Abstract
Purpose
To evaluate the clinical and histopathological parameters of pterygium to determine significant correlations between parameters that can affect management strategies.Methods
A total of 47 pterygia were clinically examined and excised for histopathological evaluation of epithelial and stromal changes. Some samples were immunostained with P53 (a protein of 53 kilodalton used as dysplastic epithelial marker), CD20 (CD/cluster of differentiation, are group of surface receptors providing targets for cellular immunophenotyping, CD20 as a B lymphocyte marker), CD 3 (as T lymphocyte marker) or vascular endothelial growth factor (VEGF/as vascular marker).Results
Most patients were male (59.6%). Cosmetic complaints (83%), grade II redness (61.7%), grade 2 extension (63.8), and associated astigmatism of <2.5 D (83%) were observed. Histopathological features included solar elastosis (100%), squamous hyperplasia (83%), increased stromal vascularity with hemorrhage (76.6%), and lymphocytic stromal infiltration, perivascular distribution, and mild epithelial lymphocytic exocytosis in 72.3%, 74.5%, and 70.2% of cases, respectively. Other changes included goblet cell hyperplasia (31.9%), prominent epithelial pigmentation (48.9%), and, most importantly, epithelial atypia (53.2%). Clinical redness was significantly correlated with vascularity, epithelial hyperplasia, and lymphocytic stromal infiltration; lymphocytic stromal infiltration was also significantly correlated with pterygium extension and with low astigmatism.Conclusion
The inflammatory response was mild in most cases and the density was not significantly correlated with any clinical parameter. Vascularity was related to clinical redness. Treatment with anti-VEGF may be beneficial, even for grade 1 pterygia that are not dominantly fibrotic.Free full text

Histopathological Parameters in Pterygia and Significant Clinical Correlations
Abstract
Purpose:
To evaluate the clinical and histopathological parameters of pterygium to determine significant correlations between parameters that can affect management strategies.
Methods:
A total of 47 pterygia were clinically examined and excised for histopathological evaluation of epithelial and stromal changes. Some samples were immunostained with P53 (a protein of 53 kilodalton used as dysplastic epithelial marker), CD20 (CD/cluster of differentiation, are group of surface receptors providing targets for cellular immunophenotyping, CD20 as a B lymphocyte marker), CD 3 (as T lymphocyte marker) or vascular endothelial growth factor (VEGF/as vascular marker).
Results:
Most patients were male (59.6%). Cosmetic complaints (83%), grade II redness (61.7%), grade 2 extension (63.8), and associated astigmatism of <2.5 D (83%) were observed. Histopathological features included solar elastosis (100%), squamous hyperplasia (83%), increased stromal vascularity with hemorrhage (76.6%), and lymphocytic stromal infiltration, perivascular distribution, and mild epithelial lymphocytic exocytosis in 72.3%, 74.5%, and 70.2% of cases, respectively. Other changes included goblet cell hyperplasia (31.9%), prominent epithelial pigmentation (48.9%), and, most importantly, epithelial atypia (53.2%). Clinical redness was significantly correlated with vascularity, epithelial hyperplasia, and lymphocytic stromal infiltration; lymphocytic stromal infiltration was also significantly correlated with pterygium extension and with low astigmatism.
Conclusion:
The inflammatory response was mild in most cases and the density was not significantly correlated with any clinical parameter. Vascularity was related to clinical redness. Treatment with anti-VEGF may be beneficial, even for grade 1 pterygia that are not dominantly fibrotic.
