Abstract
Background
Bleach baths have been proposed as a treatment for decreasing the severity of atopic dermatitis (AD). However, conflicting results have been found regarding their efficacy.Objective
To determine the efficacy of bleach vs water baths at decreasing AD severity.Methods
We performed a systematic review of all studies evaluating the efficacy of bleach baths for AD. Cochrane, EMBASE, GREAT, LILACS, MEDLINE, and Scopus were searched. Two authors independently performed study selection and data extraction.Results
Five studies were included in the review. Four studies reported significantly decreased AD severity in patients treated with bleach on at least 1 time point. However, of 4 studies comparing bleach with water baths, only 2 found significantly greater decreases in AD severity with bleach baths, 1 found greater decreases with water baths, and 1 found no significant differences. In pooled analyses, there were no significant differences observed between bleach vs water baths at 4 weeks vs baseline for the Eczema Area and Severity Index (I2 = 98%; random effect regression model, P = .16) or body surface area (I2 = 96%; P = .36).Conclusion
Although bleach baths are effective in decreasing AD severity, they do not appear to be more effective than water baths alone. Future larger-scale, well-designed randomized controlled trials are needed.Free full text

Efficacy of bleach baths in reducing severity of atopic dermatitis: A systematic review and meta-analysis
Introduction
Atopic dermatitis (AD) is a chronic, relapsing inflammatory skin disorder affecting 15–20% of children and 1–10% of adults in the United States and worldwide1–4. Deficiencies in the epidermal barrier and dysfunction of the immune system contribute to AD pathogenesis, resulting in the characteristic signs and symptoms of AD (e.g. xerosis and pruritus) and predisposing patients to cutaneous infections. AD is associated with a higher prevalence of epidermal S. aureus colonization compared with healthy controls (>70% vs. 10–20%)5, 6. In particular, AD severity is correlated with epidermal S. aureus density; AD flares are associated with cutaneous S. aureus infection, and AD exacerbations may be induced by S. aureus overgrowth even without frank infection5, 7, 8. Given the importance of S. aureus colonization and microbiome in AD, anti-bacterial treatments have been considered for managing AD9. However, the prolonged use of traditional topical and/or systemic antibiotics to suppress recolonization may be impractical given potential concerns about increased antibiotic resistance from long-term antibiotic use10, 11.
Bleach (sodium hypochlorite, NaOCl) baths are an inexpensive, widely accessible, alternative antibiotic treatment that may not worsen antibiotic resistance12. Bleach baths demonstrate in vitro and in vivo antibacterial and anti-biofilm properties13, 14, are associated with few adverse events, demonstrate no harmful effects on stratum corneum hydration, transepidermal water loss (TEWL), and epidermal pH15, and do not appear to cause antibiotic resistance12. As such, dilute bleach baths have been proposed to suppress epidermal S. aureus load and ultimately reduce AD severity. Huang et al.9 first conducted a randomized controlled trial (RCT) of bleach baths as a treatment for moderate-to-severe AD with promising results. However, subsequent RCT yielded conflicting results. Nevertheless, bleach baths are recommended in multiple clinical practice guidelines16–19. We performed a systematic review in order to determine the whether bleach baths are consistently effective in improving the severity of AD.
Methods
Literature search
This study was exempt from Institutional Review Board approval at Northwestern University Feinberg School of Medicine as data were gathered from published literature. The following databases were searched for articles prior to June 5, 2017: Cochrane Library, MEDLINE, EMBASE, Global Resource of EczemA Trials (GREAT), Latin American and Caribbean Health Sciences (LILACS) and Scopus.
Inclusion criteria were: any retrospective or prospective study, use of bleach bath or topical bleach agent interventions, evaluation of AD severity, patients with AD of any age, published online, in print, or in press, in any language before June 5, 2017. Title and abstract review were performed independently by three reviewers (R.C, P.V, and R.S.) with conflicts resolved by discussion. Studies were excluded based on the title and/or abstract if there was no clear indication they evaluated the efficacy of bleach therapy in AD patients. The remaining articles underwent full-text review for inclusion.
