Abstract
Importance
We previously described and validated a breast cancer staging system (CPS+EG, clinical-pathologic scoring system incorporating estrogen receptor-negative disease and nuclear grade 3 tumor pathology) for assessing prognosis after neoadjuvant chemotherapy using pretreatment clinical stage, posttreatment pathologic stage, estrogen receptor (ER) status, and grade. Development of the CPS+EG staging system predated routine administration of trastuzumab in patients with ERBB2-positive disease (formerly HER2 or HER2/neu).Objective
To validate the CPS+EG staging system using the new definition of ER positivity (≥1%) and to develop an updated staging system (Neo-Bioscore) that incorporates ERBB2 status into the previously developed CPS+EG.Design, setting, and participants
Retrospective review of data collected prospectively from January 2005 through December 2012 on patients with breast cancer treated with neoadjuvant chemotherapy at The University of Texas MD Anderson Cancer Center.Main outcomes and measure
Prognostic scores were computed using 2 versions of the CPS+EG staging system, one with ER considered positive if it measured 10% or higher, the other with ER considered positive if it measured 1% or higher. Fits of the Cox proportional hazards model for the 2 sets of prognostic scores were compared using the Akaike Information Criterion (AIC). Status of ERBB2 was added to the model, and the likelihood ratio test was used to determine improvement in fit.Results
A total of 2377 patients were included; all were women (median age, 50 years [range, 21-87 years]); ER status was less than 1% in 28.9%, 1% to 9% in 8.3%, and 10% or higher in 62.8%; 591 patients were ERBB2 positive. Median follow-up was 4.2 years (range, 0.5-11.7 years). Five-year disease-specific survival was 89% (95% CI, 87%-90%). Using 1% or higher as the cutoff for ER positivity, 5-year disease-specific survival estimates determined using the CPS+EG stage ranged from 52% to 98%, thereby validating our previous finding that the CPS+EG score facilitates more refined categorization into prognostic subgroups than clinical or final pathologic stage alone. The AIC value for this model was 3333.06, while for a model using 10% or higher as the cutoff for ER positivity, it was 3333.38, indicating that the model fits were nearly identical. The improvement in fit of the model when ERBB2 status was added was highly significant, with 5-year disease-specific survival estimates ranging from 48% to 99% (P < .001). Incorporating ERBB2 into the staging system defined the Neo-Bioscore, which provided improved stratification of patients with respect to prognosis.Conclusions and relevance
The Neo-Bioscore improves our previously validated staging system and allows its application in ERBB2-positive patients. We recommend that treatment response and biologic markers be incorporated into the American Joint Committee on Cancer staging system.Free full text

The Neo-Bioscore Update for Staging Breast Cancer Treated With Neoadjuvant Chemotherapy
Associated Data
Abstract
IMPORTANCE
We previously described and validated a breast cancer staging system (CPS+EG, clinical-pathologic scoring system incorporating estrogen receptor–negative disease and nuclear grade 3 tumor pathology) for assessing prognosis after neoadjuvant chemotherapy using pretreatment clinical stage, posttreatment pathologic stage, estrogen receptor (ER) status, and grade. Development of the CPS+EG staging system predated routine administration of trastuzumab in patients with ERBB2-positive disease (formerly HER2 or HER2/neu).
OBJECTIVE
To validate the CPS+EG staging system using the new definition of ER positivity (≥1%) and to develop an updated staging system (Neo-Bioscore) that incorporates ERBB2 status into the previously developed CPS+EG.
DESIGN, SETTING, AND PARTICIPANTS
Retrospective review of data collected prospectively from January 2005 through December 2012 on patients with breast cancer treated with neoadjuvant chemotherapy at The University of Texas MD Anderson Cancer Center.
MAIN OUTCOMES AND MEASURE
Prognostic scores were computed using 2 versions of the CPS+EG staging system, one with ER considered positive if it measured 10% or higher, the other with ER considered positive if it measured 1% or higher. Fits of the Cox proportional hazards model for the 2 sets of prognostic scores were compared using the Akaike Information Criterion (AIC). Status of ERBB2 was added to the model, and the likelihood ratio test was used to determine improvement in fit.
RESULTS
A total of 2377 patients were included; all were women (median age, 50 years [range, 21-87 years]); ER status was less than 1% in 28.9%, 1% to 9% in 8.3%, and 10% or higher in 62.8%; 591 patients were ERBB2 positive. Median follow-up was 4.2 years (range, 0.5-11.7 years). Five-year disease-specific survival was 89% (95% CI, 87%-90%). Using 1% or higher as the cutoff for ER positivity, 5-year disease-specific survival estimates determined using the CPS+EG stage ranged from 52% to 98%, thereby validating our previous finding that the CPS+EG score facilitates more refined categorization into prognostic subgroups than clinical or final pathologic stage alone. The AIC value for this model was 3333.06, while for a model using 10% or higher as the cutoff for ER positivity, it was 3333.38, indicating that the model fits were nearly identical. The improvement in fit of the model when ERBB2 status was added was highly significant, with 5-year disease-specific survival estimates ranging from 48% to 99% (P < .001). Incorporating ERBB2 into the staging system defined the Neo-Bioscore, which provided improved stratification of patients with respect to prognosis.
