Abstract
Background
Recent advances have enabled noninvasive mapping of cardiac arrhythmias with electrocardiographic imaging and noninvasive delivery of precise ablative radiation with stereotactic body radiation therapy (SBRT). We combined these techniques to perform catheter-free, electrophysiology-guided, noninvasive cardiac radioablation for ventricular tachycardia.Methods
We targeted arrhythmogenic scar regions by combining anatomical imaging with noninvasive electrocardiographic imaging during ventricular tachycardia that was induced by means of an implantable cardioverter-defibrillator (ICD). SBRT simulation, planning, and treatments were performed with the use of standard techniques. Patients were treated with a single fraction of 25 Gy while awake. Efficacy was assessed by counting episodes of ventricular tachycardia, as recorded by ICDs. Safety was assessed by means of serial cardiac and thoracic imaging.Results
From April through November 2015, five patients with high-risk, refractory ventricular tachycardia underwent treatment. The mean noninvasive ablation time was 14 minutes (range, 11 to 18). During the 3 months before treatment, the patients had a combined history of 6577 episodes of ventricular tachycardia. During a 6-week postablation "blanking period" (when arrhythmias may occur owing to postablation inflammation), there were 680 episodes of ventricular tachycardia. After the 6-week blanking period, there were 4 episodes of ventricular tachycardia over the next 46 patient-months, for a reduction from baseline of 99.9%. A reduction in episodes of ventricular tachycardia occurred in all five patients. The mean left ventricular ejection fraction did not decrease with treatment. At 3 months, adjacent lung showed opacities consistent with mild inflammatory changes, which had resolved by 1 year.Conclusions
In five patients with refractory ventricular tachycardia, noninvasive treatment with electrophysiology-guided cardiac radioablation markedly reduced the burden of ventricular tachycardia. (Funded by Barnes-Jewish Hospital Foundation and others.).Free full text

Noninvasive Cardiac Radiation for Ablation of Ventricular Tachycardia
Abstract
BACKGROUND
Recent advances have enabled noninvasive mapping of cardiac arrhythmias with electrocardiographic imaging and noninvasive delivery of precise ablative radiation with stereotactic body radiation therapy (SBRT). We combined these techniques to perform catheter-free, electrophysiology-guided, noninvasive cardiac radioablation for ventricular tachycardia.
METHODS
We targeted arrhythmogenic scar regions by combining anatomical imaging with noninvasive electrocardiographic imaging during ventricular tachycardia that was induced by means of an implantable cardioverter–defibrillator (ICD). SBRT simulation, planning, and treatments were performed with the use of standard techniques. Patients were treated with a single fraction of 25 Gy while awake. Efficacy was assessed by counting episodes of ventricular tachycardia, as recorded by ICDs. Safety was assessed by means of serial cardiac and thoracic imaging.
RESULTS
From April through November 2015, five patients with high-risk, refractory ventricular tachycardia underwent treatment. The mean noninvasive ablation time was 14 minutes (range, 11 to 18). During the 3 months before treatment, the patients had a combined history of 6577 episodes of ventricular tachycardia. During a 6-week postablation “blanking period” (when arrhythmias may occur owing to postablation inflammation), there were 680 episodes of ventricular tachycardia. After the 6-week blanking period, there were 4 episodes of ventricular tachycardia over the next 46 patient-months, for a reduction from baseline of 99.9%. A reduction in episodes of ventricular tachycardia occurred in all five patients. The mean left ventricular ejection fraction did not decrease with treatment. At 3 months, adjacent lung showed opacities consistent with mild inflammatory changes, which had resolved by 1 year.
CONCLUSIONS
In five patients with refractory ventricular tachycardia, noninvasive treatment with electrophysiology-guided cardiac radioablation markedly reduced the burden of ventricular tachycardia. (Funded by Barnes–Jewish Hospital Foundation and others.)
In clinical electrophysiology, noninvasive cardiac imaging methods, such as cardiac magnetic resonance imaging (MRI) and computed tomography (CT), help to identify and localize myocardial scar that may have an abnormal electrical substrate causing ventricular tachycardia.1,2 The use of multielectrode body-surface electrocardiography to create a cardiac image (electrocardiographic imaging)3 in combination with standard cardiac imaging can identify in a noninvasive manner both myocardial scar and the arrhythmogenic region from which ventricular arrhythmias arise.3-6 With the use of this technique, it is theoretically possible to develop a totally noninvasive method for ablation of ventricular tachycardia if the imaging is combined with similarly noninvasive ablative techniques.
