Abstract
Free full text

This article has been retracted. Retraction in:
Methylation-induced downregulation and tumor-suppressive role of microRNA-98 in glioma through targeting Sal-like protein 4
Abstract
MicroRNAs (miRs) have been found to play key roles in various human cancers, but the detailed regulatory mechanism of miR-98 in glioma remains largely unknown. The findings of the present study demonstrated that miR-98 was frequently downregulated in glioma tissues and cell lines (U87, U251, U373 and SHG44), and the decreased miR-98 levels were associated with DNA methylation. Treatment with 5-Aza-20-deoxycytidine, a DNA methyltransferase inhibitor, significantly increased the expression of miR-98 in glioma cells. Moreover, both miR-98 downregulation and methylation were significantly associated with a more aggressive tumor phenotype in glioma, as well as shorter survival time of glioma patients. Restoration of miR-98 expression caused a marked decrease in the migration and invasion of U87 cells, but did not affect cell proliferation. Sal-like protein 4 (SALL4) was further identified as a novel target gene of miR-98, and its protein expression was negatively regulated by miR-98 in U87 cells. Restoration of SALL4 expression reversed the suppressive effects of miR-98 on the migration and invasion of U87 cells. Furthermore, SALL4 was significantly upregulated in glioma tissues and cell lines, and an inverse correlation between miR-98 and SALL4 expression in glioma tissues was identified. In addition, the increased expression of SALL4 was significantly associated with glioma progression. Taken together, these data demonstrated that downregulation of miR-98, induced by methylation, promotes glioma cell migration and invasion via targeting SALL4. Therefore, miR-98 may become a potential therapeutic candidate for glioma.
Introduction
As the most common malignant brain tumor, glioma accounts for >80% of cancers in the brain (1,2). Despite improvements in surgical resection techniques combined with radiotherapy and chemotherapy, the prognosis of patients with advanced glioma remains poor (3,4). Deregulation of oncogenes or tumor suppressors has been implicated in the development and malignant progression of glioma, and some have been suggested as potential targets for glioma treatment (5–7).
MicroRNAs (miRs), a class of non-coding RNAs 18–25 nucleotides in length, act as key regulators of gene expression, mainly through binding to the complementary regions of their target mRNAs, resulting in mRNA degradation or protein translation inhibition (8–10). Through negative regulation of their target's expression, miRs have been implicated in a variety of physiological and pathological biological processes, including cell proliferation, survival, apoptosis, autophagy, cell cycle, migration and invasion (9,11). Certain miRs were recently demonstrated to play promoting or suppressive roles in glioma (12–15). For example, miR-146b acts as a tumor suppressor in glioma by targeting TRAF6, and its downregulation is significantly associated with poor prognosis of glioma patients (16). miR-133b contributes to arsenic-induced apoptosis in glioma cells by targeting the human ether-à-go-go-related gene channel (17). miR-503 inhibits glioma cell proliferation and invasion by inhibiting the expression of L1CAM (18).
miR-98 is an important member of the let-7/miR-98 family, which has been reported to play oncogenic or tumor suppressive roles in different human cancers (19,20). For example, Li et al reported that miR-98 exerted suppressive effects on melanoma metastasis through a negative feedback loop with interleukin (IL)-6 (21). Du et al demonstrated that miR-98 plays a suppressive role in oral squamous cell carcinoma growth and metastasis by directly targeting insulin-like growth factor 1 receptor (22). The suppressive role of miR-98 in glioma has been gradually uncovered (23). Chen et al reported that overexpression of Raf kinase inhibitor protein (RKIP) suppressed the invasion of glioma cells through upregulation of miR-98 (23). Fan et al demonstrated that miR-98 overexpression inhibited glioma cell invasion via targeting inhibitor of nuclear factor kappa-B kinase subunit ε (24). However, the regulatory mechanism of miR-98 expression in glioma remains unclear.
Therefore, the present study aimed to investigate the molecular mechanism underlying miR-98 expression in glioma and the regulatory mechanism underlying the role of miR-98 in glioma progression.
Materials and methods
Tissue collection
The present study was approved by the Ethics Committee of Xiangya Hospital, Central South University, (Changsha, China). Glioma tissues (n=84) and normal brain tissues (n=21) were collected from our hospital between May 2010 and January 2012. The patients included 52 men and 32 women, aged 23–68 years; 31 patients had WHO grade I-II, while 53 had WHO grade III-IV disease. All patients provided written informed consent. All tissue samples were immediately snap-frozen in liquid nitrogen and stored at −80°C until use.
