Abstract
Summary
Background Growth arrest-specific protein 6 signals through the TAM (TYRO-3-AXL-MERTK) receptor family, mediating platelet activation and thrombus formation via activation of the aggregate-stabilizing αIIb β3 integrin. Objective To describe the antithrombotic effects mediated by UNC2025, a small-molecule MERTK tyrosine kinase inhibitor. Methods MERTK phosphorylation and downstream signaling were assessed by immunoblotting. Light transmission aggregometry, flow cytometry and microfluidic analysis were used to evaluate the impact of MERTK inhibition on platelet activation and stability of aggregates in vitro. The effects of MERTK inhibition on arterial and venous thrombosis, platelet accumulation at microvascular injury sites and tail bleeding times were determined with murine models. The effects of combined treatment with ADP-P2Y1&12 pathway antagonists and UNC2025 were also evaluated. Results and Conclusions Treatment with UNC2025 inhibited MERTK phosphorylation and downstream activation of AKT and SRC, decreased platelet activation, and protected animals from pulmonary embolism and arterial thrombosis without increasing bleeding times. The antiplatelet effect of UNC2025 was enhanced in combination with ADP-P2Y1&12 pathway antagonists, and a greater than additive effect was observed when these two agents with different mechanisms of inhibition were coadministered. TAM kinase signaling represents a potential therapeutic target, as inhibition of this axis, especially in combination with ADP-P2Y pathway antagonism, mediates decreased platelet activation, aggregate stability, and thrombus formation, with less hemorrhagic potential than current treatment strategies. The data presented here also demonstrate antithrombotic activity mediated by UNC2025, a novel translational agent, and support the development of TAM kinase inhibitors for clinical applications.Free full text

The small molecule MERTK inhibitor UNC2025 decreases platelet activation and prevents thrombosis
Summary
Background
Gas6 signals through the TAM (TYRO-3, AXL, MERTK) receptor family mediating platelet activation and thrombus formation via activation of the aggregate-stabilizing αIIbβ3 integrin.
Objective
Here we describe anti-thrombotic effects mediated by UNC2025, a small molecule MERTK tyrosine kinase inhibitor.
Methods
MERTK phosphorylation and downstream signaling were assessed by immunoblot. Light transmission aggregometry, flow cytometry, and microfluidic analysis were used to evaluate the impact of MERTK inhibition on platelet activation and stability of aggregate formation in vitro. The effects of MERTK inhibition on arterial and venous thrombosis, platelet accumulation at microvascular injury sites, and tail bleeding times were determined using murine models. The effects of combined treatment with ADP/P2Y1&12 pathway antagonists and UNC2025 were also evaluated.
Results and Conclusions
Treatment with UNC2025 inhibited MERTK phosphorylation and downstream activation of AKT and SRC, decreased platelet activation, and protected animals from pulmonary embolism and arterial thrombosis without increased bleeding times. The anti-platelet effect of UNC2025 was enhanced in combination with ADP/P2Y1&12 pathway antagonists and a greater-than-additive effect was observed when these two agents with different mechanisms of inhibition were co-administered. TAM kinase signaling represents a potential therapeutic target since inhibition of this axis, especially in combination with ADP/P2Y pathway antagonism, mediates decreased platelet activation, aggregate stability, and thrombus formation with less hemorrhagic potential compared to current treatment strategies. The data presented here also demonstrate anti-thrombotic activity mediated by UNC2025, a novel translational agent, and support development of TAM kinase inhibitors for clinical applications.
INTRODUCTION
Stable platelet aggregation is essential for adequate hemostasis, but uncontrolled platelet activation may lead to life-threatening thromboses such as arterial ischemic stroke or myocardial infarction. Current anti-platelet therapies are complicated by hemorrhagic side effects and interpatient response variability [1], demonstrating the need to explore new pathways that may yield safer and more effective therapies.
Extracellular Growth Arrest Specific protein 6 (GAS6), stimulates the platelet surface TYRO-3/AXL/MERTK (TAM) family of transmembrane receptor tyrosine kinases [2–6] to dimerize and autophosphorylate [7–9], activating phosphatidylinositol-3-kinase (PI3K) [10, 11], RAP1 [11–13], and AKT [14, 15], triggering β3 integrin phosphorylation and amplifying outside-in signaling [2, 16, 17]. GAS6 has been reported in human platelets and plasma [3, 5, 18] and bridges membrane-bound phosphatidylserine (PtdS) [19, 20] and TAM kinases [21, 22].
Inhibition or absence of GAS6 or any TAM receptor decreases platelet aggregation and impairs thrombus formation [2, 3, 23, 24]. Gas6−/− mice form unstable platelet aggregates that are stabilized by addition of recombinant human GAS6 (rhGAS6) [5]. GAS6 enhances PI3K phosphorylation in platelets from wild type (WT) mice but not Tyro3 −/−, Mertk −/−, or Axl −/− mice [2]. Interestingly, absence of GAS6/TAM signaling does not increase spontaneous hemorrhage or bleeding time after tail clipping [2–4, 6, 25].
Here, we describe a novel mechanism of GAS6/TAM signal inhibition in platelets, targeting the MERTK tyrosine kinase active site with a highly potent and bioavailable MERTK inhibitor, UNC2025. [26–29]
MATERIALS AND METHODS
Platelet activation inhibitors
UNC2025: MERTK inhibitor synthesized as described. [26] Molecular structure is depicted in Supplemental Figure 1. The half-life of UNC2025 is 3.8h in mice with low clearance (9.2 mL/min/kg) and 100% oral bioavailability. A 3mg/kg dose results in approximately 22 nM Cmax at 30 min. [26]
PBS0739: P2Y12 receptor antagonist (Tocris, Bristol, UK).
MRS2179: P2Y1 receptor antagonist (Tocris).
2-Me SAMP: P2Y12 receptor antagonist (Sigma-Aldrich Corp., St. Louis, MO).