One such technique is stereotactic body radiation therapy (SBRT), which delivers precise, high-dose radiation to targets in the body with minimal damage to normal adjacent tissue.7 SBRT is most commonly used to treat tumors, with high rates of tumor control and low rates of toxic effects. Preclinical studies that have explored SBRT for the ablation of cardiac arrhythmia have identified doses that recapitulate the effects of catheter radiofrequency ablation with 25 to 35 Gy delivered in a single fraction. This therapy has resulted in late myocardial fibrosis and electrically inert tissue without evidence of acute or subacute injury to nearby tissues during 6 months of follow-up.8,9 Clinical case reports have shown the feasibility of the use of SBRT for this purpose.10,11
Although catheter ablation is the current state-of-the-art treatment for drug-refractory ventricular arrhythmias in patients with structural heart disease,12 it is not curative for many patients.13-22 Common reasons for catheter-ablation failures include inaccessible arrhythmogenic tissue and an inability to deliver adequate ablative energy transmurally across ventricular myocardium. In addition, catheter ablation of ventricular tachycardia is associated with high risks of procedural complication and death.23 SBRT has the potential to overcome these challenges.8,9 In this case series, we combined noninvasive mapping (electrocardiographic imaging) and noninvasive ablation (SBRT) to treat patients with refractory ventricular tachycardia.
METHODS
TREATMENT PLAN AND STUDY OVERSIGHT
The treatment described in this article was delivered to patients on the basis of their clinical circumstances, without specific testing of a research hypothesis. The treatment plan was designed by the first and last authors. All the authors participated in data collection and analysis and in the writing of the manuscript. (Details regarding the authors’ contributions are provided in the Supplementary Appendix, available with the full text of this article at NEJM.org.) The study received no industry support.
All the patients received a detailed explanation of the risks of treatment from both the treating electrophysiologist (the first author) and radiation oncologist (the last author); all the patients provided written informed consent to treatment. Institutional review board approval had previously been provided for the use of electrocardiographic imaging. At the time of the study, the SBRT device had received 510(k) premarket approval from the Food and Drug Administration, but its use in the patients reported here was considered to be off-label clinical use; this information was conveyed to the patients who were included in this study.
STUDY PATIENTS
We evaluated patients with structural heart disease, placement of an implantable cardioverter–defibrillator (ICD), and treatment-refractory ventricular tachycardia with limited conventional therapeutic options for noninvasive cardiac ablation of ventricular tachycardia (noninvasive radioablation) on a case-by-case basis. Patients were considered for noninvasive radioablation if they had had at least three episodes of ICD-treated ventricular tachycardia in the preceding 3 months, despite having received at least two antiarrhythmic medications and having undergone at least one catheter-ablation procedure (or having a contraindication to catheter ablation). Evaluation of the patients for cardiac transplantation was encouraged, according to institutional standard of care, but transplantation eligibility was not an absolute condition for consideration. Patients who had undergone placement of a left ventricular assist device were not evaluated for inclusion in the study.
PROCEDURAL WORKFLOW
The procedural workflow for noninvasive radioablation is shown in Figure 1, with full details provided in the Supplementary Appendix. Before treatment, patients underwent noninvasive electrocardiographic imaging during induced ventricular tachycardia to precisely map the ventricular tachycardia circuit. For electrocardiographic imaging, patients wore a vest of 256 electrodes (BioSemi) and underwent chest CT scanning. Patients were then brought to the electrophysiology laboratory and underwent noninvasive programmed stimulation with the use of an indwelling ICD to induce ventricular tachycardia. Data for electrocardiographic imaging maps were obtained, and the ICD was used to terminate ventricular tachycardia with a brief overdrive-pacing maneuver. Electrocardiographic imaging maps were created to identify the site of earliest electrical activation during ventricular tachycardia (the “exit site”), as described previously.3-6