INTRODUCTION
Pterygium is a common ocular disorder. It has a prevalence of 0.3% to 29% in different regions of the world.[1] It is most common in hot climates, and ultraviolet irradiation is suspected to be the most important factor for its development.[2] Primary pterygium is diagnosed by the presence of a wing of thick, reddish, fibrovascular growth encroaching on the cornea. The nasal side is more commonly affected than the temporal side.[3]
Surgical excision is mostly performed for cosmetic reasons. However, large pterygia may obscure the visual axis and may cause astigmatism,[4] and surgery is an important treatment option for primary pterygium. Complications of surgery include recurrence; recurrence rates of 15% to 75% in cases of simple excision, and of approximately 6% in free flap surgery have been reported.[5,6] Moreover, many patients refuse surgery and request conservative treatment that primarily targets the red appearance of the congested pterygium. For cases with just cosmetic complain (redness), and refuse surgery, medical treatment is the best choice and essential.[7]
In the present study, we assessed all clinical variables to determine the histopathological changes that occur in pterygium, and correlated them to determine the best management strategy for each patient.
METHODS
A total of 47 cases of primary pterygium were examined between 2015 and 2016. Histories including age, sex, occupation, duration, and complaint (cosmetic or visual) were taken.
Prior to surgery, all patients underwent a complete ocular examination including slit lamp biomicroscopy and photography. Ocular examinations included measurement of best corrected visual acuity according to the Snellen visual acuity chart. Astigmatism was assessed using a Topcon RM-8000B autorefractometer (Tokyo, Japan). The pterygium width at the limbus was measured in millimeters using a slit lamp. Pterygia were graded according to size and extent of corneal involvement, as follows: grade 1: between the limbus and a point midway between the limbus and pupillary margin; grade 2: head of the pterygium reaching the pupillary margin; and grade 3: crossing the pupillary margin.[7] Pterygia were also graded for severity of redness, as follows: grade I: no redness or faint pinkish hue; grade II: scattered areas of moderate redness; and grade III: significant and diffuse redness.[8] Exclusion criteria were previous medical treatment for pterygium including topical steroids or non-steroidal anti-inflammatory drugs, previous conjunctival surgery, conjunctival cicatricial disease, systemic autoimmune disease, and untreated dry eye disease. All patients were treated using the same surgical technique (excision and graft). Written informed consent was obtained from all participants before surgery.
The obtained tissues were preserved in 10% formalin. Each specimen was cut longitudinally through the head and the body of the pterygium. Thus, in all cases, sections passed through the corneal side as well as the canthus side. Tissue blocks were processed, cut, and stained with hematoxylin and eosin stain. Some samples were immunostained with P53 (protein of 53 kilodalton as dysplastic epithelial marker), CD 20 (cluster of differentiation as B lymphocyte marker), CD 3 (cluster of differentiation as T lymphocyte marker) or vascular endothelial growth factor (VEGF, as vascular marker). The following epithelial and stromal parameters were recorded.
Assessment of epithelial atypia was done by examination of H&E stained sections. Then, four cases only from our studied cases, (one showed no epithelial atypia, one showed reactive epithelial atypia, one showed immature squamous metaplasia and finally one showed mild looking epithelial dysplasia with no moderate or severe epithelial dysplasia were found in our studied cases), were subjected to P 53 (used as marker for epithelial dysplasia) immunohistochemical study (A mouse monoclonal antibody, concentrated 1:400 clone Bp53-12, Sigma-Adrich Lab., California, USA). Assessment of the positive nuclei was done. All studied cases were showing positive nuclear stain at basal cell layer and some suprabasal keratinocytes, thus P53 immunostaining was not further applied on the other studied cases as it didn't add value. For statistical analysis, each case was classified as with or without epithelial atypia.
Assessment of squamous epithelial hyperplasia whether mild, moderate or severe was done. For statistical purposes, it was categorized as absent or present. The number of goblet cells was determined, and was classified as few cells or prominent cells (goblet cell hyperplasia). All samples were examined for the presence and severity of lymphocytic exocytosis.
In all cases, epithelial lymphocytic exocytosis was determined as mild to moderate.
Epithelial pigmentation in basal cells and in suprabasal keratinocytes was considered absent (if none or few) or present (if prominent along basal keratinocytes or in suprabasal keratinocytes. Some authors define this as primary acquired melanosis of the conjunctiva;[9] others use the term conjunctival hypermelanosis.[10]
All cases were examined for evidence of stromal solar elastosis. They were also examined for the presence and relative amounts stromal vascularity and fibrosis. In the included cases, vascularity and fibrosis were classified into two groups. One group showed prominent vascularity that overwhelming the fibrosis, and the other group showed no significant difference between fibrosis and vascularity.