CONCLUSIONS AND RELEVANCE
The Neo-Bioscore improves our previously validated staging system and allows its application in ERBB2-positive patients. We recommend that treatment response and biologic markers be incorporated into the American Joint Committee on Cancer staging system.
Neoadjuvant chemotherapy has become increasingly used in the management of breast cancer. There are several purported benefits to this approach, including decreasing tumor size and eradicating nodal disease, allowing surgeons to limit the extent of surgery required.1–10 Neoadjuvant chemotherapy also allows for in vivo assessment of the tumor response to therapy. Multiple studies have shown that the underlying tumor biological characteristics are associated with response to therapy and subsequent outcomes. Patients with hormone receptor (HR)-negative, high-grade, or ERBB2-positive (formerly HER2 or HER2/neu) tumors are more likely to respond to neoadjuvant chemotherapy, and multiple studies have shown that achieving a pathologic complete response (pCR) to neoadjuvant chemotherapy is associated with improved disease-free survival (DFS) and overall survival (OS).11
For patients with residual disease following neoadjuvant chemotherapy, multiple studies have attempted to determine prognosis using pathologic measures of disease burden and/or biologic characteristics of the primary tumor.12–15 Our group16 previously developed the CPS+EG staging system (clinical-pathologic scoring system incorporating estrogen receptornegative disease and nuclear grade 3 tumor pathology), which incorporates pretreatment clinical stage, estrogen receptor (ER) status, grade, and posttreatment pathologic stage. Briefly, scoring points are assigned by presenting clinical stage, final pathologic stage, and the biologic markers ER and grade (Table 1). The points are added to determine a CPS+EG score, which facilitates more refined stratification by disease-specific survival (DSS) than either clinical stage or pathologic stage alone. The CPS+EG staging system has been validated in both internal and external patient cohorts.17,18
Table 1
Point Assignments for the CPS+EG and Neo-Bioscore Staging Systems
| Cancer Stage | CPS+EG Points | Neo-Bioscore Points |
|---|---|---|
| Clinical stage | ||
 I I | 0 | 0 |
 IIA IIA | 0 | 0 |
 IIB IIB | 1 | 1 |
 IIIA IIIA | 1 | 1 |
 IIIB IIIB | 2 | 2 |
 IIIC IIIC | 2 | 2 |
| Pathologic stage | ||
 0 0 | 0 | 0 |
 I I | 0 | 0 |
 IIA IIA | 1 | 1 |
 IIB IIB | 1 | 1 |
 IIIA IIIA | 1 | 1 |
 IIIB IIIB | 1 | 1 |
 IIIC IIIC | 2 | 2 |
| Tumor marker | ||
 ER negative ER negative | 1 | 1 |
 Grade 3 Grade 3 | 1 | 1 |
 ERBB2 negative ERBB2 negative | 1 |
A limitation of the CPS+EG staging system is that its development predated the routine use of trastuzumab in treating patients with ERBB2-positive breast cancer. The addition of trastuzumab to chemotherapy in the neoadjuvant setting results in higher pCR rates than are achieved with chemotherapy alone, and it has been shown that patients with ERBB2-positive breast cancer achieving a pCR with neoadjuvant chemotherapy plus trastuzumab have improved DFS and OS outcomes over patients with less than a pCR.11,19–25 Our group22 reported that, while failure to achieve a pCR is the strongest independent factor associated with recurrence in these patients, improvements in DFS and OS are seen with decreasing posttreatment pathologic stage.22 Because the CPS+EG staging system does not account for the favorable response of ERBB2-positive tumors to trastuzumab administered with chemotherapy, it cannot be used to provide prognostic information on ERBB2-positive cancer. Therefore, the current study was undertaken to update the CPS+EG staging system with a more contemporary cohort of patients to include those with ERBB2-positive disease receiving trastuzumab.
Methods
Patient Population
A prospectively maintained database was used to identify 2815 patients with nonmetastatic invasive breast cancer treated with neoadjuvant chemotherapy from January 2005 through December 2012. From this cohort, 438 patients were excluded: 209 with inflammatory breast cancer, 153 presenting with recurrent disease, 32 with ERBB2-positive breast cancer that did not receive neoadjuvant trastuzumab, and 44 with missing data on the variables of interest (ER, ERBB2, and grade). The final study population included 2377 patients. None of these patients had been included in the development or validation of the CPS+EG staging system.16–18
The institutional review board at The University of Texas MD Anderson Cancer Center approved this study and granted a waiver of informed consent.