Data extraction
The following data were collected: first author; year of publication; study design; comparison/control arm; geographical region; type of intervention; number of patients enrolled in study; number of patients completed study; age; gender; level of blinding; baseline severity instruments and means; target population; severity scale used for inclusion and whether thresholds were provided; inclusion and exclusion criteria; AD diagnostic criteria; permitted and prohibited medications; bathing protocol; bleach concentration; duration and frequency of baths; bath aftercare; non-adherence criteria; length of study; follow-up intervals; severity scores; S. aureus colonization; epidermal S. aureus density; and frequency of adverse events. Study authors were contacted in order to obtain any values unspecified or incorrectly documented in the manuscripts.
Statistical analysis
Statistical analyses were performed in SAS version 9.4 (SAS Institute; Cary, NC). Comparable outcomes across at least 3 studies were combined and pooled means were estimated. Pooled mean and standard deviations were estimated and plotted. Cohen’s D and 95% confidence intervals (CI) were used to measure effect size. Significant heterogeneity of results was detected across studies as judged by an I2 statistic >50%. Therefore, random-effect models were performed in order to generate more accurate estimates of standard error between studies. A 2-sided P-value of 0.05 was taken as significant.
Results
Study selection
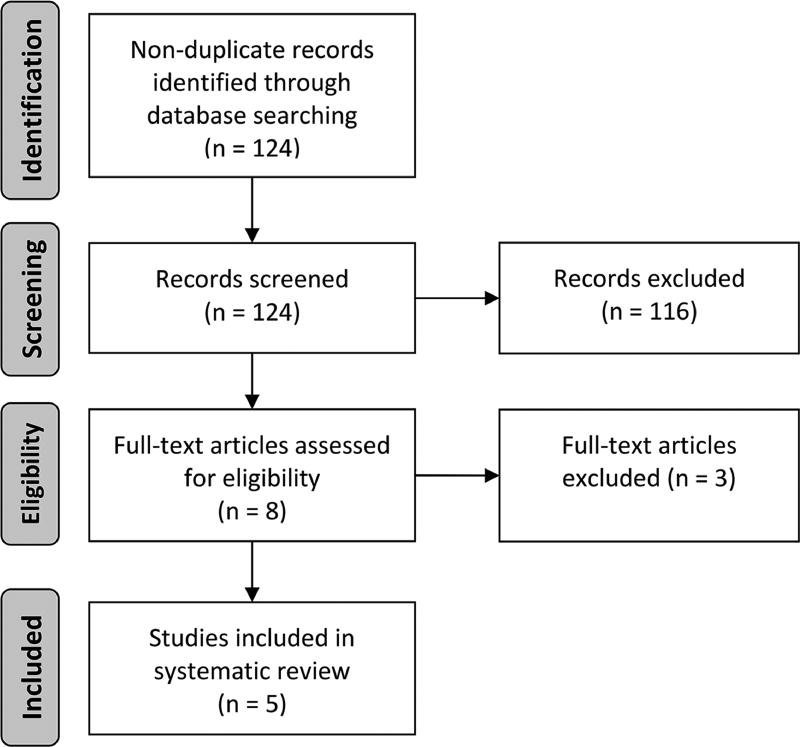
We identified 124 non-duplicate citations via our initial search strategy. The title and abstract review excluded 116 citations, while the full-text review excluded an additional 3 citations. We included 5 studies9, 20–23 in this systematic review as outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses24 flow diagram (Fig. 1).
Study characteristics
Study characteristics are described in Table 1. Among the 5 studies, 3 were RCT, one was a randomized crossover trial, and one was a single-arm pilot study. Two studies were double-blinded, two were single-blinded, and one was unblinded. Four studies were prospective and one was partially prospective and retrospective. Date of publication ranged from 2009–2016. Three studies were conducted in North America and 2 in Asia.
Table 1
Characteristics of bleach bath randomized controlled trials.
