Abstract
Importance
Depressive disorders (DDs), anxiety disorders (ADs), obsessive-compulsive disorder (OCD), and posttraumatic stress disorder (PTSD) are common mental disorders in children and adolescents.Objective
To examine the relative efficacy and safety of selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), and placebo for the treatment of DD, AD, OCD, and PTSD in children and adolescents.Data sources
PubMed, EMBASE, PsycINFO, Web of Science, and Cochrane Database from inception through August 7, 2016.Study selection
Published and unpublished randomized clinical trials of SSRIs or SNRIs in youths with DD, AD, OCD, or PTSD were included. Trials using other antidepressants (eg, tricyclic antidepressants, monoamine oxidase inhibitors) were excluded.Data extraction and synthesis
Effect sizes, calculated as standardized mean differences (Hedges g) and risk ratios (RRs) for adverse events, were assessed in a random-effects model.Main outcomes and measures
Primary outcomes, as defined by authors on preintervention and postintervention data, mean change data, and adverse event data, were extracted independently by multiple observers following PRISMA guidelines.Results
Thirty-six trials were eligible, including 6778 participants (3484 [51.4%] female; mean [SD] age, 12.9 [5.1] years); 17 studies for DD, 10 for AD, 8 for OCD, and 1 for PTSD. Analysis showed that SSRIs and SNRIs were significantly more beneficial compared with placebo, yielding a small effect size (g = 0.32; 95% CI, 0.25-0.40; P < .001). Anxiety disorder (g = 0.56; 95% CI, 0.40-0.72; P < .001) showed significantly larger between-group effect sizes than DD (g = 0.20; 95% CI, 0.13-0.27; P < .001). This difference was driven primarily by the placebo response: patients with DD exhibited significantly larger placebo responses (g = 1.57; 95% CI, 1.36-1.78; P < .001) compared with those with AD (g = 1.03; 95% CI, 0.84-1.21; P < .001). The SSRIs produced a relatively large effect size for ADs (g = 0.71; 95% CI, 0.45-0.97; P < .001). Compared with participants receiving placebo, patients receiving an antidepressant reported significantly more treatment-emergent adverse events (RR, 1.07; 95% CI, 1.01-1.12; P = .01 or RR, 1.49; 95% CI, 1.22-1.82; P < .001, depending on the reporting method), severe adverse events (RR, 1.76; 95% CI, 1.34-2.32; P < .001), and study discontinuation due to adverse events (RR, 1.79; 95% CI, 1.38-2.32; P < .001).Conclusions and relevance
Compared with placebo, SSRIs and SNRIs are more beneficial than placebo in children and adolescents; however, the benefit is small and disorder specific, yielding a larger drug-placebo difference for AD than for other conditions. Response to placebo is large, especially in DD. Severe adverse events are significantly more common with SSRIs and SNRIs than placebo.Free full text

Efficacy and Safety of Selective Serotonin Reuptake Inhibitors, Serotonin-Norepinephrine Reuptake Inhibitors, and Placebo for Common Psychiatric Disorders Among Children and Adolescents
Abstract
Importance
Depressive disorders (DDs), anxiety disorders (ADs), obsessive-compulsive disorder (OCD), and posttraumatic stress disorder (PTSD) are common mental disorders in children and adolescents.
Objective
To examine the relative efficacy and safety of selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), and placebo for the treatment of DD, AD, OCD, and PTSD in children and adolescents.
Data Sources
PubMed, EMBASE, PsycINFO, Web of Science, and Cochrane Database from inception through August 7, 2016.
Study Selection
Published and unpublished randomized clinical trials of SSRIs or SNRIs in youths with DD, AD, OCD, or PTSD were included. Trials using other antidepressants (eg, tricyclic antidepressants, monoamine oxidase inhibitors) were excluded.
Data Extraction and Synthesis
Effect sizes, calculated as standardized mean differences (Hedges g) and risk ratios (RRs) for adverse events, were assessed in a random-effects model.
Main Outcomes and Measures
Primary outcomes, as defined by authors on preintervention and postintervention data, mean change data, and adverse event data, were extracted independently by multiple observers following PRISMA guidelines.
Results
Thirty-six trials were eligible, including 6778 participants (3484 [51.4%] female; mean [SD] age, 12.9 [5.1] years); 17 studies for DD, 10 for AD, 8 for OCD, and 1 for PTSD. Analysis showed that SSRIs and SNRIs were significantly more beneficial compared with placebo, yielding a small effect size (g =
= 0.32; 95% CI, 0.25-0.40; P
0.32; 95% CI, 0.25-0.40; P <
< .001). Anxiety disorder (g
.001). Anxiety disorder (g =
= 0.56; 95% CI, 0.40-0.72; P
0.56; 95% CI, 0.40-0.72; P <
< .001) showed significantly larger between-group effect sizes than DD (g
.001) showed significantly larger between-group effect sizes than DD (g =
= 0.20; 95% CI, 0.13-0.27; P
0.20; 95% CI, 0.13-0.27; P <
< .001). This difference was driven primarily by the placebo response: patients with DD exhibited significantly larger placebo responses (g
.001). This difference was driven primarily by the placebo response: patients with DD exhibited significantly larger placebo responses (g =
= 1.57; 95% CI, 1.36-1.78; P
1.57; 95% CI, 1.36-1.78; P <
< .001) compared with those with AD (g
.001) compared with those with AD (g =
= 1.03; 95% CI, 0.84-1.21; P
1.03; 95% CI, 0.84-1.21; P <
< .001). The SSRIs produced a relatively large effect size for ADs (g
.001). The SSRIs produced a relatively large effect size for ADs (g =
= 0.71; 95% CI, 0.45-0.97; P
0.71; 95% CI, 0.45-0.97; P <
< .001). Compared with participants receiving placebo, patients receiving an antidepressant reported significantly more treatment-emergent adverse events (RR, 1.07; 95% CI, 1.01-1.12; P
.001). Compared with participants receiving placebo, patients receiving an antidepressant reported significantly more treatment-emergent adverse events (RR, 1.07; 95% CI, 1.01-1.12; P =
= .01 or RR, 1.49; 95% CI, 1.22-1.82; P
.01 or RR, 1.49; 95% CI, 1.22-1.82; P <
< .001, depending on the reporting method), severe adverse events (RR, 1.76; 95% CI, 1.34-2.32; P
.001, depending on the reporting method), severe adverse events (RR, 1.76; 95% CI, 1.34-2.32; P <
< .001), and study discontinuation due to adverse events (RR, 1.79; 95% CI, 1.38-2.32; P
.001), and study discontinuation due to adverse events (RR, 1.79; 95% CI, 1.38-2.32; P <
< .001).