Cell culture and treatment
Normal human astrocytes were purchased from the IBS Cell Bank of Fudan University (Shanghai, China) and cultured in astrocyte media (Science Cell, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS) at 37°C in a humidified incubator containing 5% CO2. Human glioma cell lines, including U87, U251, U373 and SHG44, were purchased from the Cell Bank of Central South University. Cells were cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% FBS (both from Thermo Fisher Scientific, Waltham, MA, USA) at 37°C in a humidified incubator containing 5% CO2. 5-Aza-20-deoxycytidine (5-Aza) was purchased from Sigma-Aldrich; Merck KGaA (St. Louis, MO, USA), and dissolved in phosphate-buffered saline (PBS) at indicated concentrations. Glioma cells were treated with 1 mM 5-Aza for 48 h, followed by assessment of miR-98 expression.
Cell transfection
Lipofectamine 2000 (Thermo Fisher Scientific) was used to perform cell transfection according to the manufacturer's instructions. U87 cells were transfected with scramble miR (miR-NC), miR-98 mimics, negative control (NC) inhibitor, miR-98 inhibitor, or co-transfected with miR-98 mimics and pc-DNA3.1-Sal-like protein 4 (SALL4) plasmid, or miR-98 mimics and blank pc-DNA3.1 vector. The cells were then cultured for 48 h before the following assays.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from tissues and cell lines was extracted using TRIzol reagent, then converted to cDNA using the Reverse Transcription kit (both from Thermo Fisher Scientific), according to the manufacturer's instructions. qCR was then performed by using the qPCR detection kit on ABI 7300 Plus thermocycler (both from Thermo Fisher Scientific). For miR expression detection, U6 was used as an internal reference. For mRNA detection, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as internal control. The primer sequences for SALL4 were as follows: Forward, 5′-AGCACATCAACTCGGAGGAG-3′ and reverse, 5′-CATTCCCTGGGTGGTTCACTG-3′. The primer sequences for GAPDH were as follows: Forward, 5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse, 5′-GGCTGTTGTCATACTTCTCATGG-3′. The PCR steps were 95°C for 5 min, and 40 cycles of 95°C for 30 sec and at 60°C for 30 sec. The relative expression was analyzed by the 2−ΔΔCq method (25).
Western blot assay
Cells were lysed with ice-cold lysis buffer. Protein was separated with 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred onto a polyvinylidene difluoride membrane (Thermo Fisher Scientific), which was incubated with PBS containing 5% non-fat milk (Yili, Beijing, China) for 3 h at room temperature. After washing with PBS 3 times, the membrane was incubated with rabbit anti-human SALL4 antibody (1:50, ab29112) and rabbit anti-human GAPDH antibody (1:100, ab9485) (both from Abcam, Cambridge, MA, USA) at 4°C overnight. After washing with PBS 3 times, the membrane was incubated with goat anti-rabbit secondary antibody (1:5,000, ab6721; Abcam) at room temperature for 40 min. The Chemiluminescent Substrate kit (Thermo Fisher Scientific) was used to detect signals, according to the manufacturer's instructions. The relative protein expression was analyzed by Image-Pro Plus software 6.0, and presented as the density ratio vs. GAPDH.
Detection of cell proliferation
U87 cells were plated at a density of 10,000 cells/well in 96-well plates. After being cultured for 0, 24, 48 and 72 h, the cells were incubated with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich; Merck KGaA) at a final concentration of 0.5 mg/ml for 4 h at 37°C. Following removal of the medium, 150 mM dimethyl sulfoxide solution (Sigma-Aldrich; Merck KGaA) was added. The absorbance was read at 570 nm using a BioTek™ ELX-800™ Absorbance Microplate reader (BioTek Instruments, Inc., Winooski, VT, USA).
Detection of cell migration
U87 cells were cultured to full confluence. Wounds ~1 mm wide were created using a plastic scriber. Cells were washed and then cultured in DMEM containing 10% FBS for 48 h. Subsequently, the cells were observed and photographed under a microscope.
Detection of cell invasion
Transwell assay was conducted to detect cell invasion capacity using Transwell chambers (BD Biosciences, Franklin Lakes, NJ, USA). U87 cell suspension (106 cells/ml) was prepared in DMEM without added FBS, 300 µl of the cell suspension was added into the upper chamber, and 300 µl of DMEM with 10% FBS was added into the lower chamber. After incubation at 37°C for 24 h, the U87 cells that did not migrate through the pores were carefully wiped off using a cotton-tipped swab. The filters were fixed in 90% alcohol and then stained by crystal violet (Sigma-Aldrich; Merck KGaA). The invading cells were counted using an inverted microscope (Olympus, Tokyo, Japan).