| Variables | Huang, 2009 | Wong, 2013 | Gonzales, 2015 | Hon, 2016 |
|---|---|---|---|---|
| Study design | RCT | RCT | RCT | RCT (crossover design) |
| N completed study | 22 | 36 | 18 | 40 |
| Completion rate (Treatment/Placebo) | 60.0/81.3 | 85.7/85.7 | 90.0/81.8 | 100.0/100.0 |
| Age (min-max months) | 8.4–207.6 | 36–360 | 3–60 | 145.2* |
| Diagnostic criteria | Not documented | H&R | Eichenfield modification | H&R |
| Inclusion criteria; definitions provided | Moderate-to-severe (IGA); Yes | Moderate-to-severe (R-L); No | Moderate-to-severe (IGA); Yes | Moderate-to-severe (oSCORAD); Yes |
| Signs of cutaneous infection required for inclusion | Yes | No | No | Yes |
| Interventions | Cephalexin (2 weeks) and intranasal mupirocin and bleach baths vs. cephalexin and intranasal petrolatum and water bath | Bleach baths vs. water baths | Bleach baths and TCS BID vs. water baths and TCS BID | Bleach baths vs. water baths |
| Blinding | Single | Double | Single | Double |
| Blinding protocol | Mupirocin and petrolatum ointment were dispensed in identical white jars. Bleach and water were dispensed in identical bleach bottles with identical brand-name labels. Patients and/or family members were able to distinguish bleach from water based on odor, but were directed not to reveal their treatment to investigators. Bathing in bleach did not result in lasting odor so investigators were not able to differentiate between study arms. | Bleach and distilled water were dispensed in identical bottles. | Plain white bottles were filled with bleach or water. | Bleach and water were dispensed in identical opaque, brown bottles with identical brand-name labels. Investigators were blinded to the contents of the bottles. Patients and investigators were blinded to study arm. The bleach and water dilution was pretested so that the color and odor were similar. Patients and/or family members were able to distinguish bleach from water based on odor, but were directed not to reveal their treatment to investigators. Bathing in bleach did not result in lasting odor so investigators were not able to differentiate between study arms. |
| Bathing instructions provided to patient | 0.5 cup of 6% bleach or water was added to a full bathtub of water (40 gallons). Amount of bleach or water was adjusted by the family on the basis of the bathtub size and height of bathtub water. | Clear oral and written instructions were provided. 100 mL of sodium hypochlorite 5% or 100 mL of distilled water was added to 100 L of water (e.g. half a bathtub). For children <12 years old, 50 mL was added to a ¼ tub of water (50 L). Patients instructed to soak neck down. | Instruction handout was provided. ¼ cup of household bleach (6.15% sodium hypochlorite) was added to half-full bathtub, or a ½ cup in a full bathtub (40 gallons), or ½ teaspoon per gallon of water if they were using a baby tub. | 83 ml of 6% bleach from a prepared bottle or water to 100 L of water. Amount was adjusted by the family on the basis of the bathtub size and estimated height of bathtub water. |
| Bath frequency and duration | BIW; 5–10 minutes | BIW; 10 minutes | BIW; Not documented | BIW-TIW; 10 minutes |
| Bath aftercare | Not documented | Patients should rinse off with normal tap water. | Not documented | Not documented |
| Standardization of TCS | Not documented | Not documented | BID but unrelated to bath schedule | Not permitted |
| Other interventions prohibited | topical or systemic antibiotics | systemic antibiotics or oral steroids | topical or systemic antibiotics | Systemic antibiotics, topical steroids, or antihistamines |
| Other interventions permitted | stable regimen of topical anti-inflammatories and emollients | stable regimen of topical anti-inflammatories and emollients | previously used emollients | stable regimen of emollients |
| Adherence defined | Yes | No | Yes | Yes |
| Study duration (days) | 84 | 56 | 28 | 28 |
| Outcomes assessed | Total EASI, IGA, BSA | Total EASI, BSA, VAS-itch, PRO | IGA, Total EASI, local EASI | SCORAD, oSCORAD, BSA, CDLQI, TEWL, SH |
| Follow-up assessment schedule (days) | 28, 84 | 14, 28, 56 | 28 | 28 |
| Bleach baths cause significant reduction in severity | Yes | Yes | Yes | Not documented |
| Water baths cause significant reduction in severity | Yes | Not documented | Yes | Not documented |