.001).
Conclusions and Relevance
Compared with placebo, SSRIs and SNRIs are more beneficial than placebo in children and adolescents; however, the benefit is small and disorder specific, yielding a larger drug-placebo difference for AD than for other conditions. Response to placebo is large, especially in DD. Severe adverse events are significantly more common with SSRIs and SNRIs than placebo.
Key Points
Question
Is there a scientific justification to prescribe selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors for children and adolescents, based on what is known about their efficacy and safety?
Findings
In a systematic review and meta-analysis including 36 trials (6778 participants), selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors were significantly more beneficial compared with placebo in treating common pediatric psychiatric disorders, yet also led to significantly more treatment-emergent and severe adverse events, such as suicide ideation and suicide attempts, as well as study discontinuation due to adverse events. The magnitude of the effect and adverse event profiles were disorder dependent.
Meaning
There is some evidence for the benefit of selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors in children and adolescents, but owing to the higher risk for severe adverse events, a cautious and individual cost-benefit analysis is of importance.
Introduction
Depressive disorders (DDs), anxiety disorders (ADs), obsessive-compulsive disorder (OCD), and posttraumatic stress disorder (PTSD) are among the most common mental disorders in children and adolescents. They are major public health concerns and predict long-term risk for various adverse outcomes. Thus, early diagnosis and proper treatment is of critical importance. Selective serotonin reuptake inhibitors (SSRIs) are first-line pharmaceutical treatments for these disorders, whereas serotonin-norepinephrine reuptake inhibitors (SNRIs) are considered second- or third-line treatments, given the limited available trial data to support their use. This meta-analysis compares the differential efficacy of these drugs across the disorders for which they are primarily prescribed in a pediatric population and also assesses differences in response to placebo and in adverse events.
Since the release of fluoxetine hydrochloride in the mid-1980s, the number of SSRIs and SNRIs has grown substantially. However, their use in children and adolescents is still debated, thus indicating a need for more research into their safety and efficacy and the comparative efficacy of the newer SNRIs vs SSRIs. Recent meta-analyses generate many questions about the overall benefits vs costs of using SSRIs to treat major depression in children and adolescents. The small amount of research on SNRIs for pediatric DD has had mixed results. One meta-analysis on pediatric depression found that, although SSRIs differed significantly from placebo, SNRIs and tricyclic antidepressants did not.
Although most prior reviews and meta-analyses of the effects of SSRIs and SNRIs focused on pediatric DD, considerable data also exist on pediatric AD and OCD. The latter studies suggest that most SSRIs have a favorable risk-benefit ratio, whereas there are insufficient data for the remaining SSRIs. There have been relatively few studies on SNRIs for pediatric AD, despite the fact that the only US Food and Drug Administration (FDA)–approved agent for pediatric AD, duloxetine hydrochloride, is an SNRI. To our knowledge, no double-blind, randomized clinical trials of SNRIs for pediatric OCD had been conducted as of 2016, and limited data have been reported for SSRIs and SNRIs in pediatric PTSD.
Research on safety and tolerability indicates a high risk of developing treatment-emergent adverse events (TEAEs)—most prominently headache and nausea—during treatment with an antidepressant in pediatric DD. Severe adverse events (SAEs), such as an increased risk of suicidal thoughts and behavior, in adults and youth receiving antidepressants have also been reported, leading to the implementation of a boxed warning on the labels of all antidepressants for pediatric use by the FDA in 2004, although adoption of the warning remains controversial. In addition, to date no recent meta-analyses have focused on how pediatric adverse effect profiles of SSRIs, SNRIs, and placebo might differ across disorders.
Finally, there is a growing body of literature concerning the role of placebo effects in studies of SSRIs and SNRIs, based on large placebo responses in studies of antidepressants in both adult and pediatric samples. Factors such as contact with research staff may lead to large placebo response rates in pediatric DD and may explain much of the variability in pediatric antidepressant trials. For adults with DD, a genuine placebo effect has been demonstrated, as the combination of placebo and supportive care has been shown to be more beneficial than supportive care alone. Conversely, patients in the placebo group also demonstrate TEAEs. However, how response to placebo differs across disorders or other study design features in pediatrics remains understudied.
To our knowledge, only 1 other review or meta-analysis has examined the use of SSRIs and SNRIs across pediatric DD, AD, OCD, and PTSD. However, that earlier study is now a decade old and predates 11 primary studies (n =
= 2068) that fulfill our inclusion criteria. The earlier review also did not include any studies on duloxetine, which is currently the only medication approved for pediatric AD. We therefore conducted an updated and extended review to assess the efficacy and safety of these drugs for treatment of DD, AD, OCD, and PTSD, along with between-disorder variation in drug and placebo responses. Psychological therapies are not part of this meta-analysis. However, a more recent review has compared psychological therapies alone and in combination with antidepressant medication for depression in children and adolescents.
2068) that fulfill our inclusion criteria. The earlier review also did not include any studies on duloxetine, which is currently the only medication approved for pediatric AD. We therefore conducted an updated and extended review to assess the efficacy and safety of these drugs for treatment of DD, AD, OCD, and PTSD, along with between-disorder variation in drug and placebo responses. Psychological therapies are not part of this meta-analysis. However, a more recent review has compared psychological therapies alone and in combination with antidepressant medication for depression in children and adolescents.