Clinicopathologic data were recorded, including age, clinical and pathologic stage, ER status, ERBB2 status, and nuclear grade. Clinical stage was based on physical examination, mammography, and ultrasonography of the breast and regional nodal basins at presentation. Clinical stage and pathologic stage were determined according to the guidelines set out in the seventh edition of the AJCC [American Joint Committee on Cancer] Cancer Staging Manual. Status of ER, ERBB2, and nuclear grade were obtained from each patient’s diagnostic core biopsy and reported into the pathology record by a dedicated breast pathologist. The ER status was recorded as the percentage of cells staining positive under immunohistochemical analysis and was classified into 3 groups: less than 1%, 1% to 9%, and 10% or more. The ERBB2 status was defined as positive at a reading of 3+ on immunohistochemical analysis or when gene amplification was shown on fluorescence in situ hybridization. A pCR was defined as no invasive disease in the breast or regional lymph nodes noted on the final pathology report after surgery.
Treatment
All patients received an anthracycline- and/or taxane-based neoadjuvant chemotherapy regimen; trastuzumab was administered as part of the neoadjuvant regimen for patients with ERBB2-positive disease. The study period predated the use of pertuzumab in the neoadjuvant setting. Following completion of neoadjuvant chemotherapy, patients underwent local therapy consisting of either breast-conserving therapy (which included a segmental mastectomy, axillary evaluation, and whole-breast irradiation) or mastectomy with axillary evaluation with or without postmastectomy irradiation. During the study period, postmastectomy radiation therapy was routinely recommended for patients with clinical stage III disease or pathologically node-positive disease after neoadjuvant chemotherapy, and selectively for patients with clinical T1/T2N1 tumors and high-risk features including young age, pathologic tumor size 2 cm or larger, lymphovascular invasion, high-grade disease, ER-negative disease, or treatment effect noted in multiple otherwise negative lymph nodes. No patients received additional adjuvant chemotherapy. Patients with HR-positive tumors were routinely advised to take adjuvant endocrine therapy. Patients with ERBB2-positive disease routinely completed 1 year of trastuzumab therapy.
Statistical Analysis
The CPS+EG score was determined for each patient according to the previously published staging system (Table 1).16 The score was calculated twice, once using 1% or higher as the cutoff for ER positivity and again using 10% or higher as the cutoff. The DSS was calculated from the date of diagnosis to the date of death resulting from breast cancer or censored at the date of last follow-up or death unrelated to breast cancer. Five-year DSS was calculated using the Kaplan-Meier method for patient subgroups defined using multiple staging systems: (1) AJCC clinical stage, (2) AJCC pathologic stage, (3) CPS+EG using 1% or higher as the cutoff for ER positivity, and (4) CPS+EG using 10% or higher as the cutoff. Within each staging system, DSS among subgroups was compared using the log-rank test. Fits of the Cox proportional hazards model for the CPS+EG prognostic scores computed using 2 different cutoffs for ER were compared using the Akaike Information Criterion (AIC). The ERBB2 status was added to the model, and the likelihood ratio test was used to determine improvement infit, with P < .05 indicating a significant finding. A novel staging system (Neo-Bioscore) was constructed by adding a point to the CPS+EG score for ERBB2 negativity (Table 1). Five-year DSS using the Neo-Bioscore was calculated using the KaplanMeier method. All calculations were performed with Stata software, release 14 (StataCorp LP).
Results
The study population consisted of 2377 patients; all were women. The median patient age was 50 years (range, 21-87 years). The ER status was lower than 1% in 28.9% of the patients, 1% to 9% in 8.3%, and 10% or higher in 62.8%. A total of 591 patients were ERBB2+; all of whom received trastuzumab as a component of their neoadjuvant regimen. Most patients (63.8%) had clinical stage II disease (IIA, 30.8%; IIB, 33.0%); 34.0% had clinical stage III disease (IIIA, 14.7%; IIIB, 6.6%; IIIC, 12.7%). A pCR was achieved in 26.0% of patients, with the remaining patients having pathologic stages I (18.4%), IIA (19.3%), IIB (12.7%), IIIA (15.6%), IIIB (1.1%), or IIIC (6.9%) disease. Complete clinicopathologic characteristics and treatment regimens are listed in the eTable 1 in the Supplement.