Dual luciferase reporter assay
TargetScan (www.targetscan.org) was used to predict the putative target of miR-98, according to the manufaturer's instruction. The wild-type (WT) or mutant type (MT) of SALL4 3′ untranslated region (3′UTR) was constructed and inserted into the psiCHECK2 luciferase reporter vector. U87 cells were co-transfected with WT-SALL4-3′UTR or MT-SALL4-3′UTR reporter plasmid plus miR-98 mimics or miR-NC using Lipofectamine 2000. Following co-transfection for 48 h, the luciferase activity was determined using the Dual-Luciferase Reporter assay system (Promega, Madison, WI, USA), according to the manufacturer's instructions. Renilla luciferase activity was normalized to Firefly luciferase activity.
Statistical analysis
Data are expressed as the mean ± standard deviation. Statistical analysis was performed using SPSS 20 software (SPSS, Armonk, NY, USA). The differences between two groups were analyzed using the Student's t-test. The association of gene expression or methylation status with clinical characteristics in glioma was analyzed using the Chi-squared test. P<0.05 was considered to indicate statistically significant differences.
Results
miR-98 is frequently downregulated in glioma tissues and cell lines
In the present study, the miR-98 levels in glioma tissues were first examined; normal brain tissues were used as control. RT-qPCR data indicated that miR-98 was frequently downregulated in glioma tissues compared with normal brain tissues (Fig. 1A). To confirm these findings, the miR-98 levels in glioma cell lines were then examined; normal human astrocytes were used as control. The expression of miR-98 was also reduced in glioma cell lines compared with normal astrocytes (Fig. 1B). Accordingly, the expression of miR-98 was found to be downregulated in glioma.

miR-98 is frequently downregulated in glioma tissues and cell lines. (A and B) Reverse transcription-quantitative polymerase chain reaction was used to determine the miR-98 levels in glioma tissues compared with normal brain tissues, as well as in glioma cell lines compared with normal human astrocytes. (A) **P<0.01 vs. normal; (B) **P<0.01 vs. astrocytes.
Hypermethylation causes miR-98 downregulation in glioma
The mechanism underlying miR-98 downregulation in glioma was further investigated. Methylation-specific PCR was performed to analyze the methylation status of miR-98 in glioma and normal brain tissues. It was observed that the methylation status of miR-98 was significantly higher in glioma tissues compared with that in normal brain tissues (Table I). The methylation status of miR-98 in glioma cell lines and normal human astrocytes was then examined. All glioma cell lines were positive for methylation, whereas no methylation was detectable in normal human astrocytes (Fig. 2A). Therefore, that the downregulation of miR-98 expression in glioma is likely due to the high methylation. To further confirm these findings, glioma cell lines were treated with 5-Aza, a DNA methyltransferase inhibitor, for 48 h. Following treatment, the methylation of miR-98 was significantly reduced and the expression levels of miR-98 were significantly upregulated in these glioma cell lines, which further indicates that the hypermethylation status leads to miR-98 downregulation in glioma (Fig. 2B and C).

Hypermethylation causes miR-98 downregulation in glioma. (A) The methylation status of miR-98 was examined in glioma cell lines compared with normal human astrocytes. **P<0.01 vs. astrocytes. Glioma cell lines were treated with 5-Aza-20-deoxycytidine (5-Aza) for 48 h. Non-treated cells were used as control. Treatment with phosphate-buffered saline was used as the Mock group. (B) After treatment, the methylation status of miR-98 was examined, and (C) reverse transcrition-quantitative polymerase chain reaction was used to determine the miR-98 levels. (B and C) **P<0.01 vs. control.