Methods
Search Strategy and Study Selection
The study was conducted in accordance with the PRISMA statement. We searched PubMed, EMBASE, PsycInfo, Cochrane, and Web of Science from inception until August 7, 2016; clinicaltrials.gov; and fda.gov and checked references of the included studies as well as previous reviews. Additional information on search terms is presented in the eAppendix 1 in the Supplement. In total, this search returned 4899 articles (eFigure 1 in the Supplement). The screening and selection process was conducted independently by 3 of us (C.L., H.K., and S.R.Z.). We included randomized, double-blind, placebo-controlled trials of SSRIs and SNRIs in children and adolescents younger than 18 years, including studies that examined drug vs placebo, both in the context of a psychosocial intervention, in which case the combination group was extracted only if no comparison of drug and placebo alone was given. Participants were required to have a diagnosis of a DD, AD, OCD, or PTSD, based on DSM-III, DSM-III-R, or DSM-IV-TR criteria. Comorbidity was allowed, and information about comorbid disorders was extracted.
Case reports, comments, letters, gray literature, and reviews were excluded. Non–second-generation antidepressants (eg, monoamine oxidase inhibitors, tricyclic antidepressants) were also excluded. Boston Children’s Hospital provided approval for the study.
Methodologic Quality Assessment
Two of us (C.L. and S.R.Z.) independently rated the quality of included studies based on the Cochrane Risk of Bias Assessment Tool, with final quality ratings based on consensus. Risk of bias was assessed in individual studies (eTable 1 in the Supplement) and across studies (eFigure 2 in the Supplement).
Outcome Measures and Data Extraction
The primary outcome as defined by authors was chosen as the sole outcome measure for each study. Preintervention and postintervention data or mean change data had to be available. Outcomes had to be reported on a well-validated, disorder-specific scale (eg, Children's Depression Rating Scale–Revised, Multidimensional Anxiety Scale for Children, and Children's Yale-Brown Obsessive Compulsive Scale) or on a general severity scale (ie, Clinical Global Impression–Severity Scale). We included only continuous outcome data, since dichotomizing continuous scores into categorical outcome data leads to a loss of information, reduces power, and creates an artificial boundary. We did not extract data from improvement scales, such as the Clinical Global Impression-Global Improvement Scale. Repeated attempts were made to contact the authors of studies with incomplete or insufficient data. Two studies did not include SDs or SEs, and they were imputed by way of the leaving-1-out method.
Data were extracted independently by 3 of us (C.L., H.K., and S.R.Z.). Discrepancies were resolved by consensus. Extracted data included demographic information, dropout rates, adverse events, safety information, and baseline and end point assessment points. Data from open-label extensions or follow-up after the predesignated end point were not extracted.
Statistical Analysis
Three effect sizes were calculated for each included study. First, drug-placebo difference response was assessed as the difference in mean change scores between the antidepressants and placebo. The drug and placebo responses were assessed as the mean change scores of preanalyses vs postanalyses in the drug and placebo groups, respectively. Effect sizes were calculated as Hedges g. We chose to use random-effects models rather than fixed-effects models because the studies that we included were heterogeneous and the number of studies for the subanalyses were relatively small. Heterogeneity was assessed by calculating the Q statistic, the τ2, and the I2, a transformation of Q that indicates the proportion of observed variance that can be attributed to heterogeneity rather than sampling error. The τ2 offers an estimate of the variance among true effect sizes. Effect size differences between subgroups were analyzed using a mixed-effects model. Publication bias was assessed visually by means of funnel plots and formally by means of the fail-safe N and the Begg adjusted-rank correlation test. We estimated the sensitivity of publication bias, using the trim-and-fill method.
Moderator analyses were conducted for 6 continuous moderators (treatment duration, publication year, illness duration, age of onset, number of sites, and baseline severity) and 4 categorical moderators (placebo lead-in, comorbidity, region, and primary funding source). Details of the applied statistical approaches are provided in eAppendix 2 in the Supplement.
To evaluate the risk of adverse events in the antidepressant and placebo groups, risk ratios (RRs) for TEAEs, SAEs, and study discontinuation due to adverse events across trials were calculated in a random-effects model. The RRs of SAEs were based on the percentage of patients with SAEs. Regarding RRs of TEAEs, 2 commonly used reporting methods were compared: percentage of patients with TEAEs in each group and mean number of TEAEs per patient across all reported symptoms. Comprehensive Meta-Analysis, version 3 (Biostat) and R, version 3.2.1 (R Foundation) were used for calculations and analyses.
Results
Our search identified 35 published and 1 unpublished randomized, double-blind trials including 6778 participants (3484 [51.4%] female; mean [SD] age, 12.9 [5.1] years) that compared an SSRI or an SNRI against placebo in patients younger than 18 years with a diagnosis of AD (n =
= 10), DD (n
10), DD (n =
= 17), OCD (n
17), OCD (n =
= 8), or PTSD (n
8), or PTSD (n =
= 1) (eFigure 1 in the Supplement). One study reported 2 trials that were treated independently for analyses and another compared a drug plus psychosocial intervention group vs a placebo plus psychosocial intervention group and was therefore excluded from the drug and placebo response analyses. Characteristics of the 36 included trials are presented in eTable 1 in the Supplement, and details regarding heterogeneity and publication bias can be found in the eTable 2, eAppendix 3, eFigure 2, and eFigure 3 in the Supplement.
1) (eFigure 1 in the Supplement). One study reported 2 trials that were treated independently for analyses and another compared a drug plus psychosocial intervention group vs a placebo plus psychosocial intervention group and was therefore excluded from the drug and placebo response analyses. Characteristics of the 36 included trials are presented in eTable 1 in the Supplement, and details regarding heterogeneity and publication bias can be found in the eTable 2, eAppendix 3, eFigure 2, and eFigure 3 in the Supplement.