The median follow-up time was 4.2 years (range, 0.5-11.7 years). The estimated 5-year DSS rate for the entire study population was 89% (95% CI, 87%–90%). Five-year DSS rates by clinical and pathologic stage are shown in Figure 1A and B. Disease-specific survival rates by CPS+EG score using the 2 different cutoffs for ER positivity, 1% (Figure 1C) and 10% (eFigure 1 in the Supplement), were also determined. The AIC values for the 2 models were 3333.06 for a 1% ER positivity or higher cutoff vs 3333.38 for a 10% or higher cutoff, indicating that the model fits were nearly identical though slightly better for the 1% or higher cutoff.
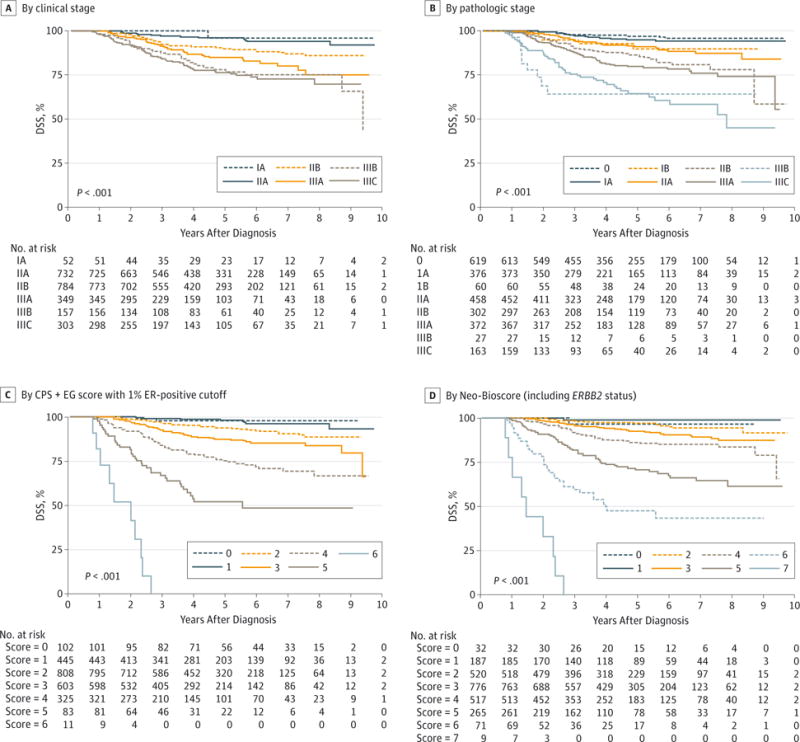
The DSS was determined based on presenting clinical stage (A), final pathologic stage (B), CPS+EG staging system (clinical-pathologic scoring system incorporating estrogen receptor-negative disease and nuclear grade 3 tumor pathology) (C),16–18 and Neo-Bioscore, which includes ERBB2 status (D).
The ERBB2 status was added to the CPS+EG model with 1% or higher ER positivity as the cutoff, consistent with current American Society of Clinical Oncology (ASCO) and College of American Pathologists (CAP) guidelines.26 Inclusion of ERBB2 status improved the fit of the model, with worse DSS for ERBB2-negative patients. Accordingly, the CPS+EG staging system was further refined to incorporate ERBB2 status by adding 1 additional point for ERBB2-negative status to define a new Neo-Bioscore (Table 1), which includes 8 distinct stages. Neo-Bioscore stratified patients with respect to DSS, with 5-year DSS estimates ranging from 48% to 99% (P < .001) (Figure 1D). Table 2 summarizes 5-year DSS rates for the study cohort stratified according to the clinical and pathologic AJCC stages, the CPS+EG staging system using the 1% cut-off, and the Neo-Bioscore. When the Neo-Bioscore was applied, there was a shift from the CPS+EG score, a more refined stratification in 1786 patients (75%). As detailed in Table 2, this shift reflects the percentage of ERBB2-negative tumors. The DSS rates for each CPS+EG score comparing ERBB2-positive with ERBB2-negative tumors are shown in eFigure 2 in the Supplement. Analyses were repeated excluding 225 ER-positive patients (9.5%) who did not receive endocrine therapy. For these analyses, patients treated prior to 2010 were considered positive if their ER values were 10% or higher, and patients treated from 2010 onward were considered positive if their ER values were 1% or higher. The Neo-Bioscore again stratified patient DSS better than did the presenting clinical stage or the final pathologic stage (Figure 2).