Table I
Methylation status of miR-98 in glioma tissues and normal brain tissues.
| Tissues | No. | Methylated | Unmethylated | P-value |
|---|---|---|---|---|
| Normal brain | 21 | 1 | 20 | <0.01 |
| Glioma | 84 | 58 | 26 |
miR-98 downregulation and methylation are associated with a progressive phenotype and shorter survival in glioma
The clinical significance of miR-98 expression in glioma was further investigated. As shown in Table II, low miR-98 levels were significantly associated with high pathological grade and low Karnofsky performance scale (KPS) index in glioma, but were not associated with age, sex, or type of surgery (gross total resection or partial resection), suggesting that reduced miR-98 expression contributes to glioma progression. Interestingly, high methylation of miR-98 was also found to be associated with high pathological grade and low KPS index in glioma (Table III). Moreover, glioma patients with low expression of miR-98 exhibited shorter overall survival compared with those with high miR-98 levels (Fig. 3A). Consistently, glioma patients with high miR-98 methylation had a poorer prognosis compared with those with low miR-98 methylation (Fig. 3B). Therefore, both miR-98 downregulation and methylation in glioma are associated with a progressive phenotype and shorter survival.
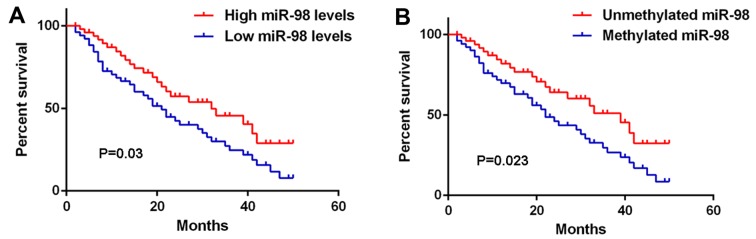
Both miR-98 downregulation and miR-98 methylation are associated with shorter survival time in glioma. (A) Glioma patients with low expression of miR-98 exhibited shorter overall survival compared with those with high miR-98 levels. (B) Glioma patients with miR-98 methylation exhibited shorter overall survival compared with those with unmethylated miR-98.
Table II
Association between miR-98 expression and clinicopathological characteristics in glioma.
| Variables | Cases (n=84) | Low miR-98 expression (n=41) | High miR-98 expression (n=43) | P-value |
|---|---|---|---|---|
| Age, years | 0.512 | |||
 <55 <55 | 39 | 21 | 18 | |
 ≥55 ≥55 | 45 | 20 | 25 | |
| Sex | 0.268 | |||
 Male Male | 52 | 28 | 24 | |
 Female Female | 32 | 13 | 19 | |
| WHO grade | 0.025 | |||
 I–II I–II | 31 | 10 | 21 | |
 III–IV III–IV | 53 | 31 | 22 | |
| KPS index | 0.013 | |||
 >90 >90 | 30 | 9 | 21 | |
 ≤90 ≤90 | 54 | 32 | 22 |
KPS, Karnofsky performance scale.
Table III
Association between methylation status of miR-98 and clinicopathological characteristics in glioma.
| Variables | Cases (n=84) | Methylated (n=58) | Unmethylated (n=26) | P-value |
|---|---|---|---|---|
| Age, years | 0.354 | |||
 <55 <55 | 39 | 29 | 10 | |
 ≥55 ≥55 | 45 | 29 | 16 | |
| Sex | 0.339 | |||
 Male Male | 52 | 38 | 14 | |
 Female Female | 32 | 20 | 12 | |
| WHO grade | 0.013 | |||
 I–II I–II | 31 | 16 | 15 | |
 III–IV III–IV | 53 | 42 | 11 | |
| KPS index | 0.001 | |||
 >90 >90 | 30 | 14 | 16 | |
 ≤90 ≤90 | 54 | 44 | 10 |
KPS, Karnofsky performance scale.
Restoration of miR-98 expression inhibits U87 cell migration and invasion
To further investigate the regulatory role of miR-98 in glioma, U87 cells were transfected with miR-98 mimic or miR-NC. Untreated cells were used as the control group. Following transfection, the miR-98 levels were significantly higher in the miR-98 group compared with those in the untreated group (Fig. 4A). The proliferation, migration and invasion of U87 cells in each group were then assessed using the MTT, wound healing and Transwell assays, respectively. Although it did not affect on cell proliferation, restoration of miR-98 expression significantly decreased U87 cell migration and invasion (Fig. 4B–D).
