICB sensitivity. Several clinical trials are now underway to test the safety and efficacy of combinations of DNA repair-targeted agents with ICB agents in both DNA repair-deficient and DNA-repair proficient settings (Table 2).[184] As clinical experience with both DNA repair targeted agents and ICB agents grows, and as the multifaceted cellular interactions between DNA repair and the immune system come into focus, it is likely that combination approaches will continue to emerge.
Table 2
Trials Combining Agents Targeting DNA Repair and ICB
| Study Identifier | Agents | Class of drugs | Design | Patients/ Estimated Accrual | Primary Endpoint |
|---|---|---|---|---|---|
| NCT02657889 | Niraparib plus Pembrolizumab | PARP inhibitor plus anti-PD-1 antibody | Phase I/II | Recurrent Triple Negative Breast Cancer (TNBC) or Ovarian Cancer (OC)N=114 | RP2D (Phase I) ORR (Phase II) |
| NCT02660034 | BGB-290 plus BGB-A317 | PARP inhibitor plus anti-PD-1 antibody | Phase IA/IB | Advanced Solid TumorsN=124 | RP2D (Phase I) ORR (Phase II) |
| NCT02849496 | Veliparib plus Atezolizumab | PARP inhibitor plus anti-PD-L1 antibody | Phase II, Open Label, Randomized | Unresectable Stage III or IV BRCA1/2-mutated TNBCN=90 | PFS |
| NCT02734004 | Olaparib plus Durvalumab | PARP inhibitor plus anti-PD-L1 antibody | Phase I/II | Advanced/Recurrent Solid TumorsN=133 | Safety and Disease Control Rate |
| NCT02484404 | Olaparib plus Durvalumab plus Cediranib | PARP inhibitor plus anti-PD-L1 antibody plus VEGFR1,2,3 inhibitor | Phase I/II | Advanced/Recurrent Solid TumorsN=338 | RP2D (Phase I) ORR or PFS (Phase II) |
| NCT02571725 | Olaparib plus Tremelimumab | PARP inhibitor plus anti-CTLA-4 antibody | Phase I/II | Recurrent BRCA1/2-mutated OCN=50 | RP2D (Phase I)ORR (Phase II) |
| NCT02953457 | Olaparib plus Durvalumab plus Tremelimumab | PARP inhibitor plus anti-PD-L1 antibody plus anti-CTLA-4 antibody | Phase I/II | Recurrent BRCA1/2-mutated OCN=39 | MTD (Phase I)PFS (Phase II) |
| NCT02264678 | AZD6738 plus Durvalumab | ATR inhibitor plus anti-PD-L1 antibody | Phase I | Advanced Head and Neck Squamous cell Carcinoma or Non Small Cell Lung CancerN=114 (all arms, including other combinations) | RP2D |
R2PD: recommended Phase 2 dose
ORR: overall response rate
MTD: maximum tolerated dose
PFS: progression-free survival
Conclusions and On-Going Efforts
Genomic instability is a hallmark of tumors and underlies many fundamental cancer cell properties. Recently, it has become clear that DNA damage and repair have a major impact on the interaction between the tumor and the immune system, and furthermore, that the DNA damage and repair landscape has important therapeutic implications in this context.
Immune-directed therapies are rapidly reshaping the landscape of clinical oncology. Immune checkpoint inhibitors are approved in a variety of settings, and numerous additional approvals are inevitable in the coming years. Although ICB induces durable responses in many patients, response rates vary significantly both within and across tumor types. Therefore, a major challenge remains to identify predictive biomarkers that can guide the use of ICB agents as monotherapy or as part of a combined treatment strategy involving DNA-damaging agents or targeted therapies.
Several lines of evidence suggest that DNA repair represents an important biomarker of ICB response. Tumors with MMR deficiency have high response rates to ICB, and the FDA recently granted Breakthrough Therapy Designation to pembroluzimab for treatment of MSI-H colorectal and non-colorectal tumors. Numerous on-going studies are now investigating activity of ICB agents in other DNA repair-deficient settings, including tumors with mutations in BRCA1/2 or POLE.
The basis for many of these studies is the observation that DNA repair deficiency often leads to increased mutational load and neoantigen burden, both of which have been correlated with ICB response in a variety of settings. However, the relationship between DNA repair and ICB response is clearly more complex. High mutational burden is neither necessary nor sufficient to drive ICB response, and it is likely that distinct DNA lesions arising from different DNA repair deficient backgrounds may produce very different immunologic effects. A more thorough understanding of the impact of genomic instability – in all its forms – on ICB response will require contributions from DNA repair experts, computational biologists, immunologists, and clinicians.
Although genomic instability has a clear association with ICB response in some settings, the most powerful tools for predicting ICB response will likely integrate multiple types of data from across platforms. For example, combining genomic instability markers such as mutational burden with transcriptional or IHC-based readouts of immune activity such as tumor and stromal T cell populations, IFNγ-related gene expression, and STING pathway activity may allow for development of “immunoscores” with improved sensitivity and specificity.[70]
Ultimately, the most common clinical setting in which ICB agents may be used is in the upfront setting in combination with standard-of-care therapies, which often include DNA-damaging agents. Little is currently known regarding the mechanistic interactions between DNA-damaging agents and ICB, and both immunosuppressive and immune-activating properties of DNA-damaging agents have been described. Progress in this area will be driven both by empiric data from on-going and planned clinical trials as well as from carefully planned in vitro and in vivo studies designed to unravel the complexities of the interaction between DNA damage and immune-modulating agents.