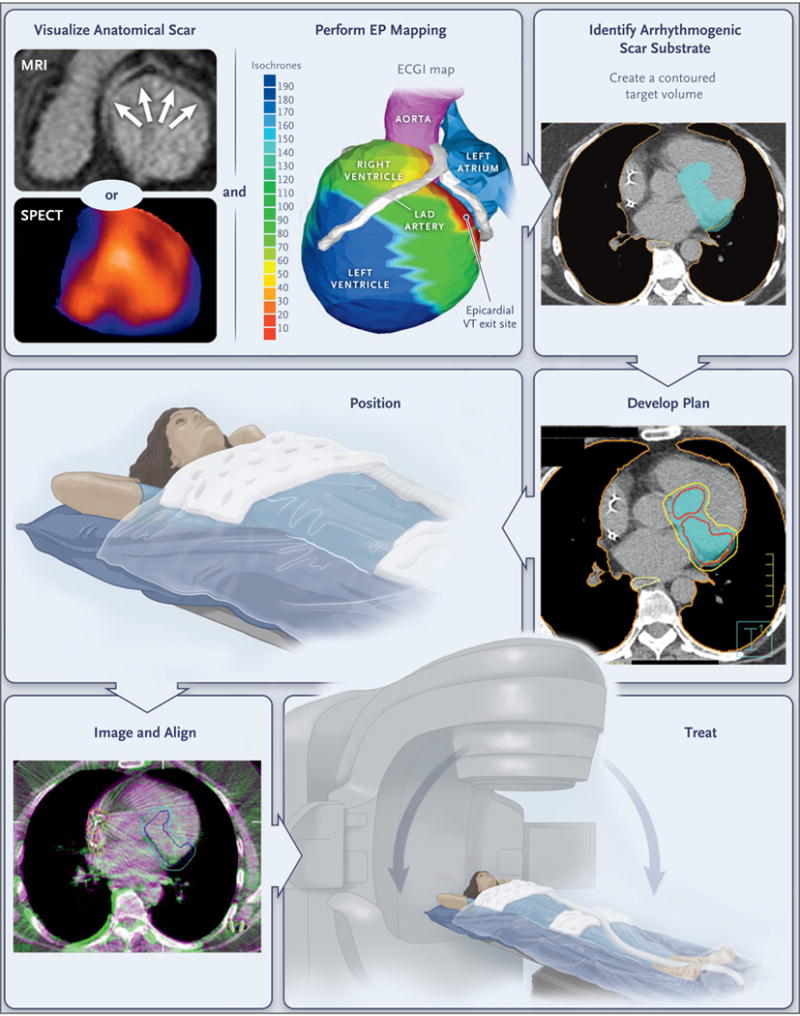
Patients undergo noninvasive visualization of the ventricular scar by means of MRI, CT, single-photon emission CT (SPECT), or a combination of methods, according to clinical routine. The zone of scarring is indicated by arrows on MRI and by blue regions on SPECT, including the base, inferior wall, and apex. Noninvasive electrophysiologic (EP) mapping is performed with electrocardiographic imaging (ECGI) of induced ventricular tachycardia (VT) with programed stimulation from the indwelling implantable cardioverter–defibrillator. The color scale shows the range of activation times of each area of the ventricular wall (isochrones), ranging from 10 msec (red) to 190 msec (deep blue) from the onset of VT activation. The electrophysiologist develops an ablation volume by targeting the full thickness of the ventricular wall harboring the first 10 msec of VT activation (the “exit site”) and the colocalized ventricular scar. (Details regarding scar imaging, EP mapping, and image fusion to develop the ablation volume are provided in the Supplementary Appendix.) The target volume is shown in light blue in the figure panel showing the arrhythmogenic scar substrate. This volume is transferred by the radiation oncologist onto a respiratory-correlated, four-dimensional CT scan, which allows an assessment of the total cardiac and pulmonary motion. In this example, a dose of 25 Gy in a single fraction is prescribed for delivery to the enhanced treatment volume, with a goal of achieving maximal coverage inside the volume while avoiding exposure to the surrounding organs at risk. The target volume is indicated in light blue in the figure panel showing the treatment plan; red and yellow boundaries indicate the distribution of zones projected to receive 2750 cGy and 2375 cGy of radiation, respectively; the lung is outlined in orange, and the yellow boundary behind the heart is the esophagus. If all plans pass standard internal physics quality assurance on a calibrated phantom, the patient is immobilized with the use of a vacuum-assisted device, and stereotactic radioablation is performed by means of an image-guided, radiotherapy-equipped linear accelerator that uses a cone-beam CT to align the radiotherapy treatment beams with the target volume. The dark blue boundary indicates the target, which includes the total cardiac and pulmonary motion. The light blue boundary indicates the target with an additional expansion to account for motion, setup uncertainty, and delivery uncertainty. Treatment is then delivered with the use of the radiotherapy delivery system. LAD denotes left anterior descending.
When clinically available, additional cardiac imaging was used to identify regions of anatomical scarring with either resting single-photon emission CT (SPECT) or contrast-enhanced cardiac MRI with the use of standard techniques (Fig. 1). Electrical information from the electrocardiographic imaging and information from the anatomical scarring were combined to build a volumetric target for radioablation that targeted the area of the first 10 msec of ventricular tachycardia (the exit site) and the full myocardial thickness of the associated ventricular scar.
In addition, before treatment, patients underwent a planning CT scan, which included immobilization of the entire body from thorax to legs with the use of a vacuum-assisted device (Body-FIX, Elekta) and acquisition of a respiration-correlated CT scan (four-dimensional CT) to assess the sum total of cardiac and pulmonary motion. A final target (planning target volume) was developed by expanding the target, as defined above, to account for motion, setup uncertainty, and delivery uncertainty. (Details about this method are provided in the Supplementary Appendix.)
A total dose of 25 Gy in a single fraction was prescribed to be administered to the planning target volume with a goal of achieving maximal dose coverage while avoiding a dose in excess of calculated dose constraints to surrounding organs, including the esophagus, stomach, lungs, and spinal cord. All plans were subjected to, and passed, standard internal physics quality assurance on a calibrated phantom before delivery.