The combined analysis between groups across all disorders yielded a small drug-placebo difference (g =
= 0.32; 95% CI, 0.25 to 0.40; P
0.32; 95% CI, 0.25 to 0.40; P <
< .001). In the between-group analysis stratified by disorder, AD (g
.001). In the between-group analysis stratified by disorder, AD (g =
= 0.56; 95% CI, 0.40 to 0.72; P
0.56; 95% CI, 0.40 to 0.72; P <
< .001) and OCD (g
.001) and OCD (g =
= 0.39; 95% CI, 0.25 to 0.54; P
0.39; 95% CI, 0.25 to 0.54; P <
< .001) did not differ significantly from each other (P
.001) did not differ significantly from each other (P =
= .14), but both yielded significantly higher (AD vs DD: P
.14), but both yielded significantly higher (AD vs DD: P <
< .001 and OCD vs DD: P
.001 and OCD vs DD: P =
= .02) drug-placebo differences than the DD group (g
.02) drug-placebo differences than the DD group (g =
= 0.20; 95% CI, 0.13 to 0.27; P
0.20; 95% CI, 0.13 to 0.27; P <
< .001) (Figure 1). Excluding the unpublished study in the DD group led to a negligible change in effect size. Between-drug analysis yielded the smallest effect sizes for citalopram (g
.001) (Figure 1). Excluding the unpublished study in the DD group led to a negligible change in effect size. Between-drug analysis yielded the smallest effect sizes for citalopram (g =
= 0.18; 95% CI, −0.18 to 0.54; P
0.18; 95% CI, −0.18 to 0.54; P =
= .33) and escitalopram (g
.33) and escitalopram (g =
= 0.18; 95% CI, 0.01 to 0.34; P
0.18; 95% CI, 0.01 to 0.34; P =
= .03) and the largest effect size for fluvoxamine (g
.03) and the largest effect size for fluvoxamine (g =
= 0.68; 95% CI, −0.05 to 1.41; P
0.68; 95% CI, −0.05 to 1.41; P =
= .07). However, owing to the small number of studies and large 95% CI, the effect size for fluvoxamine was not significant.
.07). However, owing to the small number of studies and large 95% CI, the effect size for fluvoxamine was not significant.
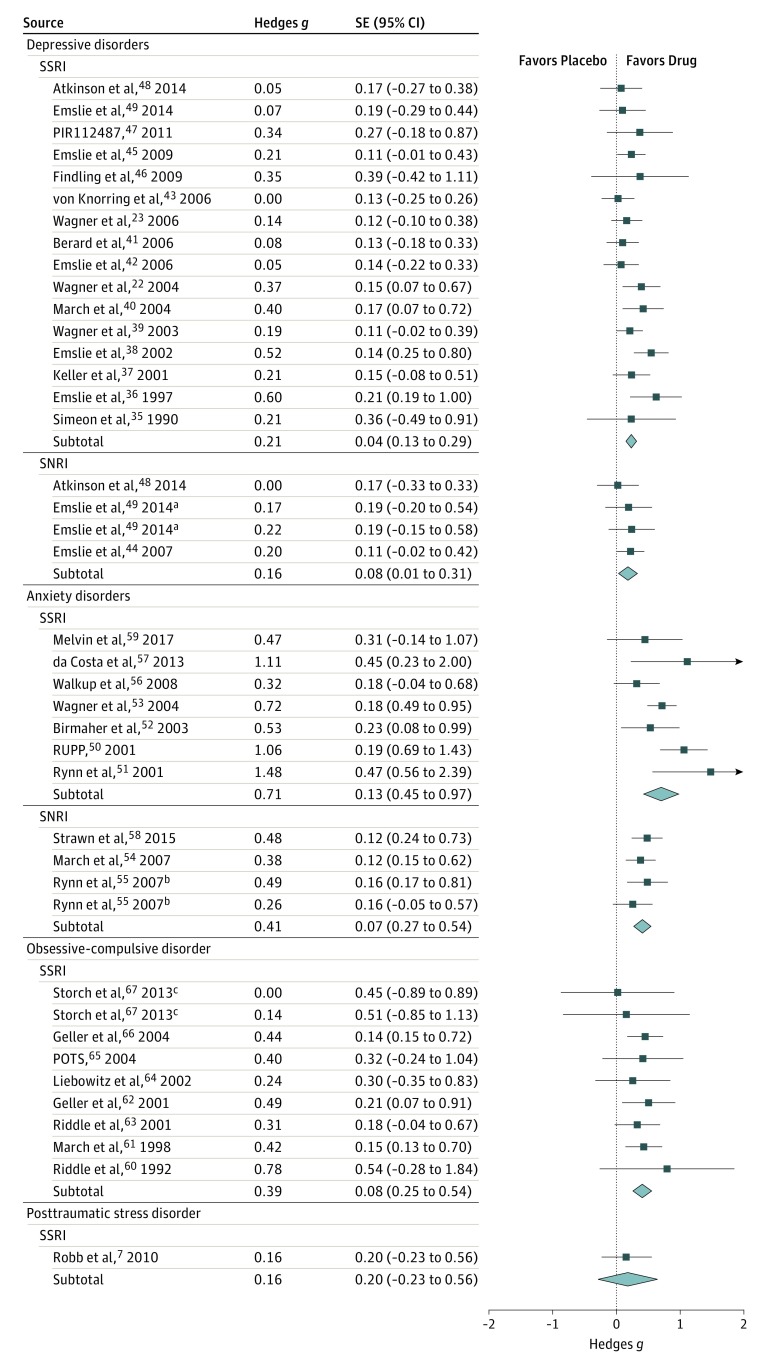
Because there was only 1 study, posttraumatic stress disorder was not included in the overall analysis. POTS indicates Pediatric OCD Treatment Study; RUPP, Research Unit on Pediatric Psychopharmacology Anxiety Study Group; SNRI, serotonin-norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
aOne study reported 2 different dosages of duloxetine.
bOne study reported 2 trials that were treated independently for analyses.
cOne study examined 2 forms of dosing. One treatment arm was sertraline at standard dosing and the second treatment arm was sertraline titrated slowly.
In the between-group analysis stratified by drug category, SSRIs and SNRIs did not differ significantly in the DD group (Q =
= 0.431; P
0.431; P =
= .51), but SSRIs were significantly better than SNRIs in the AD group (Q
.51), but SSRIs were significantly better than SNRIs in the AD group (Q =
= 4.161; P
4.161; P =
= .04). No studies investigated the use of SNRIs in OCD.
.04). No studies investigated the use of SNRIs in OCD.