All cases were examined for stromal inflammation by considering both pattern (mild perivascular, or diffuse) and type of inflammatory cells: chronic (lymphocytes with or without plasma cells) or mixed (lymphocytes with neutrophils). All cases were also examined for hemorrhage and siderophages. For any case showing siderophage-like cells, two more sections were taken from the specimen. One section was treated in ethanolic picric acid to remove any suspected formalin pigment,[11] and the other was stained using Perl's stain (Thermo Scientific Waltham, Massachusetts, USA). This parameter was classified as absent or present.
Some cases were included in an immunohistochemical study using CD20 (monoclonal mouse antihuman antibody, clone L26, concentrated 1:50, Abcam Scientific Support, San Jose, California, USA), CD3 (monoclonal rabbit antihuman antibody, concentrated 1:100, Abcam Scientific Support, USA), and VEGF (monoclonal mouse antihuman antibody, clone VG1, concentrated 1:100, Thermo Fisher Scientific, Waltham, Massachusetts, USA) to assess the type of lymphocytes in the infiltrate and the expression of this factor in the blood vessels of the lesion. The cases included in the CD20 and CD3 immunohistochemical studies were selected based on the presence of stromal and epithelial lymphocytosis (12 cases with diffuse stromal inflammation, 2 additional cases with moderate epithelial lymphocytic exocytosis; 14 cases total). Cases that were included in VEGF testing were selected based on the presence of both clinical grade 1 pterygium (9 cases) and clinical grade 3 pterygium (8 cases) as a trial to correlate the expression of this marker with the pterygium grade.
Statistical Analysis
The clinical and histopathological parameters were analyzed to determine any correlation between them. Statistical pre-coded data were entered into Microsoft Excel 2010 for Windows (Microsoft Corp., Redmond, WA, USA). Data were then transferred to IBM SPSS Statistics for Windows, Version 23.0 (IBM Corp., Armonk, NY, USA) for analysis. Quantitative variables were presented as the mean and standard deviation (SD); qualitative variables were presented as the frequency and percentage. Groups were compared using the Mann-Whitney test for quantitative variables and the Chi square and Fisher's exact tests for qualitative variables. P values < 0.05 were considered statistically significant, and values < 0.01 were considered highly significant.
RESULTS
This study included 47 patients: 28 males (59.6%) and 19 females (40.4%). The ages ranged from 25 to 46 years (mean, 34.3 ± 5.3 years). Patients were divided into two age groups: 25-35 years (34 patients, 72.3%) and 36-45 years (13 patients, 27.7%). Occupations included 40 outdoor jobs (85.1%) and 7 indoor jobs (14.9%). Thirty-nine patients (83%) had cosmetic complaints; 8 patients (17%) had visual complaints. The duration of the pterygium ranged from 4 to 60 months (mean, 20.7 ± 16.1). The pterygium extension over the cornea was grade 1 in 9 cases (19.1%), grade 2 in 30 cases (63.8%), and grade 3 in 8 cases (17%). The severity of redness was reported as grade I in 4 cases (8.5%), grade II in 29 cases (61.7), and grade III in 14 cases (17%). The width ranged from 1.4 to 7.8 mm (mean, 2.9 ± 1.6 mm). Cases were divided into two groups (<4 mm width, 39 cases, 83%) and (>4 mm width, 8 cases, 17%). Eight cases had astigmatism >2.5 D; 39 cases had astigmatism <2.5 D.