SBRT was performed with the use of an image-guided radiotherapy-equipped linear accelerator (TrueBeam, Varian Medical Systems) that uses cone-beam CT to acquire images of the thorax, which can be directly registered to the planning CT. This procedure results in accurate alignment of the heart and target volume without the need for invasive placement of a fiducial marker. During treatment, patients were placed in their custom immobilization device, which was aligned with the use of the cone-beam CT, with verification of the alignment by means of fluoroscopy. All the patients were treated without the use of any additional imaging during treatment and without sedation or anesthesia.
OUTCOME ASSESSMENTS
After treatment, patients were followed according to our standard of care for patients undergoing ablation of ventricular tachycardia. All ICDs were reprogrammed with a monitor-only zone at 100 bpm to assess for slow ventricular tachycardia. Patients were enrolled in a remote monitoring program for devices to enhance rapid identification and interpretation of any ICD-detected arrhythmias. Patients were seen in the outpatient clinic with ICD interrogations every 2 weeks for 2 months, monthly for the next 4 months, and then 1 year after treatment.
At each visit, an attempt was made to wean patients off their antiarrhythmic medications to mitigate known short-term and long-term toxic effects of these drugs. If no further ventricular arrhythmias were detected, doses of antiarrhythmic medications were reduced or stopped, with the goal of discontinuing all antiarrhythmic medications after the 6-week visit. Patients continued to receive medical therapy (including beta-blockers) for heart failure before and after treatment.
We tallied episodes of ventricular tachycardia as the sum of appropriate ICD shocks, appropriate ICD antitachycardia pacing, and sustained (>30 seconds), nontreated ventricular tachycardia in the monitor zone. The treating electrophysiologists adjudicated all ICD interrogations. Patients underwent echocardiography at baseline and at 1, 6, and 12 months after treatment to assess for cardiac adverse events. Patients also underwent chest CT at baseline and at 3 and 12 months to assess for thoracic adverse events in accordance with routine standard of care for thoracic SBRT.
RESULTS
PATIENTS
From April through November 2015, nine patients were evaluated for noninvasive radioablation; of these patients, five received the treatment. Of the four patients who did not receive treatment, two declined to participate (one chose to enter hospice care and died from complications of ventricular tachycardia 1 week later, and one chose to undergo an invasive procedure for ventricular tachycardia ablation), one died of progressive cardiogenic shock before treatment, and one underwent implantation of a left ventricular assist device, with recurrent ventricular tachycardia storm after surgery.
Table 1 outlines the demographic and clinical data for each patient; further clinical details are provided in the Supplementary Appendix. The mean age of the five treated patients was 66 years (range, 60 to 83). The mean number of episodes of ventricular tachycardia per patient in the 3 months before treatment was 1315 (range, 5 to 4312). All the patients were taking two antiarrhythmic drugs at the time of evaluation. Previous invasive catheter-ablation procedures had failed in three patients. Two patients had contraindications to invasive catheter ablation: one (Patient 2) had a new mechanical prosthetic mitral valve and evidence of epicardial ventricular tachycardia, and one (Patient 5) was deemed to be too frail for any invasive procedures. All five patients had New York Heart Association class III or IV heart-failure symptoms. The mean left ventricular ejection fraction was 23% (range, 15 to 37).
Table 1
Demographic and Clinical Characteristics of the Patients and Treatment Details.*
| Variable | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|---|---|---|---|---|
| Demographic or clinical characteristic | |||||
| Age (yr) | 61 | 60 | 65 | 62 | 83 |
| Sex | Male | Male | Male | Male | Female |
| Type of cardiomyopathy | Nonischemic | Ischemic | Nonischemic | Nonischemic | Ischemic |
| NYHA class | IV | III | IV | IV | IV |
| Left ventricular ejection fraction (%) | 37 | 17 | 22 | 26 | 15 |
| No. of previous antiarrhythmic drugs | 3 | 3 | 3 | 4 | 2 |
| No. of previous catheter ablations | 1 | 0 | 2 | 4 | 0 |
| No. of induced episodes of ventricular tachycardia | 2 | 1 | 0 | 5 | 6 |
| No. of episodes of ventricular tachycardia 3 mo before treatment | 30 | 20 | 5 | 2210 | 4312 |
| Treatment | |||||
| Ablation target region | Anterior basal left ventricle | Anterolateral basal left ventricle | Inferior left ventricle | Left ventricle outflow and septum | Inferolateral mid left ventricle |
| blation volume (ml) | 51.3 | 17.3 | 44.5 | 53.0 | 81.0 |
| Treatment time (min) | 12 | 11 | 14 | 12 | 18 |
| Length of hospital stay after treatment (days) | 2 | 1 | 2 | 2 | 1 |
| Antiarrhythmic medication at discharge | Amiodarone, mexiletine | Amiodarone, mexiletine | Amiodarone, mexiletine | Amiodarone, mexiletine | Amiodarone, mexiletine |
| No. of episodes of ventricular tachycardia during 6-wk blanking period | 0 | 3 | 0 | 355 | 322 |
| No. of episodes of ventricular tachycardia 10.5 mo after blanking period | 3 | 0 | 1 | 0 | NA |
| No. of additional ablation procedures performed by 1 yr | 0 | 0 | 0 | 1 at 4 wk | NA |
| Antiarrhythmic medication at 1 yr | None | None | Amiodarone (restarted at 9 mo) | None | NA |
NONINVASIVE RADIOABLATION PROCEDURE
Procedural details for each patient are provided in the Supplementary Appendix. All the patients underwent noninvasive electrocardiographic imaging for mapping of their ventricular tachycardia. Four patients had inducible ventricular tachycardia (mean number of circuits, three; range, one to six). Electrocardiographic imaging was performed during all induced episodes of ventricular tachycardia. In one patient (Patient 3), ventricular tachycardia could not be induced and no electrocardiographic imaging was obtainable, so the results of 12-lead electrocardiography and previous invasive catheter mapping were used to guide the creation of a volumetric target.