In some cases MRS2179 and 2-Me SAMP were used together and are collectively referred to as “P2Y receptor inhibitors” (P2Yi).
Blood Collection
Human whole blood (WB) was collected by venipuncture from healthy volunteers after informed consent under an Institutional Review Board-approved protocol.
Washed platelet (WP) and platelet-rich plasma (PRP) preparation
WB was drawn from healthy volunteers into 3.8% sodium citrate, with addition of acid-citrate-dextrose (ACD). [30, 31] WP and PRP were prepared as described. [32]
Platelet Aggregation
Human WP or PRP, or murine WP were incubated for 15 minutes with saline (vehicle), 0.5 (WP) or 5 (PRP) µM UNC2025, 0.001 µM PBS0739 (P2Y12 receptor inhibitor), or 2 µM abciximab (positive control). Samples were analyzed by light-transmission aggregometry after stimulation with 1 (WP) or 2 (PRP) µg/mL collagen, 50 ng/mL convulxin, 2µM ADP, or 1 U/mL thrombin. Maximum aggregation (%) was recorded.
Flow Cytometry
Human WP were incubated for 15 minutes with saline (vehicle), 0.5 µM UNC2025, or 2 µM abciximab and then stimulated with 50 ng/mL convulxin (Santa Cruz Bioechnlogy, Santa Cruz, CA). PAC-1 antibodies (BD Biosciences, San Jose, CA) were added immediately after convulxin, samples were incubated for 5 minutes, andplatelets were fixed with 2% paraformaldehyde (Sigma-Aldrich). Samples were analyzed using a Gallios flow cytometer (Beckman-Coulter, Indianapolis, IN). Mean fluorescence intensity was recorded.
Microfluidic Flow Assay
Microfluidic experiments were performed on a collagen surface. [33–35] WB (100 µL) was incubated for 1 minute with saline (vehicle), 1 µM UNC2025, or 2 µM Abciximab (positive control) and then pulled through the channels for 3 minutes at a physiologic wall shear rate (650s-1). Platelet adhesion and aggregation were captured in real time using an ORCA Flash4 CMOS camera (Orca-ER, Hamamatsu) and CellSense imaging software (Olympus Life Science) with images every 1 second over the duration of the experiment. Surface area coverage and aggregate size were determined for each series.
Immunoblot
Human WP were incubated with UNC2025 or saline vehicle at 37C for 5 minutes, then stimulated with 1 µg/mL collagen and resuspended in lysis buffer containing 50 mm HEPES (pH 7.5), 150 mm NaCl, 10 mm EDTA, 10% (v/v) glycerol, 1% (v/v) Triton X-100, 1 mm sodium orthovanadate, 0.1 mm sodium molybdate and protease inhibitors (Roche Diagnostics). Lysates were incubated on ice for 15–20 min and protein supernatant was collected after centrifugation. For MERTK detection, cells were treated with 120 µM pervanadate for 3 minutes prior to cell lysis to stabilize phosphoproteins. Lysates were incubated overnight with anti-MERTK antibody (MAB8912, R&D Systems) and rec-Protein G-sepharose beads (Invitrogen) and bound proteins were eluted into 2X Laemmli sample buffer (Bio-Rad). WP lysates or immunoprecipitated proteins were resolved by electrophoresis on 8% Tris-Glycine SDS-polyacrylamide gels (Invitrogen) and transferred onto nitrocellulose membranes. Membranes were blocked in tris-buffered saline with 0.1% Tween-20 and 5% milk. The following antibodies were used for immunoblot analysis according to manufacturer recommendations: anti-phospho MERTK (Phosphosolutions Inc.), anti-MERTK (ab52968, AbCam); anti-phospho-AKT (Ser473, #92715), anti-AKT (#9272S), anti-phospho-SRC (Y416, #2101S), anti-SRC (#2109S) (Cell Signaling), anti-ACTIN (#sc-1616, Santa Cruz Biotechnology). Proteins were detected by horseradish peroxidase chemiluminescence (Perkin-Elmer) using Goat anti-rabbit IgG-HRP secondary antibody (#170-6515, BioRad).
Murine Models
All experiments were approved by university animal care and use committees and involved equal numbers of 6–12 week old male and female C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME, USA).
FeCl3-Induced Carotid Artery Thrombosis
Mice were anesthetized and underwent endotracheal intubation and mechanical ventilation. [36–39] After carotid artery exposure and ultrasonic flow probe attachment, saline vehicle, 3 mg/kg UNC2025, high dose (HD) P2Yi (3 mg/kg each MRS2179 + 2-MeSAMP), low dose (LD) P2Yi (1.5 mg/kg each MRS2179 + 2-MeSAMP), or UNC2025 + LD P2Yi were intravenously injected and circulated for 30 minutes. 6% FeCl3 was applied to the artery for 3 minutes and the probe readout was captured for 60 minutes after FeCl3 application. Elapsed Time To First (initial) artery Occlusion (TTFO, mean blood flow of 0 mL/min flow for >30 seconds), and total Duration Of Occlusion (DOO, mean blood flow <20% of pre-FeCl3 baseline) were determined.
Pulmonary Embolism
Mice were treated for 30 minutes with 3 mg/kg UNC2025 or saline vehicle administered by intravenous injection and then type 1 equine fibrillar collagen (0.28mg/kg) and epinephrine (0.029 mg/kg) were administered and time to breathing cessation was recorded as described. [24, 38] Mice that remained alive 15 minutes after collagen/epinephrine were euthanized, and experimental time was recorded as 15 minutes.