The epithelial changes included epithelial atypia in 53.2% of cases ranging from reactive atypia with immature squamous metaplasia (21 cases) to mild dysplasia (4 cases); no moderate or severe dysplasia was observed. Epithelial hyperplasia was observed in 83.0% of cases, and goblet cell hyperplasia was observed in 31.9% of cases. Mild epithelial lymphocytic exocytosis was observed in 70.2% of cases; the moderate form was observed in 29.8% of cases. Stromal inflammation had a perivascular pattern in 74.5% of cases and a diffuse pattern in 25.5% of cases. Immunohistochemical studies revealed that most lymphoid cells in all 14 cases were B lymphocytes with moderate epithelial lymphocytic exocytosis, and diffuse stromal inflammation was observed in 29.78% of cases. Prominent epithelial pigmentation was present in 48.9% of cases [Figure 1].

Epithelial changes seen in pterygium: (a) goblet cell hyperplasia, hematoxylin and eosin (h and e) stain, ×40; (b) epithelial pigmentation, h and e, ×400; (c) epithelial lymphocytic exocytosis, h and e, ×400; (d) reactive epithelial atypia, h and e, ×400; (e) immature squamous metaplasia with reactive changes, h and e, ×400; (f) mildly dysplastic epithelium, h and e, ×400; (g) p53 positive nuclear stain in basal and some suprabasal keratinocytes in reactive epithelium, ×400; (h) p53 positive nuclear stain in basal and some suprabasal keratinocytes of reactive immature squamous epithelium, ×400.
Stromal solar elastosis was evident in all cases. Prominent stromal vascularity (vascularity is more dominant than fibrosis) was observed in 76.6% of cases; there was no significant difference between vascularity and fibrosis in 23.4% of cases. Stromal vascularity was further analyzed by an immunohistochemical study using VEGF in 17 cases that were clinically grade 1 (9 cases) or grade 3 (8 cases), and was found to be expressed in all lesional blood vessels. The inflammation was predominantly chronic in 72.3% and mixed in 27.7% of the studied cases. Hemorrhage was present in 76.6% of cases. Hemorrhage with sidrophages was present in 7 cases that were further examined using Perl's stain [Figure 2].

Stromal changes in pterygium. (a) mild perivascular lymphocytic infiltration, hematoxylin and eosin (h and e) stain, ×100; (b) diffuse lymphocytic infiltration, h and e, ×100; (c) prominent stromal solar elastosis, h and e, ×100; (d) stromal sidrophages, h and e, ×100; (e) sidrophages with positive iron granules, perl's stain × 100; (f) positive immunostain for vascular endothelial growth factor, ×100; (g) positive immunostain for cd20 at epithelial lymphocytic exocytosis, ×400.
Few histopathological changes were significantly correlated with clinical redness. They included epithelial hyperplasia (P = 0.039), prominent vascularity over fibrosis (P < 0.001), and the chronic inflammatory response (P < 0.001) [Table 1 and Figure 3]. A correlation of the extension of the pterygium and of astigmatism with all histopathological variables revealed a significant reverse correlation with the chronic inflammatory response (P = 0.033, P = 0.028, respectively) [Tables [Tables2,2, ,33 and Figure 4].
Table 1
Correlation of the degree of redness with histopathological findings


(a) pterygium redness grade III, clinical case; (b) significant histopathological features including prominent vascularity overwhelming fibrosis, hematoxylin and eosin (h and e) stain, ×100; (c) prominent squamous epithelial cell hyperplasia, h and e, ×100; and (d) lymphocytic (chronic) stromal inflammatory response, h and e, ×400.