Treatment characteristics are provided in Table 1. Ablation target volumes ranged from 17 to 81 ml (mean, 49). On-table treatment times ranged from 11 to 18 minutes (mean, 14).
VENTRICULAR TACHYCARDIA BURDEN
At a median follow-up of 12 months, a marked reduction in the burden of ventricular tachycardia occurred after treatment (Table 1 and Fig. 2). In aggregate, there were 6577 episodes of ventricular tachycardia in the 15 patient-months before treatment. During the 6 weeks immediately after ablation (the “blanking period,” when arrhythmias may occur because of postablation inflammation), there were 680 episodes of ventricular tachycardia. After the 6-week blanking period, there were 4 episodes of ventricular tachycardia during the next 46 patient-months, for a relative reduction of 99.9% from baseline.
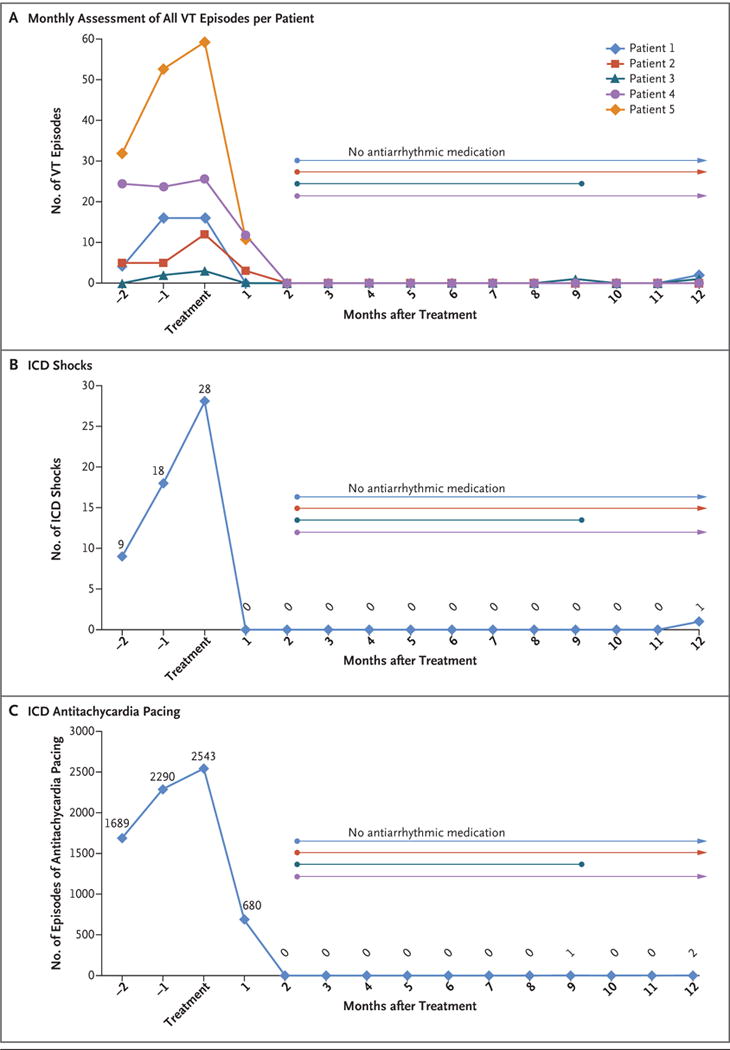
Panel A shows the total number of episodes of ventricular tachycardia (VT), including appropriate shocks from an implantable cardioverter–defibrillator (ICD), appropriate ICD antitachycardia pacing, and sustained untreated VT, in each of the five study patients, for 3 consecutive months before treatment and continuing for 12 months after treatment. In Patients 4 and 5, the numbers of VT episodes were markedly greater than in Patients 1, 2, and 3; therefore, the numbers that are shown for Patients 4 and 5 have been divided by 30 to allow comparisons on the same scale. Also shown are the total numbers of ICD shocks (Panel B) and numbers of episodes of antitachycardia pacing (Panel C) for all five patients during the same time frame. Six weeks after treatment, all four surviving patients were able to discontinue their antiarrhythmic medications, although Patient 3 restarted amiodarone 9 months after treatment of the first episode of antitachycardia pacing. Four weeks after treatment, Patient 4 underwent invasive catheter ablation because of incomplete cessation of ventricular tachycardia, with no further episodes by 12 months.