Intravital Microvascular Thrombosis
Mice underwent intravital microscopy of the cremaster muscle microcirculation. [40, 41] 30–40 µm diameter arterioles with unperturbed blood flow were studied. After a 10 minute stabilization, labeled anti-CD41 F(ab)2 fragments (0.12 µg/g body weight), anti-P-selectin (0.2 µg/g), and anti-fibrin (0.2 µg/g) were intravenously infused. Vascular injury was induced by pulsed nitrogen dye laser (SRS NL100, 440 nm), and up to five injuries and thrombi were recorded in each mouse following saline infusion. Next, 3 mg/kg UNC2025 was infused, circulated for 30 min, and a second set of injuries/thrombi were recorded. CD41, P-selectin, and fibrin were measured by summing all pixels in a frame with corresponding fluorescence intensity above background.
Murine Tail Bleed Assay. [37, 42]
Saline vehicle, 3 mg/kg UNC2025, high dose (HD) P2Yi (3 mg/kg each MRS2179 + 2-MeSAMP), low dose (LD) P2Yi (1.5 mg/kg each MRS2179 + 2-MeSAMP), or UNC2025 + LD P2Yi were injected into the retro-orbital sinus and allowed to circulate for 15 minutes. Tails were snipped 1 cm from the tip and the proximal ends were placed in a conical tube containing 37°C normal saline for 15 minutes. Total bleeding time (including rebleeds) was recorded.
Synergy Calculations
Median effect analysis was performed according to the methods of Chou and Talaly [43] using Calcusyn software (Cambridge, UK). Interactions were also assess using the Bliss Additivity equation Fa12=fa1 + fa2 – fa1×fa2, where fa = frequency of effect mediated by drug 1 or 2, respectively, and Fa12 = predicted frequency of affect for an additive interaction. An observed Fa greater than the calculated Fa12 indicates synergy.
Statistical Analyses
Data with Gaussian distribution are expressed as mean +/− SEM with significance evaluated by paired, two-tailed t test. Data with non-normal non-Gaussian distribution are expressed as median +/− interquartile range (IQR) and evaluated using Mann-Whitney rank sum tests. Experiments with human WB, PRP, or WP were performed and analyzed as paired samples. Murine assays were analyzed as unpaired samples.
RESULTS
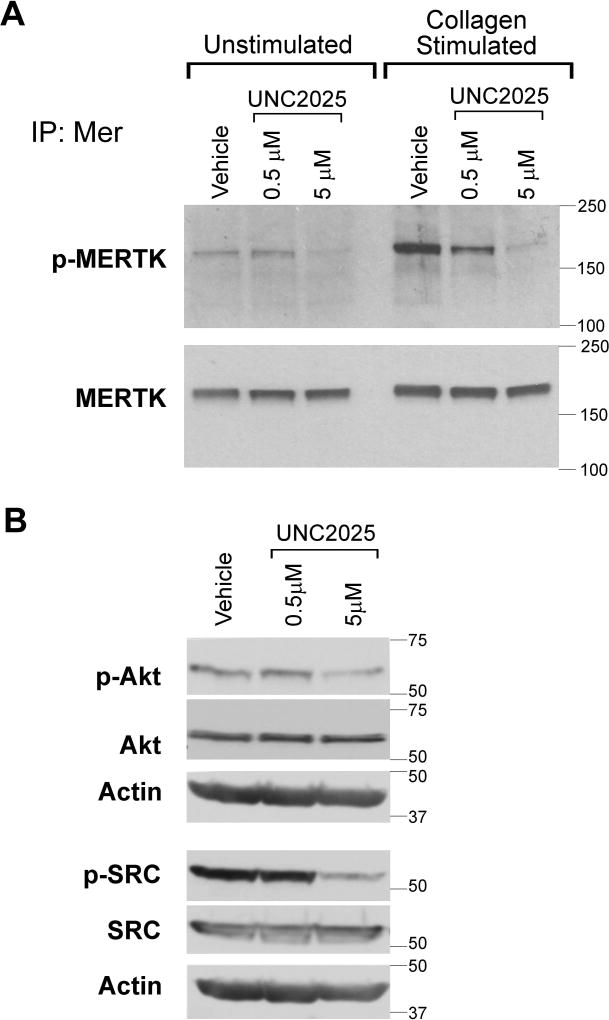
UNC2025 decreased platelet MERTK phosphorylation and downstream signaling
UNC2025 mediated dose-dependent inhibition of MERTK phosphorylation in unstimulated human platelets and after stimulation with collagen (Fig. 1A). In this context, treatment with a dose of 0.5 µM UNC2025 resulted in partial inhibition of MERTK and a dose of 5 µM was sufficient for more complete inhibition. Signaling through the AKT and SRC pathways, which are known downstream targets of MERTK, was similarly descreased in platelets treated with UNC2025 (Fig. 1B). These data demonstrate the utility of UNC2025 as a MERTK inhibitor in human platelets and define the dose of UNC2025 required for effective MERTK inhibition.