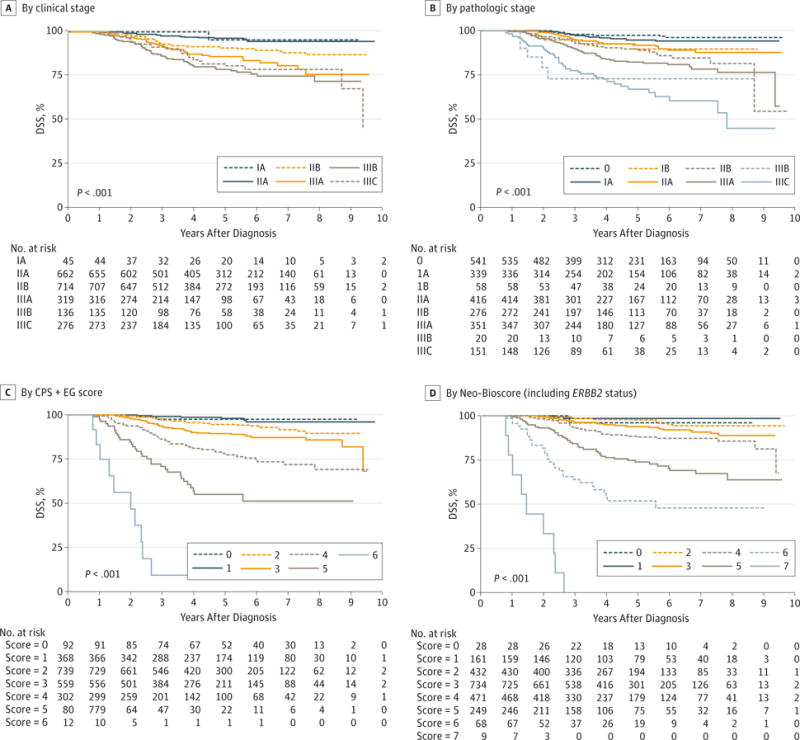
The DSS was determined based on presenting clinical stage (A), final pathologic stage (B), CPS+EG staging system (clinical-pathologic scoring system incorporating ER-negative disease and nuclear grade 3 tumor pathology) (C),16–18 and Neo-Bioscore, which includes ERBB2 status (D). For these analyses, patients treated prior to 2010 were considered ER positive at an ER value of 10% or higher Patients treated from 2010 through 2012 were considered ER positive at an ER value of 1% or higher.
Table 2
Five-Year DSS Outcomes by Clinical Stage, Pathologic Stage, CPS+EG Staging System, and Neo-Bioscore
| Clinical Stage | DSS (95% CI) | Pathologic Stage | DSS (95% CI) | CPS+EG Scorea | DSS (95% CI) | Neo-Bioscore | DSS (95% CI) |
|---|---|---|---|---|---|---|---|
| 0 | 0 (n = 619) | 97 (95–98) | 0 (n = 102) | 98 (92–100) | 0 (n = 32) | 97 (78–100) | |
| IA (n = 52) | 96 (75–99) | IA (n = 376) | 95 (92–97) | 1 (n = 445) | 98 (96–99) | 1 (n = 187) | 99 (95–100) |
| IB (NA) | NA | IB (n = 60) | 90 (76–98) | 2 (n = 808) | 94 (91–95) | 2 (n = 520) | 97 (95–98) |
| IIA (n = 732) | 96 (94–97) | IIA (n = 458) | 91 (87–94) | 3 (n = 603) | 87 (84–90) | 3 (n = 776) | 93 (90–95) |
| IIB (n = 784) | 90 (87–92) | IIB (n = 302) | 86 (81–90) | 4 (n = 325) | 75 (69–80) | 4 (n = 517) | 86 (82–89) |
| IIIA (n = 349) | 85 (80–89) | IIIA (n = 372) | 80 (75–84) | 5 (n = 83) | 52 (40–63) | 5 (n = 265) | 71 (64–77) |
| IIIB (n = 157) | 78 (70–85) | IIIB (n = 27) | 64 (42–80) | 6 (n = 11) | 0 | 6 (n = 71) | 48 (35–60) |
| IIIC (n = 303) | 76 (70–81) | IIIC (n = 163) | 64 (55–72) | 7 (n = 9) | 0 |
Discussion
Our group16 previously developed the CPS+EG staging system, which incorporates tumor biologic factors including ER status and grade into traditional pretreatment and posttreatment anatomic staging allowing for better DSS stratification of patients with breast cancer receiving neoadjuvant chemotherapy.16 The staging system was both internally and externally validated.17,18 A limitation of the CPS+EG staging system is that it cannot be used for patients with ERBB2-positive breast cancer because its development predated the routine use of ERBB2-targeted therapy. In the present study, we validated the CPS+EG staging system with a contemporary cohort and showed that it is applicable regardless of whether a 1% or 10% cutoff is used to define ER positivity–an important finding given the 2010 change in ASCO/CAP guidelines.26 In addition, we showed that ERBB2 status is an important prognostic factor in an era when patients with ERBB2-positive breast cancer are routinely treated with trastuzumab. Incorporation of ERBB2 status into the CPS+EG staging system defines a new staging system, the Neo-Bioscore, which allows for prognostic stratification of patients with breast cancer of all subtypes who receive neoadjuvant chemotherapy.