| Bleach or water baths more effective | 1 month=Bleach (EASI, BSA, IGA); 3 months=Bleach (EASI, BSA), No difference (IGA) | 1 month=No difference (total EASI, BSA, VAS itch, PRO); 2 months=Bleach (EASI, BSA), No difference (VAS itch, PRO) | 1 month= No difference (IGA, total EASI, local EASI) | 1 month (ITT)= No difference (oSCORAD, pruritus, sleep loss, SCORAD intensity, CDLQI), Bleach (BSA and [oSCORAD per protocol approach]) |
| Changes in S. aureus colonization | ||||
| Absence/Presence CFU or quantitative PCR | No significant difference | Not documented | Not documented | Not documented |
| Not documented | No significant difference | No significant difference | No significant difference | |
| Adverse events | Not documented | No significant difference | No significant difference | No significant difference |
BIW=twice weekly; TIW=thrice weekly; BID=twice daily; RCT=randomized controlled trial; DC=diagnostic criteria; IGA=Investigator Global Assessment; HR=Hanifin & Rajka criteria; R-L=Rajka and Langeland; oSCORAD=objective Scoring Atopic Dermatitis; TCS=topical corticosteroid; PRO=undefined patient reported outcome (patient overall assessment of treatment response); CFU=colony forming units; PCR=polymerase chain reaction; SH=skin hydration (unspecified methodology); TEWL=transepidermal water loss; VAS=visual analog scale; BSA=body surface area;
Four trials were included children and one included both children and adults (mean: 13.7–145.2 months; range: 3–360 months). Study populations ranged from 18 to 40 patients. AD was diagnosed by the Hanifin & Rajka (H&R) criteria in 2 studies, and Eichenfield modification of H&R criteria in one study; diagnostic criteria were not documented in 2 studies. All 5 studies recruited patients with moderate-severe AD, as judged by Investigator Global Assessments (IGA) (n=3), objective SCORing AD (oSCORAD) (n=1), or Rajka & Langeland (R-L) (n=1). Washout and concomitant treatment regimen are presented in Table 1.
Four studies evaluated the efficacy of 0.005% bleach baths, and one study evaluated a 0.0061% cleanser containing bleach. Lengths of baths ranged from 5–10 minutes with one study not documenting whether bathing times were standardized. Patients were instructed to bathe in bleach baths or use the bleach cleanser biweekly (n=3), biweekly or triweekly (n=1), and triweekly or more frequently (n=1). Allowance for additional regular water baths were not restricted (n=1) or not documented (n=4).
Study follow-up assessments ranged from 2 (n=1), 4 (n=5), 8 (n=2), and 12 weeks (n=2). Outcome measures included body surface area (BSA; n=4), eczema area and severity index (EASI; n=3), IGA (n=3), local-EASI (n=1), SCORAD/oSCORAD (n=1), children’s dermatology life quality index (CDLQI; n=1), visual analog scale (VAS)-itch (n=1), TEWL (n=1) and skin hydration using an unspecified methodology (n=1). Four studies assessed different bacteriologic parameters.
Efficacy
Four studies documented whether bleach baths or cleansers caused significant reductions in AD severity. All 4 demonstrated significant reductions in the bleach group in at least one time point. However, among 4 studies comparing bleach to water baths, only 2 found significantly greater reductions in AD severity with bleach baths, 1 with water baths, and 1 found no significant differences.
At 4 weeks, one study found a significant reduction in AD severity compared to controls (EASI, BSA, IGA), two found no significant differences (EASI [n=2], IGA [n=1], local-EASI [n=1], BSA [n=1], VAS-itch [n=1], and patient self-assessment [n=1]), and one showed mixed results. In other words, out of the aggregated 15 severity assessment evaluations, only 3 assessments demonstrated that bleach baths were more effective than water baths, 11 revealed no difference, and 1 demonstrated regular water baths to be more effective.
In one study at 8-weeks, bleach baths caused significant reductions in EASI and BSA assessments, but not VAS-itch or the patient’s self-assessment. In one study at 12-weeks, bleach baths caused significant reductions in EASI and BSA, but not IGA. Across all five studies at all follow-up periods, there were no significant differences in patient-reported outcomes (PRO) between both treatment arms.