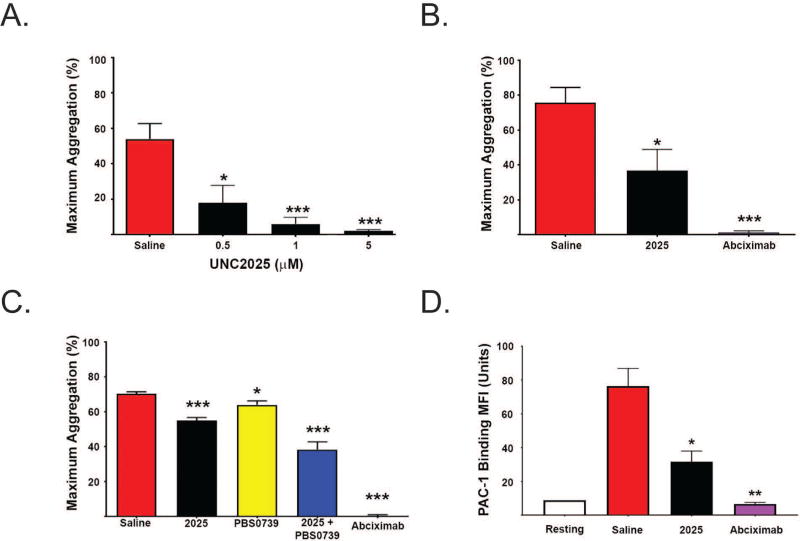
UNC2025 decreased human and murine platelet activation
UNC2025 mediated dose-dependent decreases in mean (+/− SEM) maximum aggregation of human platelets stimulated with collagen, ADP, or thrombin (Figs. 2A–B and Supp. Fig. 2). Treatment with 0.5 µM UNC2025 decreased mean (+/− SEM) maximum collagen-stimulated aggregation of human washed platelets (18.0 +/− 9.8%, n=6, p<0.05) compared to controls treated with saline vehicle (54.0 +/− 8.7%, n=6) (Fig. 2A). Similarly, Figure 2B demonstrates decreased collagen-stimulated aggregation of human platelet rich plasma (PRP) pre-treated with 5 µM UNC2025 (36.9 +/− 12.0%, n=7, p<0.05) compared to saline vehicle (75.7 +/− 8.7%, n=7). Pre-treatment with 2 µM Abciximab is shown as a positive control. Finally, treatment with 0.5 µM UNC2025 also mediated decreased collagen-stimulated aggregation of murine washed platelets (37.3 +/− 5.7%, n=10, p<0.01) compared to vehicle controls (58.2 +/− 5.8%, n=20) (Supp. Fig. 3).
Since both GAS6/TAM and ADP/P2Y signaling axes involve PI3K-mediated activation of integrin αIIbβ3, we next studied the nature of platelet inhibition and thrombosis protection after antagonism of both of these pathways. Treatment with 0.5 µM UNC2025 or 0.001 µM PBS0739, a P2Y12 inhibitor, mediated decreased collagen-induced aggregation of human WP, and the effect of combined treatment was more than additive based on analyses using both the Chou-Talaly and Bliss Independence Models (Fig. 2C and Supp. Fig. 4). Mean maximum collagen-induced aggregation in samples treated with 0.5 µM UNC2025 (55.0 +/− 1.7%, n=6, p<0.001) or 0.001 µM PBS0739 (64.1 +/− 2.3%, n=6, p<0.05), differed significantly from saline-treated negative controls (70.3 +/− 1.2%, n=6). Samples treated with a combination of PBS0739 and UNC2025 had a significantly decreased mean maximum aggregation (38.3 +/− 4.4%, n=6) compared to saline vehicle (p<0.001), UNC2025 alone (p=0.006), or PBS0739 alone (p=0.002). The combination index was determined based on the Chou-Talaly model and was <1, indicating synergy.[43] Using the Bliss additivity equation, a mean (+/− SEM) of 31 +/− 7.2% decrease in platelet aggregation was predicted for an additive interaction, but significantly greater inhibition (61 +/−8.8%, p=0.02) was observed, indicating synergistic inhibition of platelet aggregation mediated by the two compounds.
UNC2025 decreases activation of αIIbβ3
Next, we studied the effects of UNC2025 on activation of human WP as indicated by p-selectin expression (anti-p-selectin antibody binding) and conformational change of the β3 integrin (PAC-1 binding). Samples treated with 0.5 µM UNC2025 demonstrated decreased mean fluorescence intensity (MFI) (+/− standard error) of PAC-1 binding (1.3 +/− 0.3 units, p<0.005 and 0.16 +/−0.03 units p<0.01, respectively) compared to controls pre-treated with an equivalent volume of vehicle (1.9 +/− 0.26 units, n=5) (Fig. 2D). There was no significant difference in P-selectin expression (Supp. Fig. 5) between groups. The lack in changes in anti-p-selectin antibody binding suggests that alpha granule release was not affected by treatment with UNC2025.
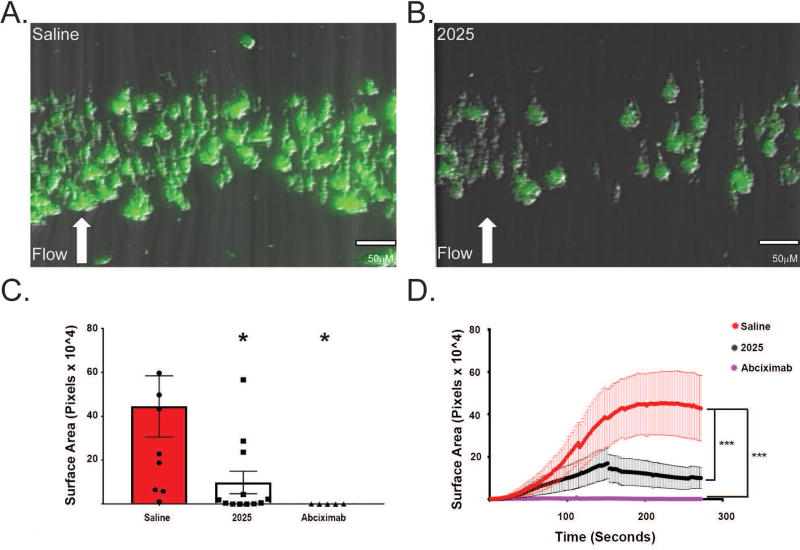
UNC2025 reduced human platelet aggregate stability under flow conditions
The impact of UNC2025-mediated MERTK inhibition on platelet aggregate formation in human WB was evaluated under physiologic flow conditions, using a microfluidic model [35, 44]. At the end of each whole blood perfusion (and buffer rinse), platelet aggregates exhibited decreased surface area coverage in samples treated with 0.5 µM UNC2025 compared to saline vehicle (Fig. 3A–B). The surface area covered by platelet aggregates increased over time in all samples but was more pronounced after vehicle treatment compared to samples treated with UNC2025 or Abciximab. Smaller aggregates were observed at the end of the run in UNC2025-treated samples, and disaggregation of large aggregates was directly visualized during the run and occurred more often in 2025-treated samples than in control samples. Review of the surface area coverage accumulation over time revealed a trend toward greater mean (+/− standard error) number of aggregate embolizations under flow conditions in samples pre-treated with UNC2025 (8.1 +/−3.2) compared to controls pre-treated with saline although this difference did not reach statistical significance (4.0 +/− 0.9, p=NS). Mean (+/− standard error) surface area coverage at the end of the run (after buffer rinse) was significantly decreased in samples treated with UNC2025 ([10.0 +/− 5.1]×104 pixels) compared to controls treated with saline ([44.5 +/− 13.9]×104 pixels, n=5, p<0.05) as seen in Figure 3C. Thus, MERTK inhibition decreased platelet aggregate stability under flow conditions.