A recent meta-analysis in which data were pooled from almost 12 000 patients enrolled in 12 neoadjuvant trials showed that a pCR, defined as no residual invasive disease in the breast or axilla (ypT0/is ypN0), was associated with improved eventfree survival and OS.11 It is important to note that, while this survival improvement was demonstrated in the patient-level analysis, when the investigators performed additional analyses to address treatment effects within the individual trials, pCR was not validated as a surrogate end point for improved event-free survival and OS.11 A second meta-analysis that included 29 neoadjuvant therapy trials provided further trial-level data that did not support the use of pCR as a surrogate end point for DFS or OS.27
One strength of the Neo-Bioscore is that it can better stratify DSS for patients who achieve a pCR by accounting for presenting clinical stage and biologic markers. A patient presenting with a stage IIA, grade 2, triple-negative breast cancer who achieves a pCR would have a 98% 5-year expected DSS calculated by CPS+EG (score = 1) and a 97% 5-year expected DSS by Neo-Bioscore (score = 2). In contrast, if a patient presenting with stage IIIC, grade 2, triple-negative breast cancer achieves a pCR, the anticipated 5-year DSS rate would be 87% by CPS+EG (score = 3) and 86% by Neo-Bioscore (score = 4). If a patient presenting with stage IIIC, ERBB2-positive, ER-negative, grade 2 disease achieves pCR, the anticipated 5-year DSS rate would be 87% by CPS+EG (score = 3) and 93% by Neo-Bioscore (score = 3). Taken together, these data emphasize the importance of the extent of disease at presentation and tumor biology in dictating outcomes as well as the value of the Neo-Bioscore over CPS+EG in further stratifying DSS outcomes in patients with ERBB2-positive disease.
The prognosis of patients not achieving pCR is less well defined. Multiple investigators have attempted to determine prognosis in these patients using pathologic measures and/or biologic characteristics. Carey et al12 have reported that assignment of pathologic stage according to the AJCC staging system can stratify patients with respect to distant DFS and OS. Symmans et al15 describe the residual cancer burden (RCB), a continuous index that combines pathologic size and cellularity of the primary tumor and the number and size of nodal metastases. The RCB score, which can range from 0 (pCR) to III (extensive residual disease), is a significant predictor of distant relapse-free survival. When applied to posttreatment AJCC stage groups, the RCB can further stratify patients into subgroups with respect to prognosis.
Jones et al13 have reported that posttreatment expression of the proliferative marker Ki67 is inversely correlated with recurrence-free and OS for patients not achieving a pCR, and more recently, Sheri et al14 have reported that adding posttreatment Ki67 to the RCB could improve long-term outcome prediction. These observations regarding the prognostic significance of post-treatment Ki67 are likely due to the fact that patients with a high Ki67 level in the resected tumor have resistant and highly proliferative disease not eradicated with chemotherapy. Interestingly, in the study by Sheri et al, the investigators looked at the additional prognostic information that could be gained from posttreatment ER expression and grade. Consistent with the CPS+EG and Neo-Bioscore, which both showed improved stratification with the inclusion of more biologic factors, they found that the addition of all variables, RCB, Ki67, ER, and grade, provided the most informative prognostic score.14
Like the CPS+EG staging system, the RCB was defined in a cohort of patients treated prior to the routine use of trastuzumab for ERBB2-positive disease. Of 220 patients in the study by Sheri et al,14 53 had ERBB2-positive disease, but fewer than 50% received neoadjuvant trastuzumab. Therefore, the utility of these previously published prognostic scores in patients with ERBB2-positive disease treated with trastuzumab is unknown. The Neo-Bioscore accounts for the favorable effect of trastuzumab on the prognosis of patients with ERBB2-positive disease by assigning an additional point to patients with ERBB2-negative breast cancer. Importantly, this suggests that the CPS+EG staging system can be used as a “backbone” and that it is possible that as new molecular markers are identified, they can be easily incorporated to refine the staging system.