Table 2
Correlation of grade with histopathological findings
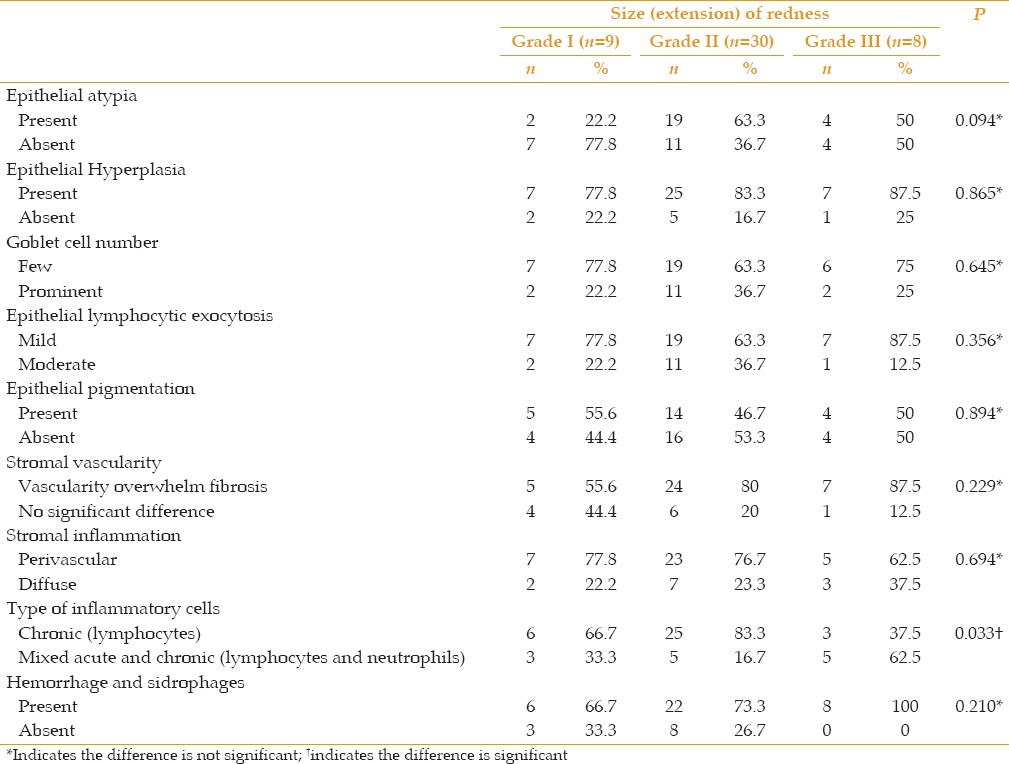
Table 3
Correlation of astigmatism with histopathological findings


(a) pterygium extension grade 3, clinical case; (b) the significant histopathological feature was mixed chronic (lymphocytes and plasma cells) and acute (neutrophils) inflammatory cells, hematoxylin and eosin stain, ×400.
A significant positive correlation was found between astigmatism and the following: pterygium with grade III redness (P = 0.011), pterygium with grade 3 corneal extension (P < 0.001), pterygium width >4 mm (P < 0.001), and longer pterygium duration (P = 0.026) [Table 4].
Table 4
Correlation of astigmatism with degree of redness, grading, and slit lamp width

No significant correlation was found between any of the examined histopathological features and age, sex, occupation, or duration of the pterygium.
DISCUSSION
The present study was designed to determine all histopathological changes in pterygium in relation to the clinical manifestations. The histopathologic changes and clinical features of pterygia may provide a better understanding of the pathogenesis and reveal additional clues for management strategies[12] (surgical or non-surgical) to reduce recurrence, severity of inflammation, tissue invasion, proliferation, and vascularization.[13]
In our study, 72.5% of pterygium patients were 25-35 years of age; 27.7% were aged 36-45. The data indicate that age is not a contributing factor. Pterygium can occur as early as 25 years of age. In this study, 59.6% of patients were men and 40.4% were women. This finding has been reported in most of the literature, indicating a higher incidence of pterygium in males, yet female predomination has also been reported.[14]
We noted that an increased duration of pterygium is correlated with increased severity of redness, degree of extension over the cornea, increased width, and astigmatism. Extension of the pterygium over the cornea is associated with the degree of severity of redness.