After 300 seconds, samples pre-treated with UNC2025 exhibited decreased area under the curve ([12.5 +/− 1.5] x104 pixels) compared to controls pre-treated with saline ([37.4 +/− 2.8]×104 pixels), and similar to that of samples pre-treated with the positive control, abciximab ([2.6 +/− 0.2]×104 pixels) (Fig. 3D).
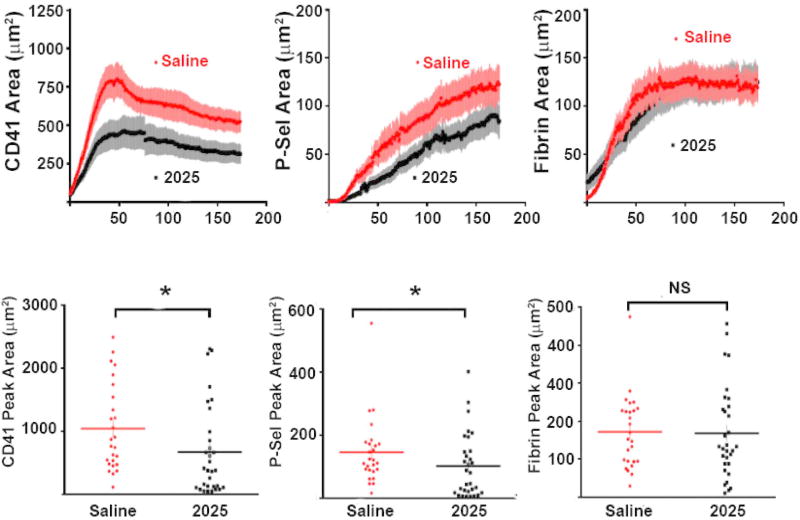
UNC2025 decreased platelet activation at murine microcirculation vascular injury sites
Next we used an in vivo murine microcirculation thrombosis model to allow better characterization of clot regional architecture-specific effects. Treatment with 3 mg/kg UNC2025 mediated significant decreases in accumulation of median (interquartile range) peak area for total/CD41-positive (384 [113–97] µm2, p=0.013) and activated/P-selectin positive (81 [18–149] µm2, p=0.047) platelets at sites of laser-induced vascular injury in the mouse cremaster muscle microcirculation, compared to saline-treated controls (822 [485–1596] µm2 and 117 [89–174] µm2, respectively, but fibrin accumulation was not affected (134 [88–239] µm2 compared to 162 [95–239] µm2, respectively) (Fig. 4). Supplemental Videos IA and IB demonstrate the increase in total and activated platelet accumulation at the injury site in saline-treated controls compared to UNC2025-treated mice, respectively.
UNC2025 decreased murine arterial thrombosis
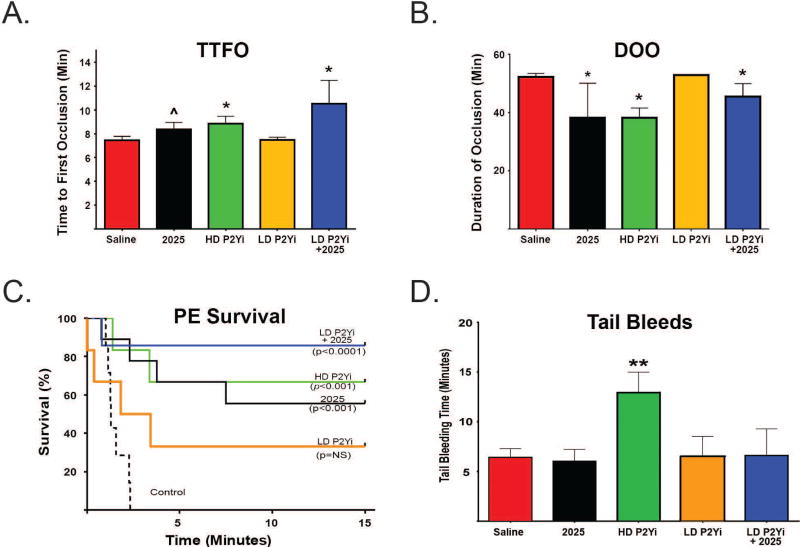
Next, we evaluated the effects of UNC2025 on larger vessels. Mice pre-treated with 3 mg/kg UNC2025 30 minutes prior to 6% FeCl3 application to the carotid artery exhibited prolonged time to initial clot formation (Fig. 5A) and decreased thrombus stability (Fig. 5B) compared to saline (vehicle)-treated controls. Mean (+/−SEM) Time To First (initial) arterial Occlusion (TTFO) in saline-treated controls (7.3 +/− 0.3 min, n=19) was significantly shorter than in animals treated with UNC2025 (8.4 +/− 0.6 min, n=10, p=0.05) (Fig. 5A). Median (IQR) Duration Of artery Occlusion (DOO), defined as the total time of vessel occlusion during a 60-minute experiment, was significantly longer in saline-treated controls (52.3 [47.8–53.3] min], n=19) than in UNC2025-treated mice (38.2 [9.9–50.1] min, n=10, p=0.02) (Fig. 5B). These results demonstrate UNC2025-mediated protection of mice from arterial clots likely due, at least in part, to decreased stability of the initial platelet aggregate.Review of the ultrasound probe tracings revealed a trend toward increased mean (+/− standard error) clot embolizations in mice pre-treated with UNC2025 (2.1 +/− 1.0 emboli/mouse) compared to controls pre-treated with saline vehicle (0.9 +/− 0.5 emboli/mouse, p=NS).