Figure 2A shows the monthly number of episodes of ventricular tachycardia on a per-patient basis. Every patient had a reduction in ventricular tachycardia burden. Of the four patients who were alive at 12 months, three were not receiving any antiarrhythmic medication. Patient 3 restarted amiodarone 9 months after treatment after the first episode of antitachycardia pacing. Patient 4 underwent an additional invasive catheter ablation procedure at 4 weeks after treatment because of incomplete cessation of ventricular tachycardia, with no further episodes thereafter. Improvement was observed with respect to both the number of ICD shocks (an aggregate number of 55 ICD shocks before treatment vs. 1 shock after treatment) (Fig. 2B) and ICD antitachycardia pacing (6577 episodes before treatment vs. 3 episodes after treatment) (Fig. 2C).
ADVERSE EVENTS
No complications occurred during the treatment or index hospitalization. Three patients reported fatigue on the day after treatment. No acute heart-failure exacerbations occurred in the immediate period after treatment. Patients were discharged home 1 to 3 days after treatment.
No adverse effects were observed in ICD system performance, lead thresholds, or lead impedances at any point after treatment. Serial echocardiography showed no pericardial effusions. The mean change in the left ventricular ejection fraction at the last follow-up visit was an absolute increase of 6 percentage points (range, −2 to 22) (Fig. 3A). No pulmonary symptoms occurred after treatment. Serial CT at 3 months showed findings that were consistent with inflammatory changes in the adjacent lung tissue that were typical of thoracic SBRT, with near-complete resolution at 12 months (Fig. 3B). At 12 months, there was no chest pain or apparent changes to the myocardium or coronary arteries on CT in the region targeted for treatment.
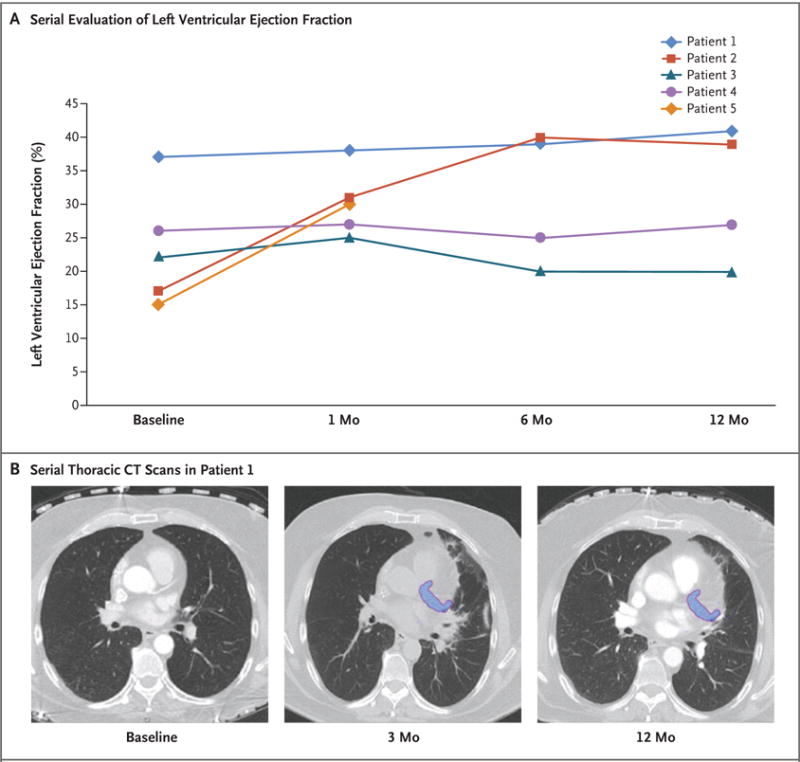
Panel A shows serial evaluation of the left ventricular ejection fraction after treatment in each of the study patients, as assessed on echocardiography. The mean value increased by 6 percentage points (range, −2 to 22). Panel B shows serial thoracic CT scans after treatment in Patient 1. The treatment area is shown in blue. At 3 months, there were adjacent local inflammatory changes in the lung parenchyma, effects that had nearly resolved at 12 months. A similar pattern was observed in the other study patients.