There is a growing body of literature differentiating ERBB2-positive breast cancer by HR expression. In the meta-analysis from Cortazar et al,11 the effect of achieving a pCR on eventfree survival and OS was most pronounced in aggressive breast cancer subtypes including ERBB2-positive, HR-negative disease as well as triple-negative disease. That meta-analysis included 3 trials that reported the administration of trastuzumab for ERBB2-positive patients, the GeparQuattro,25 NOAH,20 and TECHNO24 trials. The pCR rate in the meta-analysis for patients with ERBB2-positive, HR-positive disease was 31% (95% CI, 26%–36%), and the hazard ratio for OS for those achieving a pCR vs those who did not was 0.56 (95% CI, 0.23-1.37). For patients with ERBB2-positive, HR-negative disease, the pCR rate was 50% (95% CI, 45%–56%), and the hazard ratio for OS was 0.08 (95% CI, 0.03-0.22).11 The meta-analysis also included 1157 patients with triple-negative breast cancer, and in these patients, the pCR rate was 34% (95% CI, 31%–36%), and the hazard ratio for OS for those achieving a pCR vs those who did not was 0.16 (95% CI, 0.11-0.25).11 Similarly, in a report of approximately 1100 patients receiving neoadjuvant chemotherapy at MD Anderson between 1985 and 2004, prior to routine evaluation of ERBB2 status and use of trastuzumab, patients with triple-negative breast cancer were found to have significantly higher pCR rates than patients with non-triple-negative breast cancer, 22% vs 11%.28 Interestingly, patients with triple-negative breast cancer who achieved a pCR had a 3-year OS rate of 94%, not significantly different than the 98% in patients with non-triple-negative breast cancer. However, for patients with triple-negative breast cancer who did not achieve a pCR, the 3-year OS rate was 68%. In contrast, for patients with non-triple-negative breast cancer not achieving a pCR, the 3-year OS rate was 88%. Taken together, these data emphasize the importance of achieving a pCR in patients with aggressive subtypes of breast cancer lacking HR expression. This is likely because these patients have already received the majority of their systemic therapy prior to surgery and have residual disease, with the caveat that ERBB2-positive patients currently complete 1 year of trastuzumab therapy. In addition, because they lack HR expression, they are not going to benefit from administration of endocrine therapy. This biologic information is captured by the Neo-Bioscore.
In addition, to meet the growing interest in treating patients with residual disease following neoadjuvant chemotherapy, the Neo-Bioscore could be used to identify high-risk patients for clinical trial participation or to stratify patients in such studies. Two ongoing trials are using the CPS+EG staging system to identify high-risk ER-positive patients with residual disease after neoadjuvant chemotherapy. The PENELOPE-B trial (NCT01864746) trial evaluating the addition of palbociclib to standard endocrine therapy and the OlympiA (NSABP B-55/BIG 6-13; NCT02032823) evaluating olaparib in BRCA-mutated, non-ERBB2-positive breast cancer are both using a CPS+EG score of 3 or higher to define high-risk ER-positive patients. The present study further validates the CPS+EG staging system regardless of the threshold used to define ER positivity and provides the Neo-Bioscore, which could be used in a similar fashion to identify high-risk ERBB2-positive patients to be considered for additional adjuvant therapy trials.
Conclusions
This work represents a continued effort by our group to demonstrate the importance of tumor biology in dictating survival outcomes for patients with breast cancer. In addition to the CPS+EG staging system and the newly defined Neo-Bioscore, which apply to patients receiving neoadjuvant chemotherapy, we have published data for patients undergoing surgery as an initial intervention showing the importance of ER status and grade on prognosis.29 Work is ongoing to update that staging system as well, incorporating ERBB2-positive patients who received trastuzumab. Goals of the AJCC staging system include providing a common language for physicians to communicate regarding a patient’s disease and providing prognostic information. A robust staging system is also critical for identifying and stratifying clinical trials. We therefore suggest that biologic factors and, for patients receiving neoadjuvant chemotherapy, the response to treatment be incorporated into the AJCC staging system, which is currently being revised.
Acknowledgments
Funding/Support: This work was supported in part by a cancer center support grant (CA016672) from the National Cancer Institute to The University of Texas MD Anderson Cancer Center. Dr Mittendorf is an R. Lee Clark Fellow of The University of Texas MD Anderson Cancer Center supported by the Jeanne F. Shelby Scholarship Fund. Dr Jose Vila is supported by a grant from the Carlos III Health Institute.
Role of the Funder/Sponsor: The funding institutions had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Disclosures
Dr Mittendorf is the principal investigator for clinical trials supported by Antigen Express, Galena BioPharma, and Genentech. Dr Chavez-MacGregor is a consultant for Novartis, Pfizer, and Roche. Dr Symmans has stock and intellectual property in Nuvera Biosciences. Dr Hortobagyi is the principal investigator for clinical trials supported by Novartis and is a consultant to Antigen Express, AstraZeneca, Genentech, Novartis, Peregrine, and Pfizer. Dr Hunt is a consultant for Armada Health.
Footnotes
Author Contributions: Drs Mittendorf and Tucker had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Mittendorf, Tucker, Sahin, Hunt.Acquisition, analysis, or interpretation of data: Mittendorf, Vila, Tucker, Chavez-MacGregor, Smith, Symmans, Sahin, Hortobagyi, Hunt.
Drafting of the manuscript: Mittendorf, Vila, Tucker, Hunt.