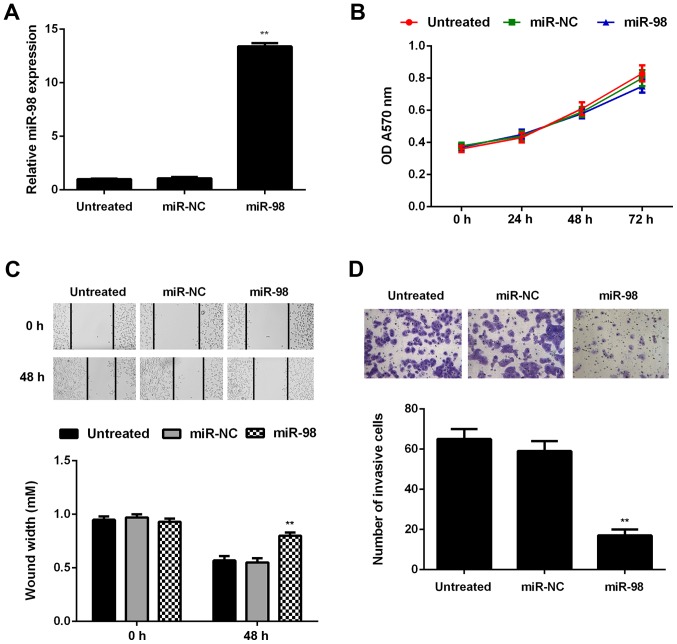
miR-98 overexpression inhibits the migration and invasion of U87 cells. U87 cells were transfected with miR-98 mimic or scramble miR (miR-NC). (A) Reverse transcription-quantitative polymerase chain reaction was conducted to examine miR-98 expression. (B) MTT assay, (C) wound healing assay and (D) Transwell assay were conducted to evaluate cell proliferation, migration and invasion. Untreated cells were used as the control group. **P<0.01 vs. untreated group. OD, optical density.
SALL4 is a target gene of miR-98 in U87 cells
Subsequently, the potential targets of miR-98 in glioma were examined. SALL4 was predicted to be a potential target of miR-98 (Fig. 5A). To confirm this association, luciferase reporter plasmids containing WT or MT of SALL4 3′UTR were generated (Fig. 5B). The luciferase reporter gene assay revealed that luciferase activity was markedly downregulated in U87 cells co-transfected with the WT-SALL4-3′UTR luciferase reporter plasmid and miR-98 mimic compared with the control group, which was eliminated by transfection with MT-SALL4-3′UTR luciferase reporter plasmid (Fig. 5C). These findings confirm that SALL4 is a direct target gene of miR-98 in U87 cells.
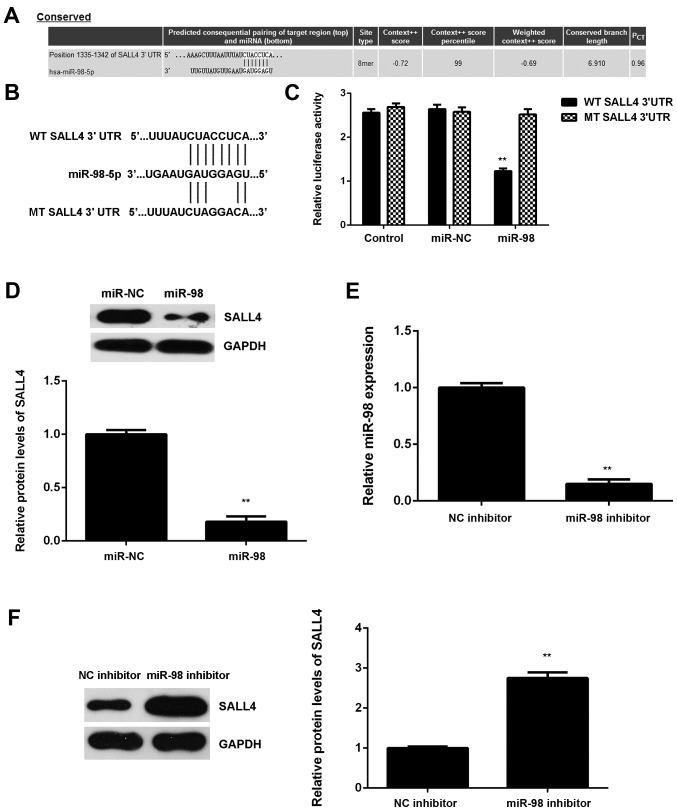
Sal-like protein 4 (SALL4) is a target gene of miR-98 in U87 cells. (A) TargetScan predicts that SALL4 is a potential target gene of miR-98. (B) Luciferase reporter plasmids containing the wild-type (WT) or mutant type (MT) of SALL4 3′ untranslated region (3′UTR) were constructed. (C) Luciferase activity was significantly decreased in U87 cells co-transfected with the WT-SALL4-3′UTR luciferase reporter plasmid and miR-98 mimic compared with the control group, which was eliminated by transfection with MT-SALL4-3′UTR luciferase reporter plasmid. **P<0.01 vs. control. (D) Western blotting was conducted to examine the SALL4 protein expression in U87 cells transfected with miR-98 mimic or scramble miR (miR-NC). **P<0.01 vs. miR-NC. (E) Reverse transcription-quantitative polymerase chain reaction was used to determine the miR-98 levels in U87 cells transfected with miR-98 inhibitor or negative control (NC) inhibitor. (F) Western blotting was then conducted to examine the SALL4 protein levels. (E-F) **P<0.01 vs. NC inhibitor.