Acknowledgments
K.W.M. is funded by The American Society of Radiation Oncology (ASTRO), the Bladder Cancer Advocacy Network (BCAN), and the KL2/Catalyst Medical Research Investigator Training award (an appointed KL2 award) from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award KL2 TR001100).
M.S.G. is funded by the Ovarian Cancer Research Fund Alliance (Liz Tilberis Scholar), the Melanoma Research Alliance (Team Science Awards), and the Susan F. Smith Center for Women’s Cancers.
P.K. is funded by the Department of Defense Ovarian Cancer Research Program (DOD OCRP), award number W81XWH-15-1-0564.
A.D.D. is funded by the Stand Up To Cancer-Ovarian Cancer Research Fund- Ovarian Cancer National Alliance-National Ovarian Cancer Coalition Dream Team Translational Research Grant (Grant Number: SU2C-AACR-DT16-15). Stand Up To Cancer is a program of the Entertainment Industry Foundation. Research grants are administered by the American Association for Cancer Research, the Scientific Partner of SU2C.
This work was also supported by grants from the U.S. National Institutes of Health (R01DK43889, R37HL052725, P01HL048546, P50CA168504), the U.S. Department of Defense (BM110181), the Leukemia and Lymphoma Society (6237-13), the Breast Cancer Research Foundation, and the Fanconi Anemia Research Fund.
Footnotes
Conflicts of Interest: P.K. has served on advisory boards for Vertex, Pfizer, and Merck.
References
Full text links
Read article at publisher's site: https://doi.org/10.1158/2159-8290.cd-17-0226
Read article for free, from open access legal sources, via Unpaywall:
https://cancerdiscovery.aacrjournals.org/content/candisc/7/7/675.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Tumor organoids improve mutation detection of pancreatic ductal adenocarcinoma.
Sci Rep, 14(1):25468, 26 Oct 2024
Cited by: 0 articles | PMID: 39462012 | PMCID: PMC11513084
Synergistic Potential of Nanomedicine in Prostate Cancer Immunotherapy: Breakthroughs and Prospects.
Int J Nanomedicine, 19:9459-9486, 02 Oct 2024
Cited by: 0 articles | PMID: 39371481 | PMCID: PMC11456300
Review Free full text in Europe PMC
Personalized treatment approach for HER2-positive metastatic breast cancer.
Med Oncol, 41(11):252, 25 Sep 2024
Cited by: 1 article | PMID: 39320608
Review
Inflammation-related markers and prognosis of alpha-fetoprotein producing gastric cancer.
World J Gastrointest Oncol, 16(9):3875-3886, 01 Sep 2024
Cited by: 0 articles | PMID: 39350978 | PMCID: PMC11438777
Application of PARP inhibitors combined with immune checkpoint inhibitors in ovarian cancer.
J Transl Med, 22(1):778, 21 Aug 2024
Cited by: 0 articles | PMID: 39169400 | PMCID: PMC11337781
Review Free full text in Europe PMC
Go to all (380) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials (Showing 10 of 10)
- (1 citation) ClinicalTrials.gov - NCT02734004
- (1 citation) ClinicalTrials.gov - NCT02660034
- (1 citation) ClinicalTrials.gov - NCT02953457
- (1 citation) ClinicalTrials.gov - NCT02571725
- (1 citation) ClinicalTrials.gov - NCT02657889
- (1 citation) ClinicalTrials.gov - NCT02849496
- (1 citation) ClinicalTrials.gov - NCT02264678
- (1 citation) ClinicalTrials.gov - NCT01876511
- (1 citation) ClinicalTrials.gov - NCT02484404
- (1 citation) ClinicalTrials.gov - NCT02912572
Show less
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Diverse immune response of DNA damage repair-deficient tumors.
Cell Rep Med, 2(5):100276, 18 May 2021
Cited by: 15 articles | PMID: 34095878 | PMCID: PMC8149377
DNA repair defects and implications for immunotherapy.
J Clin Invest, 128(10):4236-4242, 01 Oct 2018
Cited by: 51 articles | PMID: 30272580 | PMCID: PMC6159999
Review Free full text in Europe PMC
The aberrant expression of rhythm genes affects the genome instability and regulates the cancer immunity in pan-cancer.
Cancer Med, 9(5):1818-1829, 12 Jan 2020
Cited by: 14 articles | PMID: 31927791 | PMCID: PMC7050078
Error-prone DNA repair pathways as determinants of immunotherapy activity: an emerging scenario for cancer treatment.
Int J Cancer, 147(10):2658-2668, 01 Jun 2020
Cited by: 11 articles | PMID: 32383203
Review
Funding
Funders who supported this work.
NCATS NIH HHS (1)
Grant ID: KL2 TR001100
NCI NIH HHS (1)
Grant ID: P50 CA168504
NHLBI NIH HHS (3)
Grant ID: R01 HL052725
Grant ID: P01 HL048546
Grant ID: R37 HL052725
NIDDK NIH HHS (2)
Grant ID: R56 DK043889
Grant ID: R01 DK043889