The within-drug group analysis stratified by disorder yielded no significant difference (P =
= .07) between studies of AD (g
.07) between studies of AD (g =
= 1.68; CI, 1.56-1.79; P
1.68; CI, 1.56-1.79; P <
< .001) and DD (g
.001) and DD (g =
= 1.85; 95% CI, 1.7-2.0; P
1.85; 95% CI, 1.7-2.0; P <
< .001), yet both yielded significantly larger drug responses (P
.001), yet both yielded significantly larger drug responses (P <
< .001) than studies of OCD (g
.001) than studies of OCD (g =
= 1.01; 95% CI, 0.88-1.14; P
1.01; 95% CI, 0.88-1.14; P <
< .001). When stratified by drug, duloxetine yielded the largest response (g
.001). When stratified by drug, duloxetine yielded the largest response (g =
= 1.95; 95% CI, 1.73-2.18; P
1.95; 95% CI, 1.73-2.18; P <
< .001) and fluvoxamine the smallest response (g
.001) and fluvoxamine the smallest response (g =
= 1.22; 95% CI, 0.41-2.02; P
1.22; 95% CI, 0.41-2.02; P =
= .003); however, the difference between those 2 drugs was not significant (Q
.003); however, the difference between those 2 drugs was not significant (Q =
= 3.021; P
3.021; P =
= .08). The combined analysis across all disorders for the within-group analysis yielded a drug response of g
.08). The combined analysis across all disorders for the within-group analysis yielded a drug response of g =
= 1.65 (95% CI, 1.52-1.78; P
1.65 (95% CI, 1.52-1.78; P <
< .001). The SSRIs and SNRIs did not differ significantly in both the DD group (Q
.001). The SSRIs and SNRIs did not differ significantly in both the DD group (Q =
= 2.351; P
2.351; P =
= .13) and the AD group (Q
.13) and the AD group (Q =
= 0.341; P
0.341; P =
= .56).
.56).
The within-placebo group analysis stratified by disorder yielded a large placebo response for studies of DD (g =
= 1.57; 95% CI, 1.36-1.78; P
1.57; 95% CI, 1.36-1.78; P <
< .001), which was significantly larger (P
.001), which was significantly larger (P <
< .001) than the placebo response in studies of AD (g
.001) than the placebo response in studies of AD (g =
= 1.03; 95% CI, 0.84-1.21; P
1.03; 95% CI, 0.84-1.21; P <
< .001). The moderate placebo response in the OCD group (g
.001). The moderate placebo response in the OCD group (g =
= 0.63; 95% CI, 0.47-0.79; P
0.63; 95% CI, 0.47-0.79; P <
< .001) was significantly lower than in both the DD (P
.001) was significantly lower than in both the DD (P <
< .001) and AD (P
.001) and AD (P =
= .002) groups (Figure 2). The combined analysis across all disorders for the within-group analysis yielded a placebo response size of g
.002) groups (Figure 2). The combined analysis across all disorders for the within-group analysis yielded a placebo response size of g =
= 1.23 (95% CI, 1.06-1.39; P
1.23 (95% CI, 1.06-1.39; P <
< .001).
.001).
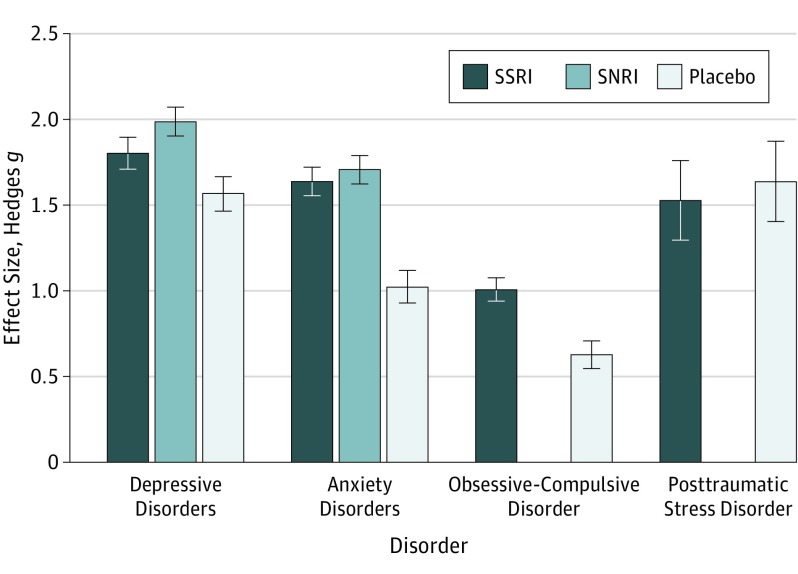
Because there was only 1 study, posttraumatic stress disorder (PTSD) was not included in subgroup analyses. Responses to selective serotonin reuptake inhibitors (SSRIs) were significantly larger in depressive disorders (DDs) and anxiety disorders (ADs) compared with obsessive-compulsive disorder (OCD) (both P <
< .001). The placebo response was significantly larger in DDs compared with ADs (P
.001). The placebo response was significantly larger in DDs compared with ADs (P <
< .001) and OCD (P
.001) and OCD (P <
< .001) and significantly larger in ADs compared with OCD (P
.001) and significantly larger in ADs compared with OCD (P <
< .002). SNRI indicates serotonin-norepinephrine reuptake inhibitor.
.002). SNRI indicates serotonin-norepinephrine reuptake inhibitor.
Adverse Event Analysis
Twenty-six trials reported the percentage of patients with TEAEs (reporting method 1), 26 trials reported the mean number of TEAEs per patient across symptoms (reporting method 2), and 15 trials reported both reporting methods. The 2 reporting methods differed significantly (across all 52 trials: P =
= .002; within the 15 studies reporting both reporting methods: P
.002; within the 15 studies reporting both reporting methods: P =
= .045), indicating higher RRs with reporting method 2. Patients taking an antidepressant reported significantly more TEAEs (reporting method 1: RR, 1.07; 95% CI, 1.01-1.12; P
.045), indicating higher RRs with reporting method 2. Patients taking an antidepressant reported significantly more TEAEs (reporting method 1: RR, 1.07; 95% CI, 1.01-1.12; P =
= .01; reporting method 2: RR, 1.49; 95% CI, 1.22-1.82; P
.01; reporting method 2: RR, 1.49; 95% CI, 1.22-1.82; P <
< .001) and SAEs (RR, 1.76; 95% CI, 1.34-2.32; P
.001) and SAEs (RR, 1.76; 95% CI, 1.34-2.32; P <
< .001) compared with placebo. No significant differences in TEAEs or SAEs were found between SSRIs and SNRIs. The RRs for TEAEs stratified by drug and disorder are displayed in Table 1. Discontinuation of treatment due to adverse events was significantly more common in the antidepressant group compared with the placebo group (RR, 1.79; 95% CI, 1.38-2.32; P
.001) compared with placebo. No significant differences in TEAEs or SAEs were found between SSRIs and SNRIs. The RRs for TEAEs stratified by drug and disorder are displayed in Table 1. Discontinuation of treatment due to adverse events was significantly more common in the antidepressant group compared with the placebo group (RR, 1.79; 95% CI, 1.38-2.32; P <
< .001). The RRs for study discontinuation and SAEs stratified by drug and disorder are summarized in Table 2. Mean rates of TEAEs, SAEs, and study discontinuation can be found in eTable 3 in the Supplement.