The regulatory effect of miR-98 on the protein expression of SALL4 in U87 cells was then studied. The protein levels of SALL4 were significantly lower in the miR-98 group compared with those in the miR-NC group, indicating that overexpression of miR-98 inhibited SALL4 protein expression in U87 cells (Fig. 5D). To further confirm these data, U87 cells were then transfected with miR-98 inhibitor. Transfection with NC inhibitor was used as the control group. Following transfection, the miR-98 levels were significantly lower in the miR-98 inhibitor group compared with the NC inhibitor group (Fig. 5E). Western blot data further demonstrated that downregulation of miR-98 caused a significant increase in the SALL4 protein expression in U87 cells (Fig. 5F). Based on these findings, miR-98 appears to directly bind to the 3′UTR of SALL4 mRNA and downregulate its protein expression in U87 cells.
Restoration of SALL4 expression attenuates the miR-145-mediated inhibition of U87 cell migration and invasion
Subsequently, we investigated whether SALL4 was involved in the miR-98-mediated malignant phenotypes of U87 cells. miR-98-overexpressing U87 cells were transfected with pcDNA3.1-SALL4 plasmid. miR-98-overexpressing U87 cells transfected with blank pcDNA3.1 vector were used as the control group. After transfection, the mRNA and protein levels of SALL4 were found to be significantly higher in the miR-98 + SALL4 group compared with the miR-98 + blank group (Fig. 6A and B). As overexpression of miR-98 promoted U87 cell migration and invasion, these two phenotypes were examined by conducting wound healing and Transwell assays. The migration and invasion of U87 cells were significantly upregulated in the miR-98 + SALL4 group compared with those in the miR-98 + blank group (Fig. 6C and D). According to the abovementioned data, it was demonstrated that restoration of SALL4 expression attenuates the miR-145-mediated inhibition of migration and invasion of glioma cells. Therefore, the inhibitory effects of miR-98 on glioma are at least partly mediated through direct targeting of SALL4.

Restoration of Sal-like protein 4 (SALL4) expression attenuates the miR-145-mediated inhibition of migration and invasion of U87 cells. miR-98-overexpressing U87 cells were transfected with SALL4 expression plasmid or blank vector. (A) Reverse transcription-quantitative polymerase chain reaction and (B) western blotting were performed to examine the mRNA and protein levels of SALL4. (C) Wound healing assay and (D) Transwell assay were conducted to examine cell migration and invasion. **P<0.01 vs. miR-98 + blank.
SALL4 is upregulated in glioma, with an inverse correlation to miR-98 levels
Finally, the expression levels of SALL4 in glioma was examined. The mRNA levels of SALL4 were significantly higher in glioma tissues compared with those in normal brain tissues (Fig. 7A). Moreover, our findings demonstrated that the increased expression of SALL4 was associated with malignant progression of glioma (Table IV). Interestingly, an inverse correlation between the SALL4 and miR-98 levels in glioma tissues was observed (Fig. 7B). These findings suggest that the decreased miR-98 expression contributes to the increased expression of SALL4 in glioma. In addition, the protein levels of SALL4 were also higher in glioma cell lines compared with those in normal astrocytes (Fig. 7C).