Gas6/TAM inhibition decreased fatal murine pulmonary embolism
Similarly, we evaluated the effects of UNC2025 in a collagen/epinephrine-induced pulmonary embolism (PE) model. Median (IQR) survival time in saline-treated controls (2.5 min [2.0–3.1 min], n=27) was shorter than in mice treated with 3 mg/kg UNC2025 (15 [1.6–15] min, n=16, p<0.001). (Fig. 5C) 56% of UNC2025-treated mice were alive 15 minutes after collagen-epinephrine injection, while all saline-treated controls developed fatal emboli in less than 5 minutes. These data demonstrate UNC2025-mediated protection of mice from pulmonary embolism.
UNC2025 did not increase murine tail bleeding times
Significant differences in median (IQR) bleeding times were not observed in saline-treated control mice (6.2 [5.9–8.5] minutes, n=8) and mice treated with 3 mg/kg UNC2025 (5.9 [4.8–8.5] min, n=3) ( Fig. 5D).
Combined antagonism of GAS6/TAM and ADP/P2Y12 allows for inhibitor dose reduction in vivo
Similar to the synergistic inhibition of platelet activation observed in aggregation assays, combined antagonism of P2Y and MERTK decreased thrombus formation in vivo. In the arterial thrombosis model, longer TTFO (Fig. 5A) was observed in mice treated with HD P2Yi (8.8 +/− 0.6 min, n=5, p<0.05) or 3 mg/kg UNC2025 (8.4 +/− 0.6 min, n=10, p<0.05) alone, compared to saline-treated controls (7.3 +/− 0.6 min, n=19) Mice treated with LD P2Yi, exhibited TTFO similar to vehicle control (7.5 +/−0.2 min, n=5, p=NS). Combining UNC2025 and the sub-therapeutic LD P2Yi (9.9 +/− 0.4 min, n=5, p<0.05), however, recapitulated the significantly longer TTFO mediated by HD P2Yi.
Similarly, shorter DOO (Fig. 5B) was observed in mice treated with HD P2Yi (38.2 [17.7–41.6] min, p=0.01) or UNC2025 (38.2 [9.9–50.1] min, p=0.02), compared to saline-treated controls (52.3 [47.8–53.3] min). Mice treated with LD P2Yi exhibited a DOO similar to vehicle control (52.9 [52.4–52.9] min). Combining UNC2025 and LD P2Yi (41.3 [14.5–46.8] min, p=0.03) recapitulated the shorter DOO mediated by HD P2Yi.
In the pulmonary embolism model (Fig. 5C) prolonged survival (median +/− IQR) was observed following collagen/epinephrine injection in mice treated with HD P2Yi (15.0 [2.9–15.0] min, n=12, p<0.001) or UNC2025 (15.0 [1.6–15.0] min, n=16, p<0.001) compared to vehicle (2.5 [2.0–4.2] min, n=27). Median survival was not significantly diferent in LD P2Yi-treated mice (6.9 [2.2–15.0] min, n=5), but treatment with the combination of UNC2025 and LD P2Yi (15.0 [15.0–15.0] min, n=6, p<0.0001) recapitulated the longer survival mediated by HD P2Yi. 68% of HD P2Yi-treated mice (p<0.001), 40% of LD P2Yi-treated mice (p=NS), 56% of UNC2025-treated mice (p<0.001), and 90% of LD P2Yi + UNC2025-treated mice (p<0.0001) were alive 15 min after collagen/epinephrine administration, while all control animals developed fatal emboli within 5 min. These data indicate that adding UNC2025 allows P2Yi dose reduction in murine models without compromising protection from arterial or venous thrombosis.
Mean (+/− SEM) tail bleeding times (Fig. 5D) were similar in mice pre-treated with vehicle (6.4 +/− 0.9 min, n=8), UNC2025 (6.0 +/− 1.2 min, n=3, p=NS), LD P2Yi (6.5 +/− 2.0 min, n=3, p=NS), or LD P2Yi combined with UNC2025 (6.6 +/− 2.7 min, n=3, p=NS). Only HD P2Yi (12.9 +/− 2.1 min, n=3, p<0.05) significantly prolonged bleeding.
DISCUSSION
We show herein that pharmacologic inhibition of GAS6/TAM signaling efficiently abrogated platelet activation responses, leading to decreased aggregate stability and reduced thrombosis in animal models without increased bleeding. Additionally, we demonstrated a synergistic anti-platelet effect in the context of ADP/P2Y inhibition, consistent with a previous report suggesting that interruption of αIIbβ3 activation decreases stability of platelet aggregates. [45]
UNC2025 mediated anti-thrombotic and direct anti-platelet activity in a variety of in vitro and in vivo assays, both alone and in combination with P2Y inhibitors‥ UNC2025-treated platelets exhibited decreased activation in platelet aggregation assays and reduced activity under physiologic shear stress. In the microfluidic assay platelet adhesion to collagen in the first 60 seconds was not affected (Fig. 3D), but the binding of flowing platelets to collagen-adherent platelets was decreased and large platelet aggregates dislodged more rapidly. These effects correlated with direct inhibiton of MERTK phosphorylation in platelets and reduced downstream signaling through AKT and SRC (Figures 1A–B), implicating MERTK inhibition as a mechanism of UNC2025-mediated functional effects. Additionally, the observed decrease in SRC signaling, a known pro-thrombotic mediator in platelets [48] and a downstream target of TAM kinase signaling, [49] suggests a biochemical mechanism by which TAM kinase inhibition mediates anti-thrombotic effects. However, a direct effect on SRC cannot be ruled out. Of note, UNC2025 is equipotent against MERTK and FLT3 with fifty-fold greater selectivity in cell-based assays relative to AXL, the next most potently inhibited kinase[26]; however, FLT3 expression has not been reported in human or murine megakaryocytes or platelets and thus, the effects of treatment with UNC2025 are likely not mediated by FLT3 inhibition. Treatment with UNC2025 phenocopies the effects of genetic TAM kinase deletion in mouse platelets. Specifically, platelets from Gas6−/− and Mertk−/− mice exhibit reduced aggregation in vitro and decreased clot stability in vivo [3, 50, 51], similar to UNC2025’s effects reported here.