.001). The RRs for study discontinuation and SAEs stratified by drug and disorder are summarized in Table 2. Mean rates of TEAEs, SAEs, and study discontinuation can be found in eTable 3 in the Supplement.
Table 1.
| Disorder and Intervention | Reporting Method 1b | Reporting Method 2c | ||||
|---|---|---|---|---|---|---|
| No. of Trials | RR (95% CI) | P Value | No. of Trials | RR (95% CI) | P Value | |
| Overall | ||||||
| SSRI vs placebo | 19 | 1.07 (1.02-1.13) | .006 | 24 | 1.52 (1.22-1.88) | <.001 |
| SNRI vs placebo | 7 | 1.07 (0.94-1.22) | .33 | 2 | 1.56 (0.48-5.04) | .46 |
| Stratified by Disorder | ||||||
| DDs | ||||||
| SSRI vs placebo | 11 | 1.06 (0.98-1.14) | .13 | 11 | 1.46 (1.03-2.07) | .03 |
| SNRI vs placebo | 4 | 1.12 (0.84-1.50) | .44 | |||
| Combined vs placebo | 15 | 1.06 (0.98-1.15) | .13 | |||
| ADs | ||||||
| SSRI vs placebo | 3 | 1.23 (0.86-1.76) | .25 | 4 | 1.39 (0.85-2.26) | .19 |
| SNRI vs placebo | 3 | 1.06 (0.90-1.24) | .49 | 2 | 1.56 (0.48-5.04) | .46 |
| Combined vs placebo | 6 | 1.08 (0.97-1.21) | .16 | 6 | 1.40 (0.93-2.12) | .11 |
| OCD | ||||||
| SSRI vs placebo | 4 | 1.08 (0.96-1.21) | .19 | 8 | 1.89 (1.23-2.88) | .003 |
| SNRI vs placebo | ||||||
| PTSD | ||||||
| SSRI vs placebo | 1 | 1.00 (0.83-1.22) | .97 | 1 | 1.28 (0.42-3.88) | .67 |
| SNRI vs placebos | ||||||
Abbreviations: ADs, anxiety disorders; DDs, depressive disorders; OCD, obsessive-compulsive disorder; PTSD, posttraumatic stress disorder; RR, risk ratio; SNRI, serotonin-norepinephrine reuptake inhibitors SSRI, selective serotonin reuptake inhibitor; TEAEs, treatment-emergent adverse events.
Table 2.
| Disorder and Intervention | Discontinuationb | SAEc | ||||
|---|---|---|---|---|---|---|
| No. of Trials | RR (95% CI) | P Value | No. of Trials | RR (95% CI) | P Value | |
| Overall | ||||||
| SSRI vs placebo | 27 | 1.84 (1.38-2.44) | <.001 | 17 | 1.71 (1.22-2.40) | .002 |
| SNRI vs placebo | 6 | 1.56 (0.83-2.94) | .17 | 7 | 2.10 (1.19-3.69) | .01 |
| Stratified by Disorder | ||||||
| DDs | ||||||
| SSRI vs placebo | 14 | 1.40 (0.99-1.98) | .06 | 11 | 1.72 (1.12-2.63) | .01 |
| SNRI vs placebo | 3 | 2.95 (1.61-5.40) | <.001 | 3 | 4.43 (1.73-11.32) | .002 |
| Combined vs placebo | 17 | 1.66 (1.20-2.28) | .002 | 14 | 1.99 (1.33-2.97) | .001 |
| ADs | ||||||
| SSRI vs placebo | 5 | 3.45 (1.34-8.86) | .01 | 2 | 2.22 (0.45-10.87) | .33 |
| SNRI vs placebo | 3 | 0.78 (0.39-1.56) | .48 | 4 | 1.37 (0.67-2.78) | .39 |
| Combined vs placebo | 8 | 1.38 (0.73-2.60) | .33 | 6 | 1.48 (0.77-2.83) | .24 |
| OCD | ||||||
| SSRI vs placebo | 7 | 3.59 (1.89-6.84) | <.001 | 3 | 1.35 (0.47-3.92) | .58 |
| SNRI vs placebo | ||||||
| PTSD | ||||||
| SSRI vs placebo | 1 | 2.31 (0.47-11.49) | .31 | 1 | 13.90 (0.81-238.36) | .07 |
| SNRI vs placebo | ||||||
Abbreviations: ADs, anxiety disorders; DDs, depressive disorders; OCD, obsessive-compulsive disorder; PTSD, posttraumatic stress disorder; RR, risk ratio; SAEs, severe adverse events; SNRI, serotonin-norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
Moderator Analysis
Univariate analyses indicated larger effect sizes as a function for earlier trials, fewer sites, longer illness duration, and nonindustry funding. However, none of the moderators was found to be significant in a multivariate meta-regression (eAppendix 3 and eTables 4-6 in the Supplement).
Discussion
Our meta-analysis addresses the response and safety profile of SNRIs, SSRIs, and placebo in pediatric DD, AD, OCD, and PTSD. Results indicate that SSRIs and SNRIs are more beneficial than placebo in treating these commonly diagnosed conditions in children and adolescents. However, the overall drug-placebo difference is small and varies significantly by disorder, with a larger response in AD than DD, especially for SSRIs (g =
= 0.71; 95% CI, 0.45-0.97; P
0.71; 95% CI, 0.45-0.97; P <
< .001). This difference in drug-placebo difference response is mainly due to a higher placebo response in pediatric DD. Furthermore, patients with OCD exhibit a significantly smaller response to both drug treatment and placebo treatment compared with AD and DD.