Pooled analysis of efficacy
EASI and BSA assessments at 4-weeks were the only end-points and time-points in common across at least 3 studies (Fig. 2A, B). In pooled analyses, the mean ± std. dev. EASI and BSA decreased at 4 weeks vs. baseline in both the bleach (EASI: 11.0±7.8 vs. 24.9±25.3; BSA: 42.8%±12.7% vs. 49.8%±18.1%) and water bath (EASI: 14.2±7.7 vs. 25.2±12.3; BSA: 41.1%±18.1% vs. 45.6%±17.9%) groups, respectively (Fig. 2C, D). There were no significant differences observed between bleach vs. water baths at 4 weeks vs. baseline for EASI (I2=98%; random effect regression model, P=0.16) or BSA (I2=96%; P=0.36).a
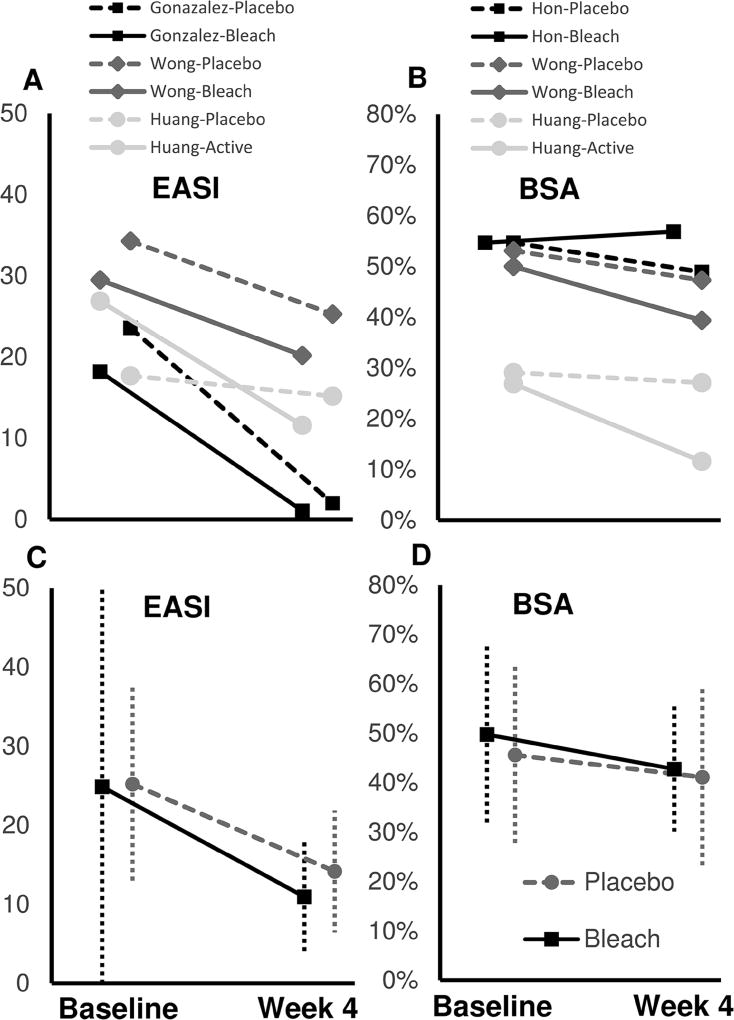
Mean (A) EASI and (B) BSA of bleach baths (solid lines) and water baths (dashed lines) are presented for individual studies. Pooled mean ± std. dev. (C) EASI and (D) BSA of bleach baths (solid lines) and water baths (dashed lines) are presented for 3 combined studies.
Bacteriologic assessments
All 5 studies evaluated the effect of bleach baths or cleansers on S. aureus status, but only 4 were able to reliably assess bacteriologic parameters. One study found no reduction in the proportion of patients colonized in both groups. Three of 3 studies found reductions in S. aureus density after both bleach and normal baths, but with no significant differences between groups. Two studies found no significant differences of antibiotic resistance between groups.
Safety, tolerability, and adherence
Adverse events (AE) of bleach baths and cleansers were documented in 4 of 5 studies. AE included stinging/burning (6/56; 11%), itch (6/57; 0.5%), xerosis (4/38; 10.5%), erythema (2/29; 6.9%), urticaria (1/20; 5%), oozing (1/20; 5%). There were no differences of AE between bleach and water baths.
Adherence was assessed in 3 studies, but was measured differently across all studies (completion of cephalexin therapy, frequency and concentration of bleach baths, frequency and duration of intranasal mupirocin application, whether patients bathed <2 times per week, delayed follow-up, or using oral antibiotics during the study). There were no significant differences in adherence between patients in both treatment arms.
Discussion
This systematic review found that bleach baths/cleansers and water baths alone significantly reduced AD severity. However, water baths were as effective as bleach baths at 4 weeks in pooled analyses, with only one study of efficacy at later time-points. There were no differences of S. aureus density in patients treated with bleach vs. water baths. Together, the results suggest that much of the efficacy of bleach baths at reducing AD severity is attributable to water baths and less the bleach per se.