A significant positive association was found between lesion dimensions over the cornea and vascular density. The same study showed that pterygium redness and fleshiness were positively correlated with lesion dimensions over the cornea. However, larger lesions over the cornea may be associated with increased thickness and volume of the pterygium body, resulting in higher grades of redness and fleshiness.[8]
We found that astigmatism is correlated with pterygium grade III redness, grade 3 corneal extension, width >4 mm, and longer duration. We also found that pterygium extension over the cornea had the strongest correlation to astigmatism. Pterygium extension has a stronger correlation with corneal astigmatism than with width.
Previous work has suggested an association between the increasing size of a pterygium and increasing degrees of corneal astigmatism.[14] When a primary pterygium reaches more than 1.0 mm in size from the limbus, it induces significant astigmatism. This astigmatism tends to increase with increasing lesion size, and is improved by successful removal of the pterygium.[15,16]
This study found many histopathological variables of pterygia. The most important variable is epithelial atypia. Many studies discuss this issue.[17] In the present study, epithelial atypia was found in 53.2% of cases and ranged from reactive atypia to mild dysplasia. The results concur with Gaton et al,[17] who also found only mild dysplasia in a few cases of pterygium (3 of 45, 6.6%). In the cases of this study, P53 immune staining was done on four cases only (with no atypia, with reactive atypia, with immature looking squamous metaplasia, and mild looking dysplasia). No staining difference was detected between the four studied cases, so no more cases were subjected to P53 immune staining.
This result concurred with Kang et al,[18] who studied the effect of P53 on reactive, immature, and dysplastic uterine cervix squamous epithelium and found limited significant value in differentiation. Van Der Pols et al,[13] reported that P53 expression is increased in the basal layer and in the whole epidermis with increased time spent outdoors.
Chan et al[19] found that pterygium commonly showed squamous metaplasia (73.2%); hyperplasia was associated with goblet cell hyperplasia (87.5%). This concurred with our results that found epithelial hyperplasia in 83% of the cases, but our study showed less evidence of goblet cell hyperplasia: just 31.9% of the cases had prominent goblet cells.
Epithelial pigmentation was evident in 48.9% of the cases in our study. Melanocytic lesions can be related to sun exposure according to Dodd et al[20] Thus, it is not strange to find increased melanin pigment in pterygium epithelium that is related to ultraviolet radiation exposure. Moreover, Perra et al[21] studied melanocytic pigmented lesions in 80 cases of pterygia and found nine cases of pigmented lesions.
In the present study, both epithelial and stromal inflammation were detected, but the inflammatory responses were mild (not prominent) and chronic (rather than mixed or acute) in both the epithelium and the stroma. This concurred with Nassar et al,[2] who reported an inflammatory response in all studied pterygium cases; a mild inflammatory response, whether epithelial (84.2%) or stromal (71.8%), was most common. Our results were also in agreement with Safi et al,[8] who detected a significant stromal inflammatory response in only 38.6% of their studied cases. No correlation was found between clinical pterygium redness and the epithelial or stromal inflammation, even if it was pathologically very evident.
Sankar et al[22] found that all pterygium cases showed elastoid basophilic degeneration. This was in agreement with our results, as solar elasosis was evident in all of our studied cases. They also found that this solar elastosis is an important diagnostic histopathological clue for any pterygium case.
Stromal vascularity of a pterygium is an important and promising therapeutic target. In the present study, prominent vascularity that overcame fibrosis was evident in most of the studied cases (76.6%), and the remaining cases showed both vascularity and fibrosis with significant clinico-pathological correlation. Thus, pterygium vascularity can be used as a target for treatment, especially for patients with cosmetic complaints. A few years ago, many studies attempted to use anti-VEGF as target therapy for pterygium.[2,23,24] Hoshino et al[25] reported that VEGF has a narrow target cell range and is specific for endothelial cells, because VEGF binds to the high-affinity cell surface receptors KDR/Flk and Flt, which are predominantly expressed by endothelial cells. This concurred with our results, which showed positive staining for VEGF in the wall of the blood vessels of all studied lesions (17 cases). These selected cases presented grade 1 pterygium (9 cases) and grade 3 pterygium (8 cases). There was no significant difference between VEGF expression and the pterygium grade.