The similar inhibition of activation responses observed in both human and mouse platelets validates the use of UNC2025 for translational application in mouse models of thrombosis. The increased embolization we noted in the microfluidic flow assay and arterial thrombosis model is reminiscent of the transient re-bleeding after tail-clip in Mertk−/− mice noted previously [2] and is consistent with previous observations that Gas6−/− platelets form unstable aggregates under flow [5]. While the TTFO was minimally prolonged for inhibitor-treated mice, a significant difference was seen in the DOO between UNC2025-treated animals and controls. Since DOO is directly proportional to aggregate stability in this model, these results reflect relatively normal initial platelet adhesion and accumulation, but subsequent inability to stabilize aggregates in the setting of GAS6/TAM inhibition, consistent with what we observed in vitro. Results from the murine intravital laser-induced microvascular injury model demonstrated that GAS6/TAM inhibition decreases accumulation of activated (P-selectin-positive) platelets at injury sites by affecting platelet-platelet interactions, but not initial platelet adhesion or fibrin accumulation (a surrogate for thrombin activity). When considered in the context of hierarchical clot organization [40], these data suggest that GAS6/TAM inhibition may primarily affect the core of a newly formed clot. Although the in vitro experiments show that UNC2025 affects platelet function (human and murine) specifically, endothelial cells also express TAM receptors and we cannot rule out a vascular effect of the compound in vivo. Future studies using transgenic models and transfused platelets will further elucidate any contribution of the vasculature to the observed phenotype.
UNC2025 also mediated a synergistic decrease in platelet aggregation in combination with an inhibitor of the ADP receptors P2Y1 and P2Y12 (P2Yi). This effect was recapitulated in vivo in the FeCl3-induced arterial and pulmonary embolism thrombosis models in which the addition of UNC2025 allowed for 50% reduction in P2Yi dose without compromising thrombosis protection, consistent with prior evidence of synergy between Gas6- and ADP receptor inhibitor-evoked AKT phosphorylation [5]. Interestingly, inhibition of platelet aggregation and degranulation by MERTK antagonism can be overcome by high concentrations of ADP or thrombin receptor agonist peptide [6] and co-infusion of ADP can overcome reduced aggregate formation under flow in Gas6−/−, Tyro3−/−, and Axl−/− mice [5], suggesting complimentary roles for ADP/P2Y and Gas6/TAM signaling. Thus, combined Gas6/TAM and ADP/P2Y signaling is expected to mediate more effective inhibition of common downstream pathways, such as AKT and β3 integrin, and our data support the idea that addition of a Gas6/TAM inhibitor to P2Yi treatment regimens could allow for P2Yi dose reduction leading to decreased risk of concomitant bleeding while maintaining the anti-thrombotic effect of high dose P2Yi therapy [52, 53]. In addition, due to the moderate inhibition of platelet activation mediated by UNC2025, GAS6/TAM inhibitors may be most useful as an adjunct to standard anti-platelet therapy, in a role similar to that considered for anti-thrombotic agents such as the protease activated receptor 1 antagonist Vorapaxar [54–56] or protein disulfide isomerase inhibitors [57].
Treatment with UNC2025 has been well-tolerated in mice for up to 150 days and the primary side-effects associated with high dose UNC2025 treatment were anemia and leukopenia. [28] These effects were not dose-limiting and were likely due primarily to FLT3 inhibition in the bone marrow compartment. More extensive studies to assess the impact of MERTK inhibition on inflammation, infection, and auto-immunity are needed to assess the potential consequences of long-term treatment with UNC2025.
In summary, GAS6/TAM pathway inhibition is an effective anti-thrombotic strategy in preclinical models, both alone and in combination with ADP/P2Y inhibitors. In addition, our data demonstrate therapeutic activity mediated by a novel translational agent without increased bleeding and support continued development of GAS6/TAM inhibitors for clinical application where they may be useful in the context of short-term treatment when reduction of bleeding risk is imperative, such as pro-thrombotic surgical procedures, pulmonary vein ablation and stent placement.
Supplementary Material
Supp FigS1
Supp FigS2
Supp FigS3
Supp FigS4
Supp FigS5
Supp VideoS1a
Supp VideoS1b
Acknowledgments
The authors wish to thank Kristen Allison for technical assistance.
Sources of Funding: This study was supported in part by the National Hemophilia Foundation/Baxter Clinical Research Fellowship (BB), American Society of Hematology Scholar Award (BB), CSL Behring/Prof. Heimburger Award in Hemostasis (BB), NIH K12HD068372-03 Child Health Research Career Development grant (BB), Hemostasis & Thrombosis Research Society Mentored Research Award sponsored by Shire (BB), 5H30MC00008-20-00 HRSA/MCHB (BB), and the Postle Chair and NIHR01 HL084086 (JD). This work was also supported by the University Cancer Research Fund and Federal Funds from the National Cancer Institute, National Institute of Health, under Contract HHSN261200800001E.