Bleach baths are compound interventions with multiple steps: water bath, exposure to bleach during the bath, and application of emollients and/or topical anti-inflammatory agents after the bath. Each of these 3 steps may be effective at reducing AD severity. Water baths alone may hydrate and sooth the skin, and can wash away scale and serum-crust. The bleach may have anti-staphylococcal and other disinfecting properties. Although, the results of the present study indicate that water baths were similarly effective as bleach baths at disinfecting the skin. Application of emollients and/or topical anti-inflammatories after the bath (referred to as prehydration, “soak and seal”, or “soak and smear”) may seal moisture in the stratum corneum, increased permeability, and enhance drug absorption25, 26. Previous studies showed good efficacy for soak and smears27–29, though a recent RCT found no greater efficacy than TCS on dry skin30. Water bath alone or the soak and smear regimen may be effective without the addition of bleach.
Bleach baths are commonly recommended because they are inexpensive, relatively safe and easily accessible. Upon reviewing the results of this study, clinicians might wonder what difference does it make whether bleach baths or water baths alone should be recommended? However, there are several important considerations. Exposure to dilute bleach can result in stinging, burning and transient worsening of itch. Ocular exposure to bleach can be particularly uncomfortable and hinders prehydration of the face. There is additional cost and time spent acquiring bleach, albeit fairly minimal. Bleach may stain towels, linens and/or clothing. Bleach baths emit caustic fumes. One of the authors (JS) had two patients with reported asthma exacerbations due to inhalation of fumes from bleach baths. It is important to recognize that water baths alone might accomplish similar efficacy as bleach baths without these additional concerns.
Comparison of study results is hindered by differences of inclusion criteria, patient phenotype, interventions, and outcome measures across studies. Different diagnostic criteria and severity assessments were used to recruit patients, which may result in study populations with differing phenotype31, 32. Active cutaneous infections were used as inclusion criteria in half of studies and exclusion criteria in the other half. Blinding protocols and success varied across studies. Only half of RCT exclusively compared bleach to water baths without other concurrent interventions. One study concurrently administered mupirocin and cephalexin, which may be confounding factors9. Different severity measures were used to evaluate efficacy outcomes across studies, showing inconsistent efficacy between studies and within individual studies. Differences in bathing protocol may have impacted study results. Bathing is a multicomponent process comprised of soap, water and bleach exposure factors (e.g. duration, frequency, temperature, etc.), and aftercare such as drying, emollients and topical medications. There was no documented efforts to standardize most of these variables, except frequency and duration of baths in all studies and soap-use in one study. Only one study standardized TCS use, and no studies coordinated the use of TCS and emollient with bath regimens. As the principal intervention of interest, bathing protocol should be thoroughly documented, standardized, and isolated from the effect of confounding factors such as additional concurrent interventions. Future studies are needed that properly address these concerns.
Current primary care provider recommendations advocate for infrequent bathing in AD33. However, the results of this systematic review indicate that regular bathing and emollient use is an effective treatment for AD and better than infrequent bathing. These results provide evidence to support the AAD guidelines’ recommendation for regular bathing in the care of AD34. The AAD guidelines also recommend use of dilute bleach baths in patients with frequent bacterial infections34. Interestingly, bleach baths were not more effective than water baths in studies that recruited patients with clinically infected AD. It may be that water baths alone can reduce risk of bacterial infection in AD patients and future studies are needed to elucidate this point.
The studies included in this systematic review showed no significant difference between bleach and water bath interventions in reducing S. aureus epidermal colonization or density. These findings are unexpected as AD exacerbations are correlated with epidermal S. aureus density and bleach baths are hypothesized to improve AD severity via their antibacterial properties14, 35. In contrast, bleach baths and mupirocin were found to eradicate S. aureus colonization in an RCT of non-AD patients with community acquired staphylococcal skin/soft tissue infections compared to controls at 1 and 4 months (63% vs. 38% and 71% vs. 48%, respectively)36. A recent Cochrane review concluded there are no discernable benefits of anti-staphylococcal interventions in reducing S. aureus density in AD patients compared to anti-inflammatory medications37, which is consistent with the results of our study.