During follow-up, after amiodarone was discontinued, sinus-node function recovered in three patients. Heart rates in sinus rhythm occasionally exceeded the ICD programmed cutoff rate for detection (100 bpm). This situation required ICD reprogramming to avoid inappropriate therapy.
One patient (Patient 5) had a fatal stroke 3 weeks after treatment. This 83-year-old woman had a history of atrial fibrillation, severe cardiomyopathy, and other risk factors for stroke. Because of a risk of frailty-related bleeding, oral anticoagulants were not prescribed for stroke prevention. In the 3 weeks after treatment, her burden of ventricular tachycardia was reduced by 82% (from 1777 episodes of antitachycardia pacing in the month before treatment to 322 episodes after treatment). Her left ventricular ejection fraction had increased from 15% to 30%. No intracardiac thrombus was seen on echocardiography or during pathological assessment. It remains unclear whether the stroke was associated with SBRT or with preexisting medical conditions that placed her at high risk for stroke. (Additional information about this patient is provided in the following section.)
CARDIAC RADIOBIOLOGIC EFFECT
Consent was obtained for postmortem cardiac pathological assessment in Patient 5. Prominent ectatic blood vessels were identified at the interface of dense scar and viable myocardium (scar border zone) (Fig. 4A). This pattern has been described as a component of the acute vascular injury that is usually observed in the early weeks after radiation exposure. In such cases, the injury pattern is typically accompanied by endothelial-cell swelling, vacuolization, and perivascular tissue edema.24 However, in this patient, the endothelial lining of these vessels appeared to be normal and nonreactive, without evidence of an acute vasculitis or tissue edema (Fig. 4B). We observed no evidence of acute myocyte necrosis, hemorrhage, or acute inflammation. The relative contributions of remote myocardial infarction and acute cardiac SBRT to the formation of dense scar are unknown.
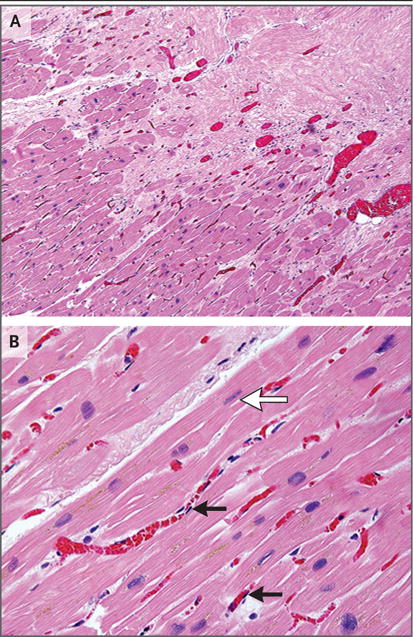
Panel A shows prominent small-vessel ectasis at the interface of dense fibrosis (upper right) and viable myocardium (lower left) in postmortem cardiac samples obtained from Patient 5, who had a fatal stroke 3 weeks after treatment. There is no acute myocardial inflammation or acute cellular necrosis. Panel B from the transition region shows occasional rectangular “boxcar” nuclei (white arrow) and hypertrophic cardiomyocytes, which are observed in chronic stages of heart failure. Endothelial cells are normal in appearance (black arrows), showing long, thin, nonreactive nuclei. (Hematoxylin and eosin staining was used in both panels.)
DISCUSSION
In this case series involving five patients with treatment-refractory ventricular tachycardia, a marked reduction in the burden of ventricular tachycardia was achieved with a noninvasive method for mapping and treating the arrhythmogenic area of the heart. The patients were selected for this treatment because they had end-stage disease, with a low likelihood of survival free from further episodes of ventricular tachycardia. The average treatment time was under 15 minutes. Most of the patients were able to stop antiarrhythmic medications several weeks after treatment, and the antiarrhythmic effect continued throughout the first year.
Worldwide, invasive catheter ablation is increasingly performed to treat ventricular tachycardia.12 The procedure is largely effective in the absence of ventricular scar (idiopathic ventricular tachycardia). However, catheter ablation for cardiomyopathic ventricular tachycardia is associated with recurrence rates as high as 50% at 6 months.13-22 Patients with recurrence of ventricular tachycardia despite catheter ablation have a poor prognosis associated with progressive heart failure and irrepressible ventricular tachycardia, with a risk of death that is four to six times the risk among patients who do not have such a recurrence.13,15,17,25
Recurrences of ventricular tachycardia occur for several reasons, including the presence of multiple coexisting ventricular tachycardia circuits, the presence of circuits that extend deep within the myocardium outside the reach of standard catheter-ablation energy, and the formation of new circuits after the ablation procedure. Success rates are higher with more extensive ablation, including the use of combined endocardial and epicardial approaches in an effort to “homogenize” the scar.26-28 For patients with ventricular tachycardia that cannot be treated effectively with standard catheter ablation, investigators are developing and testing invasive alternative treatments with higher procedural risks, including surgical access to the epicardial space for catheter ablation, surgical sympathetic denervation, transcoronary ethanol injection, and catheter ablation with an extendable needle.29 Thus, if a noninvasive approach to ablation of ventricular tachycardia is shown to be safe and effective, it would be a potentially important therapeutic advance.