Hemorrhage with/without sidrophages in the pterygium was observed in 76.6% of cases. Recurrent hemorrhage in a pterygium may elicit a chronic inflammatory response. This may explain the dominance of the chronic inflammatory response in most of our studied cases (72.3%).
Clinical redness was significantly correlated with histopathological vascular content, as vascularity overwhelmed fibrosis in grade II (79.3%) and in grade III (92.9%) specimens. Safi et al[8] showed that pterygium redness was significantly correlated with vascular density, but not with other histopathologic features in their study.
Epithelial hyperplasia was significantly correlated with redness, as evident in grade II (93.1%) and grade III (71.4%) cases. Chronic inflammation was also evident in all pterygium cases with different redness grades, but was more evident in low extension grades: 66.7% in grade 1 extension and 83.3% in grade 2 extension. Mixed inflammation dominated in grade 3 extensions. Clinical cases with high astigmatism (>2.5 D) were also significantly correlated with the mixed inflammatory response. Whether the acute inflammation is the exaggerating factor that increases pterygium extension and induces more astigmatism, or it occurs secondary to other initiating factors requires further studies.
Age, sex, occupation (indoors or outdoors), and duration of pterygium were not related to histopathological parameters.
In conclusion, this study illustrates many histopathological variables of pterygium that may affect the patient plan of treatment, including new therapeutic targets such as anti-VEGF for treatment of pterygium with different grades of redness, and the follow-up.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
Articles from Journal of Ophthalmic & Vision Research are provided here courtesy of Ophthalmic Research Center
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.4103/jovr.jovr_31_17
Article citations
A Phase IIa Multicenter, Randomized, Vehicle-Controlled, Dose Escalating Study to Evaluate the Safety, Efficacy, and Pharmacokinetics of CBT-001 Ophthalmic Solution in Patients With Primary or Recurrent Pterygium.
Ophthalmol Sci, 4(4):100502, 04 Mar 2024
Cited by: 0 articles | PMID: 38883924 | PMCID: PMC11179250
Transcriptome Analysis of Pterygium and Pinguecula Reveals Evidence of Genomic Instability Associated with Chronic Inflammation.
Int J Mol Sci, 22(21):12090, 08 Nov 2021
Cited by: 7 articles | PMID: 34769520 | PMCID: PMC8584501
Pterygium-The Good, the Bad, and the Ugly.
Cells, 10(7):1567, 22 Jun 2021
Cited by: 17 articles | PMID: 34206333 | PMCID: PMC8305200
Review Free full text in Europe PMC
Pterygium: an update on pathophysiology, clinical features, and management.
Ther Adv Ophthalmol, 13:25158414211020152, 01 Jan 2021
Cited by: 39 articles | PMID: 34104871 | PMCID: PMC8170279
Review Free full text in Europe PMC
Does pterygium morphology affect corneal astigmatism?
Ther Adv Ophthalmol, 13:25158414211030423, 01 Jan 2021
Cited by: 2 articles | PMID: 34291187 | PMCID: PMC8278454
Go to all (7) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Correlations Between Histopathologic Changes and Clinical Features in Pterygia.
J Ophthalmic Vis Res, 11(2):153-158, 01 Apr 2016
Cited by: 17 articles | PMID: 27413494 | PMCID: PMC4926561
Overexpression of fractalkine and its histopathological characteristics in primary pterygium.
Graefes Arch Clin Exp Ophthalmol, 257(12):2743-2750, 21 Oct 2019
Cited by: 1 article | PMID: 31637486
Differential expression of vascular endothelial growth factor implies the limbal origin of pterygia.
Ophthalmology, 112(6):1023-1030, 01 Jun 2005
Cited by: 36 articles | PMID: 15885787
P53 expression in altered limbal basal cells of pingueculae, pterygia, and limbal tumors.
Curr Eye Res, 16(12):1179-1192, 01 Dec 1997
Cited by: 88 articles | PMID: 9426949
Review