.001). This difference in drug-placebo difference response is mainly due to a higher placebo response in pediatric DD. Furthermore, patients with OCD exhibit a significantly smaller response to both drug treatment and placebo treatment compared with AD and DD.
The small effect size between SSRIs and SNRIs vs placebo in pediatric DD might be owing to the lack of a clear depression phenotype. This was apparent in DSM-5 field trials on major depressive disorder (MDD), which found a low test-retest reliability (κ =
= 0.28) for children, adolescents, and adults. Furthermore, there is high comorbidity between pediatric DD and other disorders, especially AD. A recent review on the use of SSRIs and SNRIs in pediatric populations reported that approximately 25% of patients with MDD had a comorbid AD. In our meta-analysis, although not all included studies reported comorbidity rates, those doing so reported comorbidity rates in AD ranging between 6% and 56% in patients with DD. Yet, attempts by the DSM-5 work group to create a “mixed anxiety and depression disorder” resulted in an unacceptable rate of test-retest reliability (κ
0.28) for children, adolescents, and adults. Furthermore, there is high comorbidity between pediatric DD and other disorders, especially AD. A recent review on the use of SSRIs and SNRIs in pediatric populations reported that approximately 25% of patients with MDD had a comorbid AD. In our meta-analysis, although not all included studies reported comorbidity rates, those doing so reported comorbidity rates in AD ranging between 6% and 56% in patients with DD. Yet, attempts by the DSM-5 work group to create a “mixed anxiety and depression disorder” resulted in an unacceptable rate of test-retest reliability (κ =
= −0.004) when tested in the DSM-5 field trials.
−0.004) when tested in the DSM-5 field trials.
Although it appears that the response to placebo is robust in pediatric DD, children and adolescents with ADs, who respond to pharmacologic treatment to the same degree as those with DD, do not appear to exhibit such a robust placebo response. While in line with older reviews in children, this finding is in contrast to adult studies that found no significant differences in placebo effect size between depression and anxiety. This contrast is not unique: placebo responses between children and adults differ significantly for binary outcomes across a wide variety of diseases. One explanation might be that children and adolescents with major DD may be more demoralized than patients with AD and are therefore more sensitive to changes in hope and favorable meanings. However, because no pediatric trial included a no-treatment arm that could serve as a control for the natural course of the disorders, the difference in placebo response may also reflect differences in the probability of spontaneous improvement between the 2 pediatric disorders rather than differences in the placebo effect. Owing to the small number of studies in children, we could not estimate the drug and placebo response for the individual ADs, yet a recent adult study found drug and placebo effect sizes to be roughly equivalent across ADs. In pediatric patients, however, those with panic disorder seem to experience a greater placebo response compared with patients with generalized AD or social phobia.
Our results are very similar to those of a recent meta-analysis of 5 decades of research on youth psychological therapy, which found that mean effect sizes at posttreatment were strongest for AD (g =
= 0.61), weakest for DD (g
0.61), weakest for DD (g =
= 0.29), and nonsignificant for multiproblem treatment (g
0.29), and nonsignificant for multiproblem treatment (g =
= 0.15), indicating a general difficulty in establishing a clinically relevant benefit in the treatment of pediatric depression. The substantial placebo response in MDD indicates that depressed children and adolescents might benefit from innovative treatment modalities that harness the power of the placebo effect in an ethical fashion, including clinician contact and other common factors, such as the patients’ expectations of improvement, their desire for relief, and the exposure to treatment rituals. Placebo response also offers several implications for research design in antidepressant trials. Alternative designs, such as a discontinuation design or n-of-1 trials, might be recommended when establishing efficacy, yet also have their individual shortcomings. Differences between 2 medication groups could provide information about the magnitude of expectancy effects. In this regard, response and remission rates to antidepressants have been shown to be significantly higher in comparator trials compared with placebo-controlled trials. Future instructive studies could incorporate designs in which people who respond to placebo continue to receive placebo.
0.15), indicating a general difficulty in establishing a clinically relevant benefit in the treatment of pediatric depression. The substantial placebo response in MDD indicates that depressed children and adolescents might benefit from innovative treatment modalities that harness the power of the placebo effect in an ethical fashion, including clinician contact and other common factors, such as the patients’ expectations of improvement, their desire for relief, and the exposure to treatment rituals. Placebo response also offers several implications for research design in antidepressant trials. Alternative designs, such as a discontinuation design or n-of-1 trials, might be recommended when establishing efficacy, yet also have their individual shortcomings. Differences between 2 medication groups could provide information about the magnitude of expectancy effects. In this regard, response and remission rates to antidepressants have been shown to be significantly higher in comparator trials compared with placebo-controlled trials. Future instructive studies could incorporate designs in which people who respond to placebo continue to receive placebo.
With regard to adverse events, our finding that patients receiving any antidepressant reported more TEAEs, SAEs, and study discontinuation compared with those receiving placebo is in line with other meta-analyses reporting increased suicidality (odds ratio, 2.39; 95% CI, 1.31- 4.33), suicidal ideation, and suicide attempts (risk difference: antidepressant vs placebo, 0.7%; 95% CI, 0.1%-1.3%) in children and adolescents receiving SSRIs and SNRIs compared with placebo. This finding is mainly due to the large amount of significant SSRI studies, although patients receiving SNRIs reported significantly more SAEs than did those receiving placebo. Thus, our results support concerns about the safety of antidepressants in children and adolescents. Evaluating the mean number of adverse events provides a more sensitive measure than the percentage of patients exhibiting at least 1 adverse event and might be recommended as the primary reporting method in future clinical trials.