Because of the novelty of noninvasive radioablation, its potential for harm, and the limited number of patients who were included in this analysis, this procedure should not be considered to be suitable for clinical use, pending the results of further research studies. Furthermore, there are well-described late toxic effects of radiotherapy to the heart for large-field fractionated-dose treatments, as has been reported in the treatment of lymphoma and breast cancer.25,30 The potential late effects of high-dose SBRT exclusively to focal areas of previously injured heart are unknown. The volumes of myocardium that are subjected to radiotherapy in these patients (from 17 to 81 ml) are large enough that effects on specialized cardiac structures (papillary muscles, coronary arteries, conduction system, and valves) are of potential concern, as is the risk of overall effects on ventricular function, although no such effects were seen during the 12-month follow-up period in the four surviving patients in our study. The risk of thromboembolism, as observed in Patient 5, warrants cautious consideration. We have initiated a prospective phase 1–2 trial (ENCORE-VT) to evaluate the safety and efficacy of SBRT (ClinicalTrials.gov number, NCT02919618).
In conclusion, in five patients with intractable ventricular tachycardia, the use of noninvasive stereotactic cardiac radiotherapy as an ablation technique resulted in a marked reduction in the burden of ventricular tachycardia after treatment.
Acknowledgments
Supported by a grant from the Barnes–Jewish Hospital Foundation (to Dr. Cuculich), by Washington University (the Department of Radiation Oncology, to Dr. Robinson; and the Cardiovascular Division of the Department of Medicine, to Dr. Cuculich), and by a grant (R01 HL033343, to Dr. Rudy) from the National Institutes of Health.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
Full text links
Read article at publisher's site: https://doi.org/10.1056/nejmoa1613773
Read article for free, from open access legal sources, via Unpaywall:
https://www.nejm.org/doi/pdf/10.1056/NEJMoa1613773?articleTools=true
Citations & impact
Impact metrics
Article citations
Cardiac arrhythmias during and after thoracic irradiation for malignancies.
Cardiooncology, 10(1):81, 14 Nov 2024
Cited by: 0 articles | PMID: 39543778 | PMCID: PMC11562486
Case of Successful Sympathetic Nerve Modulation by Targeted Heavy Ion Radiotherapy for Idiopathic Ventricular Tachycardia.
Ann Noninvasive Electrocardiol, 29(6):e70020, 01 Nov 2024
Cited by: 0 articles | PMID: 39425937 | PMCID: PMC11490255
Advancing the Collaboration Between Imaging and Radiation Oncology.
Semin Radiat Oncol, 34(4):402-417, 01 Oct 2024
Cited by: 0 articles | PMID: 39271275
Review
Stereotactic arrhythmia radioablation (STAR) opens a new era in the treatment of arrhythmias?
Front Cardiovasc Med, 11:1449028, 27 Sep 2024
Cited by: 0 articles | PMID: 39399514 | PMCID: PMC11469775
Review Free full text in Europe PMC
Implementing stereotactic arrhythmia radioablation with STOPSTORM.eu consortium support: intermediate results of a prospective Israeli single-institutional trial.
Strahlenther Onkol, 16 Sep 2024
Cited by: 0 articles | PMID: 39283343
Go to all (267) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT02919618
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Phase I/II Trial of Electrophysiology-Guided Noninvasive Cardiac Radioablation for Ventricular Tachycardia.
Circulation, 139(3):313-321, 01 Jan 2019
Cited by: 150 articles | PMID: 30586734 | PMCID: PMC6331281
Clinical experience of stereotactic body radiation for refractory ventricular tachycardia in advanced heart failure patients.
Heart Rhythm, 17(3):415-422, 01 Oct 2019
Cited by: 50 articles | PMID: 31585181
Stereotactic arrhythmia radioablation for refractory scar-related ventricular tachycardia.
Heart Rhythm, 17(8):1241-1248, 06 Mar 2020
Cited by: 59 articles | PMID: 32151737
Cardiac stereotactic body radiation therapy for ventricular tachycardia: Current experience and technical gaps.
J Cardiovasc Electrophysiol, 32(11):2901-2914, 05 Oct 2021
Cited by: 4 articles | PMID: 34587335
Review