ADDENDUM
B. R. Branchford- designed and performed experiments, analyzed/synthesized data
T. J. Stalker- designed and performed experiments, analyzed/synthesized data, reviewed/edited manuscript
L. Law- performed experiments, analyzed/synthesized data, reviewed manuscript
G. Acevedo- performed experiments, analyzed/synthesized data, reviewed manuscript
S. Sather- designed and performed experiments, analyzed/synthesized data, reviewed manuscript
C. Brzezinski- performed experiments, analyzed/synthesized data, reviewed manuscript
S. R. Lentz- designed experiments, provided training and equipment, reviewed/edited manuscript
K. M. Wilson- performed experiments, analyzed/synthesized data, reviewed manuscript
K. Minson- performed experiments, analyzed/synthesized data, reviewed manuscript
W. Zhang- designed and tested experimental compound, reviewed manuscript
A. B. Lee-Sherick- performed experiments, analyzed/synthesized data, reviewed manuscript
P. Davizon-Castillo- performed experiments, analyzed/synthesized data, reviewed manuscript
C. Ng- performed experiments, analyzed/synthesized data, reviewed manuscript
K. B. Neeves- provided training and equipment, analyzed/synthesized data, reviewed/edited manuscript
X. Wang- oversaw design and testing of experimental compound, reviewed/edited manuscript
S. V. Frye- oversaw design and testing of experimental compound, reviewed/edited manuscript
H. Shelton Earp III- oversaw design and testing of experimental compound, reviewed/edited manuscript
D. DeRyckere- initial concept, experimental design, data analysis, manuscript editing
L. F. Brass- initial concept, experimental design, data analysis, manuscript editing
D. K. Graham- initial concept, experimental design, experimental oversight, data analysis, manuscript editing
J. A. Di Paola - initial concept, experimental design, data analysis, manuscript editing, provided equipment and lab space
Footnotes
Disclosures: B. Branchford, T. J. Stalker, L. Law, G. Acevedo, C. Brzezinski, K. M. Wilson, K. Minson, A. B. Lee-Sherick, P. Davizon-Castillo, C. Ng, B. Neeves, S. R. Lentz, K. Neeves, S. R. Lentz, L. Brass, and J. A. DiPaola have no relevant conflicts of interest. D. K. Graham, D. DeRyckere, S. Sather and H. S. Earp have filed patents on targeting of the Mer tyrosine kinase as cancer therapy. X. Wang, W. Zhang, and S. V. Frye have filed patent applications on UNC2025.
References
Full text links
Read article at publisher's site: https://doi.org/10.1111/jth.13875
Read article for free, from open access legal sources, via Unpaywall:
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/jth.13875
Citations & impact
Impact metrics
Article citations
MERTK Inhibition as a Targeted Novel Cancer Therapy.
Int J Mol Sci, 25(14):7660, 12 Jul 2024
Cited by: 0 articles | PMID: 39062902 | PMCID: PMC11277220
Review Free full text in Europe PMC
Design, Synthesis, and Biological Evaluation of 2-Substituted Aniline Pyrimidine Derivatives as Potent Dual Mer/c-Met Inhibitors.
Molecules, 29(2):475, 18 Jan 2024
Cited by: 0 articles | PMID: 38257391 | PMCID: PMC10819570
Regulation of brain endothelial cell physiology by the TAM receptor tyrosine kinase Mer.
Commun Biol, 6(1):916, 07 Sep 2023
Cited by: 8 articles | PMID: 37673933 | PMCID: PMC10482977
Targeted Phagocytosis Induction for Cancer Immunotherapy via Bispecific MerTK-Engaging Antibodies.
Int J Mol Sci, 23(24):15673, 10 Dec 2022
Cited by: 0 articles | PMID: 36555321 | PMCID: PMC9779728
Immunothrombosis and the Role of Platelets in Venous Thromboembolic Diseases.
Int J Mol Sci, 23(21):13176, 29 Oct 2022
Cited by: 15 articles | PMID: 36361963 | PMCID: PMC9656618
Review Free full text in Europe PMC
Go to all (18) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Tyro3, Axl, and Mertk receptors differentially participate in platelet activation and thrombus formation.
Cell Commun Signal, 16(1):98, 12 Dec 2018
Cited by: 14 articles | PMID: 30541554 | PMCID: PMC6291976
Potentiating role of Gas6 and Tyro3, Axl and Mer (TAM) receptors in human and murine platelet activation and thrombus stabilization.
J Thromb Haemost, 8(8):1797-1808, 07 Jun 2010
Cited by: 67 articles | PMID: 20546121
Ginsenoside-Rp3 inhibits platelet activation and thrombus formation by regulating MAPK and cyclic nucleotide signaling.
Vascul Pharmacol, 109:45-55, 08 Jun 2018
Cited by: 16 articles | PMID: 29890296
Reversible Platelet Integrin αIIbβ3 Activation and Thrombus Instability.
Int J Mol Sci, 23(20):12512, 19 Oct 2022
Cited by: 8 articles | PMID: 36293367 | PMCID: PMC9604507
Review Free full text in Europe PMC
Funding
Funders who supported this work.
American Society of Hematology
CSL Behring
Child Health Research Career Development grant
Federal Funds
Hemostasis and Thrombosis Research Society
NCI NIH HHS (1)
Grant ID: P30 CA046934
NHLBI NIH HHS (5)
Grant ID: P01 HL120846
Grant ID: R01 HL120728
Grant ID: R01 HL084086
Grant ID: P01 HL040387
Grant ID: T32 HL007439
NICHD NIH HHS (1)
Grant ID: K12 HD068372
National Cancer Institute
National Hemophilia Foundation
National Institutes of Health (3)
Grant ID: HHSN261200800001E
Grant ID: K12HD068372-03
Grant ID: R01 HL084086
Postle Chair
Shire (1)
Grant ID: 5H30MC00008-20-00 HRSA/MCHB