Limitations
Our study has some limitations. First, none of the randomized clinical trials included directly compared effectiveness across disorders. Accordingly, we could only make indirect conclusions with regard to disorder specificity. Second, although our meta-analysis included unpublished trials, reporting bias could lead to an overly positive representation of findings in the literature. In this regard, many concerns have been raised about the accuracy of the data of 1 study in particular: Paxil Study 329. A reanalysis of the original data found that paroxetine did not show efficacy for MDD in adolescents and that the initial study underplayed the drug’s potential to increase suicidal thoughts among adolescents. Third, there was variability in the mean age and age distribution between studies, which may have had an effect on results. Response to SSRIs and SNRIs has been shown to be lower in children than in adolescents, in part related to a higher placebo response in children. Fourth, the Begg and Eggers tests used to assess publication bias are valid only when there are 10 or more studies being evaluated, and our OCD group consisted of only 8 trials. However, no evidence of publication bias was found in the respective funnel plot. The different reporting methods of adverse events led to subgroup analyses based on only a few studies and should therefore be considered preliminary, requiring further investigation. Furthermore, restrictive inclusion criteria of clinical trials, such as noninclusion of comorbidity and a higher symptom severity threshold, make it difficult to generalize results to real-world populations. Finally, because only 1 study met our inclusion criteria for PTSD, no categorical analysis of SSRIs and SNRIs for the treatment of pediatric PTSD was possible.
Conclusions
The main findings of this meta-analysis present multiple avenues for further analyses. First, the nearly identical response rate for pediatric DD and AD deserves further investigation and perhaps the revision of federal prescribing guidelines for these 2 conditions. Although several SSRIs and SNRIs have been approved for the treatment of pediatric DD and OCD, only 1—duloxetine—has recently received FDA approval for treatment of pediatric ADs. Second, the substantial differential response to both drug treatment and placebo treatment in OCD compared with AD and DD highlights underlying differences in the etiologies and pathogeneses of the disorders that may require additional interventions for pediatric patients with OCD. It is our hope that a research domain criteria approach will help to elucidate the above-mentioned points and could lead to better treatment outcomes. Third, additional research into the factors that moderate the efficacy of SSRIs and SNRIs in children is warranted, as is the need for more comprehensive reporting of population and illness details (eg, age at onset, duration of illness) in clinical and pragmatic trials. Finally, the significant variability in the assessment and reporting of adverse events highlights the need for a standardized method of reporting TEAEs and SAEs. Given the potential for life-threatening events in young children and adolescents, understanding the extent to which these medications pose a genuine risk to youth is urgent. This need would allow future research to deviate from the current line of studies estimating the magnitude and differences between drug and placebo effects and focus more on precision medicine-driven questions, such as which treatment or combination thereof may be most advantageous for certain patient subgroups in certain clinical settings.
Notes
Supplement.
eAppendix 1. Search Terms
eAppendix 2. Moderator Analyses
eAppendix 3. Stratified by Disorder
eFigure 1. Flow Chart
eFigure 2. Risk of Bias Assessment
eFigure 3. Funnel Plots Stratified by Disorder
eTable 1. Characteristics of Included Studies
eTable 2. Study Heterogeneity
eTable 3. Adverse Effects
eTable 4. Continuous Univariate Moderator Analyses
eTable 5. Categorical Univariate Moderator Analyses
eTable 6. Multivariate Meta-regression Analyses
References
Full text links
Read article at publisher's site: https://doi.org/10.1001/jamapsychiatry.2017.2432
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5667359
Citations & impact
Impact metrics
Article citations
Expert recommendations for Germany's integration of psychedelic-assisted therapy.
BMC Med Educ, 24(1):1202, 24 Oct 2024
Cited by: 0 articles | PMID: 39443907 | PMCID: PMC11515625
Review Free full text in Europe PMC
Dorsal raphe dopaminergic neurons target CaMKII<sup>+</sup> neurons in dorsal bed nucleus of the stria terminalis for mediating depression-related behaviors.
Transl Psychiatry, 14(1):408, 02 Oct 2024
Cited by: 0 articles | PMID: 39358336 | PMCID: PMC11447211
Plasma alpha-trypsin inhibitor heavy chain 4 as an age-specific biomarker in the diagnosis and treatment of major depressive disorder.
Front Psychiatry, 15:1449202, 11 Sep 2024
Cited by: 0 articles | PMID: 39323962 | PMCID: PMC11422199
Efficacy of selective serotonin reuptake inhibitors-related antidepressants in Alzheimer's disease: a meta-analysis.
Eur J Med Res, 29(1):438, 29 Aug 2024
Cited by: 0 articles | PMID: 39210432 | PMCID: PMC11360319
Low-frequency Transcranial Magnetic Stimulation Ameliorates Anhedonic Behaviors and Regulates the Gut Microbiome in Mice Exposed to Chronic Unpredictable Mild Stress.
Alpha Psychiatry, 25(4):493-501, 01 Aug 2024
Cited by: 0 articles | PMID: 39360304 | PMCID: PMC11443298
Go to all (126) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Selective serotonin reuptake inhibitors, and serotonin and norepinephrine reuptake inhibitors for anxiety, obsessive-compulsive, and stress disorders: A 3-level network meta-analysis.
PLoS Med, 18(6):e1003664, 10 Jun 2021
Cited by: 15 articles | PMID: 34111122 | PMCID: PMC8224914
Review Free full text in Europe PMC
Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for the prevention of migraine in adults.
Cochrane Database Syst Rev, 4:CD002919, 01 Apr 2015
Cited by: 14 articles | PMID: 25829028 | PMCID: PMC6513227
Review Free full text in Europe PMC
Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for the prevention of tension-type headache in adults.
Cochrane Database Syst Rev, (5):CD011681, 01 May 2015
Cited by: 16 articles | PMID: 25931277 | PMCID: PMC6864942
Review Free full text in Europe PMC
Incidence of adverse events and comparative tolerability of selective serotonin reuptake inhibitors, and serotonin and norepinephrine reuptake inhibitors for the treatment of anxiety, obsessive-compulsive, and stress disorders: a systematic review and network meta-analysis.
Psychol Med, 53(9):3783-3792, 06 Jun 2023
Cited by: 5 articles | PMID: 37278215
Review
Funding
Funders who supported this work.
Intramural NIH HHS (1)
Grant ID: ZIA MH002781-08
NLM NIH HHS (1)
Grant ID: T15 LM007092
 1,2,7
1,2,7