Our study has several strengths including the use of a comprehensive search strategy and examination of studies from all countries and languages. Pooled analysis was performed at 4 weeks using random-effects regression models to address issues of heterogeneity. This pooled analysis provided novel insight by demonstrating that water baths were also effective at reducing AD severity. However, there are several limitations to our study, including a small number of studies, small study populations, short follow-up durations, and each were conducted at a single center. Moreover, there was a lack of uniformity with respect to AD diagnostic and inclusion criteria, demographics, study interventions, and outcome measures across studies, which require cautious interpretation.
In conclusion, while bleach baths are effective in reducing AD severity, they do not appear to be more effective than water bath alone. However, there are a number of limitations with the currently available studies. Future, larger-scale RCT are needed that address these limitations.
Abbreviations used
| AD | atopic dermatitis |
| RCT | randomized controlled trial |
| SCORAD index | Scoring Atopic Dermatitis index |
| EASI | Eczema Area and Severity Index |
| IGA | Investigator’s Global Assessment |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
JI Silverberg had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Study concept and design: JI Silverberg
Acquisition of Data: P Vakharia, R Chopra R Sacotte
Analysis and interpretation of data: JI Silverberg, P Vakharia, R Chopra, R Sacotte, Drafting of the manuscript: JI Silverberg, P Vakharia, R Chopra, R Sacotte
Critical revision of the manuscript for important intellectual content: JI Silverberg, P Vakharia, R Chopra, R Sacotte
Statistical analysis: R Chopra, J Silverberg
Obtained funding: JI Silverberg
Administrative technical or material support: None
Study supervision: None
Financial disclosures: None
Funding Support: This publication was made possible with support from the Agency for Healthcare Research and Quality (AHRQ), grant number K12 HS023011, and the Dermatology Foundation.
Design and conduct of the study? No
Collection, management, analysis and interpretation of data? No
Preparation, review, or approval of the manuscript? No
Decision to submit the manuscript for publication? No
Conflicts of interest: There are no conflicts of interest to declare for JI Silverberg, PP Vakharia, R Chopra, or R Sacotte.
Trial registration: Not applicable
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.anai.2017.08.289
Read article for free, from open access legal sources, via Unpaywall:
http://www.annallergy.org/article/S1081120617309584/pdf
Citations & impact
Impact metrics
Article citations
Adding Fuel to the Fire? The Skin Microbiome in Atopic Dermatitis.
J Invest Dermatol, 144(5):969-977, 26 Mar 2024
Cited by: 0 articles | PMID: 38530677
Review
Secondary Bacterial Infections in Patients with Atopic Dermatitis or Other Common Dermatoses.
Am J Clin Dermatol, 25(4):623-637, 05 Apr 2024
Cited by: 0 articles | PMID: 38578398
Review
Bathing in Atopic Dermatitis in Pediatric Age: Why, How and When.
Pediatr Rep, 16(1):57-68, 08 Jan 2024
Cited by: 0 articles | PMID: 38251315 | PMCID: PMC10801494
Review Free full text in Europe PMC
Fruit vinegar as a promising source of natural anti-inflammatory agents: an up-to-date review.
Daru, 32(1):307-317, 01 Dec 2023
Cited by: 0 articles | PMID: 38040916
Review
Pathogenic role of the staphylococcal accessory gene regulator quorum sensing system in atopic dermatitis.
Front Cell Infect Microbiol, 13:1178650, 14 Apr 2023
Cited by: 4 articles | PMID: 37124047 | PMCID: PMC10140505
Review Free full text in Europe PMC
Go to all (35) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Efficacy of sodium hypochlorite (bleach) baths to reduce Staphylococcus aureus colonization in childhood onset moderate-to-severe eczema: A randomized, placebo-controlled cross-over trial.
J Dermatolog Treat, 27(2):156-162, 13 Aug 2015
Cited by: 30 articles | PMID: 26270469
Bleach baths for atopic dermatitis: A systematic review and meta-analysis including unpublished data, Bayesian interpretation, and GRADE.
Ann Allergy Asthma Immunol, 128(6):660-668.e9, 30 Mar 2022
Cited by: 7 articles | PMID: 35367346
Review
Does daily bathing or showering worsen atopic dermatitis severity? A systematic review and meta-analysis.
Arch Dermatol Res, 313(9):729-735, 16 Nov 2020
Cited by: 3 articles | PMID: 33196889
Review
Bleach for Atopic Dermatitis.
Dermatitis, 29(3):120-126, 01 May 2018
Cited by: 15 articles | PMID: 29762205
Review