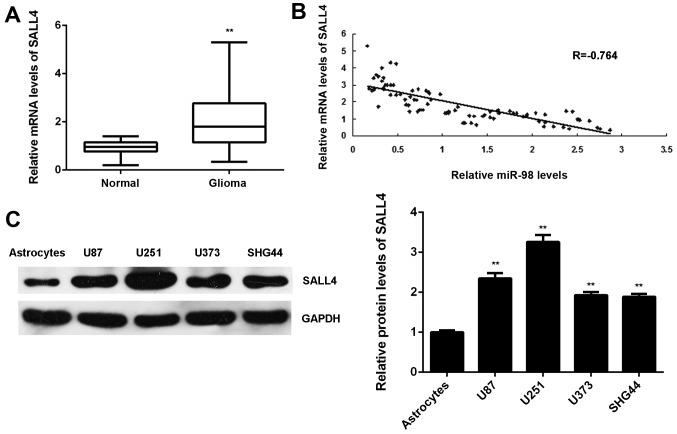
Sal-like protein 4 (SALL4) was upregulated in glioma, with an inverse correlation to miR-98 levels. (A) Reverse transcription-quantitative polymerase chain reaction was conducted to examine the SALL4 mRNA levels in glioma tissues compared with those in normal brain tissues. **P<0.01 vs. normal. (B) The SALL4 mRNA levels were inversely correlated with the miR-98 levels in glioma tissues. (C) Western blotting was conducted to examine the SALL4 protein levels in glioma cell lines compared with those in normal human astrocytes. **P<0.01 vs. astrocytes.
Table IV
Association between SALL4 expression and clinicopathological characteristics in glioma.
| Variables | Cases (n=84) | Low SALL4 expression (n=44) | High SALL4 expression (n=40) | P-value |
|---|---|---|---|---|
| Age, years | 0.282 | |||
 <55 <55 | 39 | 23 | 16 | |
 ≥55 ≥55 | 45 | 21 | 24 | |
| Sex | 0.263 | |||
 Male Male | 52 | 30 | 22 | |
 Female Female | 32 | 14 | 18 | |
| WHO grade | 0.024 | |||
 I–II I–II | 31 | 11 | 20 | |
 III–IV III–IV | 53 | 33 | 20 | |
| KPS index | 0.012 | |||
 >90 >90 | 30 | 10 | 20 | |
 ≤90 ≤90 | 54 | 34 | 20 |
SALL4, Sal-like protein 4; KPS, Karnofsky performance scale.
Discussion
Recently, miR-98 was found to play a suppressive role in glioma (23,24), but the underlying mechanism remains largely unknown. In the present study, it was demonstrated that miR-98 was frequently downregulated in glioma, which was associated with DNA methylation. Both miR-98 downregulation and methylation were found to be significantly associated with a more aggressive tumor phenotype in glioma, as well as shorter survival of glioma patients. Further investigation revealed that miR-98 inhibited the migration and invasion of U87 cells at least partly via directly targeting SALL4, which was upregulated in glioma, and its upregulation was associated with glioma progression.
Recently, miR-98 was found to act as a tumor suppressor in glioma (23,24). Chen et al reported that the expression of RKIP and miR-98 in glioma tissues was significantly lower compared with that in normal brain tissues, and overexpression of RKIP may promote the expression of miR-98, which further inhibits glioma cell invasion, possibly through targeting HMGA2 (23). Another study revealed that overexpression of miR-98 inhibited glioma cell migration and invasion through inhibition of IκB kinase and matrix metalloproteinase-9 expression, as well as nuclear factor-κB p65 nuclear translocation (24). However, the regulatory mechanism of miR-98 expression in glioma and its clinical significance remain unknown. In the present study, miR-98 was frequently found to be downregulated in glioma tissues and cell lines. Moreover, DNA methylation may be the main cause of the reduced miR-98 expression in glioma, as the methylation status of miR-98 was found to be significantly higher in glioma tissues compared with that in normal brain tissues, and treatment with DNA methyltransferase inhibitor significantly upregulated miR-98 expression in glioma cell lines. Furthermore, it was demonstrated that both miR-98 downregulation and methylation were significantly associated with higher WHO grade, low KPS and shorter survival in glioma, suggesting that miR-98 expression or methylation status may be used as important predictors of prognosis of glioma patients. Furthermore, it was observed that restoration of miR-98 expression inhibited the migration and invasion of glioma U87 cells, but did not affect cell proliferation, which was consistent with the findings of previous studies (23,24).
SALL4, a zinc finger transcription factor, was then identified as a novel target gene of miR-98 in glioma cells by using luciferase reporter gene assay. In fact, SALL4 was previously reported to be an important marker for stem cells, as it plays a key role in maintaining the self-renewal capacity of embryonic stem cells (26). In recent years, SALL4 was reported to be frequently upregulated in some cancer types, and to act as an oncogene in esophageal squamous cell carcinoma, gastric cancer, hepatocellular carcinoma and intrahepatic cholangiocarcinoma (27–30). Moreover, the promoting role of SALL4 in glioma has been gradually revealed. Zhang et al found that the SALL4 expression levels in glioma were significantly higher compared with those in normal brain tissues, and the expression of SALL4 was closely correlated with glioma pathological grade (31), which was consistent with our data. In addition, they demonstrated that high SALL4 expression was correlated with poor prognosis of glioma patients. In the present study, the expression of SALL4 was found to be inversely correlated with miR-98 expression in glioma tissues, suggesting that downregulation of miR-98 may contribute to SALL4 upregulation.