Critical revision of the manuscript for important intellectual content: Mittendorf, Tucker, Chavez-MacGregor, Smith, Symmans, Sahin, Hortobagyi, Hunt.
Statistical analysis: Mittendorf, Tucker, Chavez-MacGregor, Hunt.
Administrative, technical, or material support: Mittendorf, Vila, Sahin, Hortobagyi, Hunt.
Study supervision: Mittendorf, Symmans, Hortobagyi, Hunt.
Supplemental content at jamaoncology.com
Conflict of Interest: No other conflicts are reported.
Previous Presentation: This work was presented in part at the 2015 San Antonio Breast Cancer Symposium; December 11, 2015; San Antonio, Texas.
Contributor Information
Elizabeth A. Mittendorf, Department of Breast Surgical Oncology, The University of Texas MD Anderson Cancer Center, Houston.
Jose Vila, Department of Breast Surgical Oncology, The University of Texas MD Anderson Cancer Center, Houston.
Susan L. Tucker, Department of Bioinformatics and Computational Biology, The University of Texas MD Anderson Cancer Center, Houston.
Mariana Chavez-MacGregor, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston. Department of Health Services Research, The University of Texas MD Anderson Cancer Center, Houston.
Benjamin D. Smith, Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston.
W. Fraser Symmans, Department of Pathology, The University of Texas MD Anderson Cancer Center, Houston.
Aysegul A. Sahin, Department of Pathology, The University of Texas MD Anderson Cancer Center, Houston.
Gabriel N. Hortobagyi, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston.
Kelly K. Hunt, Department of Breast Surgical Oncology, The University of Texas MD Anderson Cancer Center, Houston.
References
Full text links
Read article at publisher's site: https://doi.org/10.1001/jamaoncol.2015.6478
Read article for free, from open access legal sources, via Unpaywall:
https://jamanetwork.com/journals/jamaoncology/articlepdf/2504172/coi150119.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1001/jamaoncol.2015.6478
Article citations
Impact of the CPS-EG score as a new prognostic biomarker in triple-negative breast cancer patients who received neoadjuvant chemotherapy.
BMC Cancer, 24(1):1338, 30 Oct 2024
Cited by: 0 articles | PMID: 39478493 | PMCID: PMC11526593
Fluctuations in serum lipid levels during neoadjuvant treatment as novel predictive and prognostic biomarkers for locally advanced breast cancer: a retrospective analysis based on a prospective cohort.
Lipids Health Dis, 23(1):261, 22 Aug 2024
Cited by: 0 articles | PMID: 39175000 | PMCID: PMC11340160
Neoadjuvant Therapy: Current Landscape and Future Horizons for ER-Positive/HER2-Negative and Triple-Negative Early Breast Cancer.
Curr Treat Options Oncol, 25(9):1210-1224, 15 Aug 2024
Cited by: 0 articles | PMID: 39145854 | PMCID: PMC11416407
Review Free full text in Europe PMC
Circulating tumor cell phenotype detection based on epithelial-mesenchymal transition markers combined with clinicopathological risk has potential to better predict recurrence in stage III breast cancer treated with neoadjuvant chemotherapy: a pilot study.
Breast Cancer Res Treat, 207(3):517-527, 11 Jul 2024
Cited by: 0 articles | PMID: 38990453
Impact of Presenting Stage on Overall Survival in Patients Treated with Neoadjuvant Chemotherapy for Triple Negative Breast Cancer.
Ann Surg Oncol, 31(8):5132-5140, 13 Jun 2024
Cited by: 0 articles | PMID: 38872043
Go to all (66) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials (2)
- (1 citation) ClinicalTrials.gov - NCT02032823
- (1 citation) ClinicalTrials.gov - NCT01864746
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Incorporation of Treatment Response, Tumor Grade and Receptor Status Improves Staging Quality in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy.
Ann Surg Oncol, 24(12):3510-3517, 21 Aug 2017
Cited by: 14 articles | PMID: 28828583
Validation of the CPS+EG and Neo-Bioscore staging systems after preoperative systemic therapy for breast cancer in a single center in China.
Breast, 40:29-37, 17 Apr 2018
Cited by: 8 articles | PMID: 29677568
Validation of CPS+EG, Neo-Bioscore, and modified Neo-Bioscore staging systems after preoperative systemic therapy of breast cancer: Protocol of a retrospective multicenter cohort study in China.
Thorac Cancer, 9(11):1565-1572, 17 Sep 2018
Cited by: 4 articles | PMID: 30296013 | PMCID: PMC6209787
Incorporating Biologic Factors into the American Joint Committee on Cancer Breast Cancer Staging System: Review of the Supporting Evidence.
Surg Clin North Am, 98(4):687-702, 21 May 2018
Cited by: 15 articles | PMID: 30005768
Review