Moreover, the protein expression of SALL4 was found to be negatively regulated by miR-98 in U87 cells. Therefore, it was hypothesized that SALL4 may be involved in the miR-98-mediated migration and invasion of glioma cells. To confirm this hypothesis, miR-98-overexpressing U87 cells were transfected with SALL4 expression plasmid, and it was observed that restoration of SALL4 expression significantly attenuated the inhibitory effects of miR-98 overexpression on U87 cell migration and invasion. Accordingly, it was demonstrated that miR-98 plays a suppressive role in the migration and invasion of U87 cells at least partly through direct targeting of SALL4. In fact, similar findings were reported in hepatocellular carcinoma. Zhou et al recently found that miR-98 plays a suppressive role in the proliferation, migration, invasion and epithelial-to-mesenchymal transition of hepatocellular carcinoma cells via direct inhibition of SALL4 (32). Therefore, the present study highlights the significance of miR-98/SALL4 signaling in human cancers.
To the best of our knowledge, this is the first study to demonstrate that high methylation induces the downregulation of miR-98 in glioma, which further promotes glioma cell migration and invasion via targeting SALL4. Therefore, miR-98 may be a potential therapeutic candidate for glioma.
Footnotes
Competing interests
The authors declare that they have no competing interests.
References
Articles from International Journal of Molecular Medicine are provided here courtesy of Spandidos Publications
Full text links
Read article at publisher's site: https://doi.org/10.3892/ijmm.2018.3464
Read article for free, from open access legal sources, via Unpaywall:
https://www.spandidos-publications.com/10.3892/ijmm.2018.3464/download
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/104747417
Article citations
CircSFMBT2 Plays an Oncogenic Role in Lung Adenocarcinoma Depending on the miR-1305/SALL4 Axis.
Biochem Genet, 62(5):3485-3503, 21 Dec 2023
Cited by: 0 articles | PMID: 38127171
Retraction: Methylation-induced downregulation and tumor suppressive role of microRNA-29b in gastric cancer through targeting LASP1.
Oncotarget, 14:173, 11 Mar 2023
Cited by: 0 articles | PMID: 36913271 | PMCID: PMC10010624
SALL4: An Intriguing Therapeutic Target in Cancer Treatment.
Cells, 11(16):2601, 20 Aug 2022
Cited by: 9 articles | PMID: 36010677 | PMCID: PMC9406946
Review Free full text in Europe PMC
SALL4 Oncogenic Function in Cancers: Mechanisms and Therapeutic Relevance.
Int J Mol Sci, 23(4):2053, 12 Feb 2022
Cited by: 19 articles | PMID: 35216168 | PMCID: PMC8876671
Review Free full text in Europe PMC
Interaction among long non-coding RNA, micro-RNA and mRNA in glioma.
Ibrain, 7(2):141-145, 28 Jun 2021
Cited by: 1 article | PMID: 37786911 | PMCID: PMC10528991
Review Free full text in Europe PMC
Go to all (7) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
MicroRNA-181b Inhibits Cellular Proliferation and Invasion of Glioma Cells via Targeting Sal-Like Protein 4.
Oncol Res, 25(6):947-957, 17 Nov 2016
Cited by: 18 articles | PMID: 27938503 | PMCID: PMC7841051
MicroRNA-16 inhibits the proliferation, migration and invasion of glioma cells by targeting Sal-like protein 4.
Int J Mol Med, 38(6):1768-1776, 17 Oct 2016
Cited by: 13 articles | PMID: 27748823
miR-103/miR-195/miR-15b Regulate SALL4 and Inhibit Proliferation and Migration in Glioma.
Molecules, 23(11):E2938, 10 Nov 2018
Cited by: 34 articles | PMID: 30423818 | PMCID: PMC6278493
SALL4 Oncogenic Function in Cancers: Mechanisms and Therapeutic Relevance.
Int J Mol Sci, 23(4):2053, 12 Feb 2022
Cited by: 19 articles | PMID: 35216168 | PMCID: PMC8876671
Review Free full text in Europe PMC




