Abstract
Purpose
One hallmark of chronic fatigue syndrome (ME/CFS) is task related worsening of fatigue. Global brain hypoperfusion, abnormal regional activation, and altered functional connectivity of brain areas associated with cognition and memory have been reported but remain controversial.Methods
We enrolled 17 female participants fulfilling the CDC Criteria for ME/CFS and 16 matched healthy controls (HC). Using a 3T-Phillips Achieva MRI-scanner, pseudo-continuous arterial spin-labeling (pCASL), was used to study the dynamics of regional cerebral blood flow (rCBF) and their relationship to mental fatigue in ME/CFS patients and HC during a demanding cognitive task, i.e. modified Paced-Auditory-Serial-Addition-Testing (PASAT).Results
ME/CFS subjects reported more fatigue than HC at baseline (p < .01). Global brain perfusion of ME/CFS and HC subjects was similar at rest. The PASAT resulted in significantly increased fatigue in ME/CFS participants and HC. Although not different between groups, overall CBF significantly increased over the first 3 min of the PASAT and then decreased thereafter. Regional CBF (rCBF) changes were significantly different between groups during the post-task recovery period. Whereas improvement of fatigue of ME/CFS subjects was associated with decreased rCBF in both superior temporal gyri (STG), precuneus, and fusiform gyrus, it was associated with increased rCBF in the same areas in HC.Conclusions
Our results suggest that ME/CFS is associated with normal global CBF at rest and during a strenuous task (PASAT); however rCBF of several brain regions associated with memory, goal-oriented attention, and visual function was differentially associated with recovery from fatigue in ME/CFS patients and HC.Free full text

Task Related Cerebral Blood Flow Changes of Patients with Chronic Fatigue Syndrome: An Arterial Spin Labeling Study
Abstract
Purpose
One hallmark of chronic fatigue syndrome (ME/CFS) is task related worsening of fatigue. Global brain hypoperfusion, abnormal regional activation, and altered functional connectivity of brain areas associated with cognition and memory have been reported but remain controversial.
Methods
We enrolled 17 female participants fulfilling the CDC Criteria for ME/CFS and 16 matched healthy controls (HC). Using a 3T-Phillips Achieva MRI-scanner, pseudo-continuous arterial spin-labeling (pCASL), was used to study the dynamics of regional cerebral blood flow (rCBF) and their relationship to mental fatigue in ME/CFS patients and HC during a demanding cognitive task, i.e. modified Paced-Auditory-Serial-Addition-Testing (PASAT).
Results
ME/CFS subjects reported more fatigue than HC at baseline (p < .01). Global brain perfusion of ME/CFS and HC subjects was similar at rest. The PASAT resulted in significantly increased fatigue in ME/CFS participants and HC. Although not different between groups, overall CBF significantly increased over the first 3 min of the PASAT and then decreased thereafter. Regional CBF (rCBF) changes were significantly different between groups during the post-task recovery period. Whereas improvement of fatigue of ME/CFS subjects was associated with decreased rCBF in both superior temporal gyri (STG), precuneus, and fusiform gyrus, it was associated with increased rCBF in the same areas in HC.
Conclusions
Our results suggest that ME/CFS is associated with normal global CBF at rest and during a strenuous task (PASAT); however rCBF of several brain regions associated with memory, goal-oriented attention, and visual function was differentially associated with recovery from fatigue in ME/CFS patients and HC.
1. Introduction
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is characterized by often disabling fatigue lasting for more than six months, accompanied by numerous somatic symptoms, affecting nearly 0.5% of the general population [1,2]. Although multiple biological and psychological mechanisms for ME/CFS have been investigated, little is known about the underlying neural underpinnings that initiate and sustain chronic fatigue.
ME/CFS has been linked to aberrant autonomic nervous system activity involving the hypothalamic-pituitary-adrenal axis; pain-related pathways; and abnormal brain activity in parietal, cingulate, inferior frontal and superior temporal cortices, and the cerebellum [3–8]. Similar abnormalities have been reported in individuals with fatigue related to multiple sclerosis [3,9], Parkinson’s disease [10,11], and cancer [12,13]. These brain regions are known to contribute to cognitive functioning, including working memory [14–19].
Previous functional imaging studies of resting state cerebral blood flow (CBF) have reported inconsistent findings in ME/CFS patients. Using single-photon emission computed tomography (SPECT), Xenon-Computed Tomography, and arterial spin labeling (ASL) MRI, several studies demonstrated global cerebral hypoperfusion in ME/CFS patients [20–23], whereas other investigations found no CBF abnormalities [24–26]. Additionally, neuroimaging studies have been used to inform the neurobiological underpinnings of task related fatigue, i.e. compared to healthy controls (HC) ME/CFS patients have shown significantly increased brain activity in several cortical and subcortical regions during motor, auditory, and working memory tasks [27,28]. Similar to several other investigations of ME/CFS patients [5,29] these studies revealed positive relationships of fatigue with the activation of fronto-parietal, cingulate, temporal, and cerebellar brain areas.
We used ASL to study the dynamics of regional CBF (rCBF) changes related to mental fatigue in ME/CFS patients. We hypothesized that rCBF changes during a fatiguing cognitive task would differ between ME/CFS and HC participants in areas related to attention and memory. Because previous work from our group suggested that ME/CFS was associated with alterations in functional connectivity (FC) in a number of brain areas [30–32], we postulated that the same brain regions would be associated with fatigue related blood flow abnormalities during the performance of a cognitive task. If confirmed, such findings would improve our understanding of the neurobiological basis of task related worsening of fatigue in ME/CFS and could serve as biomarkers for patients diagnosed with this condition.
2. Methods
2.1 Subjects
Seventeen ME/CFS subjects [16 females, 1 male; average age (SD) 49.25 (11.43) years], and 16 HC [16 females; average (SD) age 49.60 (10.00) years] participated in this study. All study procedures were approved by the University of Florida Institutional Review Board prior to study enrollment and conformed to the ethical guidelines of the 2013 Declaration of Helsinki. Prior to enrollment, all subjects were told about the nature of the study and provided written consent. All ME/CFS subjects met the 1994 CDC Criteria and the 2003 Canadian Criteria for ME/CFS. ME/CFS subjects could not have a history of heart disease, systemic sclerosis, malignancy, or other systemic disorders including psychiatric illnesses that would be exclusionary for a diagnosis of ME/CFS [33]. Healthy subjects were not allowed to take any medications except vitamins. They were excluded if they had a previous history of chronic fatigue, depression or cancer. The subjects were not allowed to take any analgesics, stimulants, hypnotics, or anti-depressants. Maintenance medications for hypertension or hyperlipidemia were permissible. No caffeine intake was allowed for at least 12 hours prior to the study. All subjects were recruited from University of Florida outpatient clinics or through advertising.
2.2 Experimental Design
The primary purpose of this study was to investigate the relationship between task-induced mental fatigue and quantitative changes in cerebral blood flow as measured by ASL. Our experimental protocol (described below) was designed to maximize group differences in the relationship between rCBF and mental fatigue among HC and ME/CFS subjects. To this end, ASL data were acquired before, during and after the cognitive task lasting for 18 minutes. This scan allowed the examination of relevant differences in rCBF related to a fatiguing task over time. Mental fatigue was induced in all subjects using a modified version of the Paced Auditory Serial Addition Test (PASAT), which is a difficult cognitive task involving attention, working memory, and executive function [34].
2.3 Ratings of Fatigue, Pain, Anxiety, and Depression
Prior to fMRI scanning the subjects used mechanical visual analogue scales (VAS; 0-10) for ratings of clinical fatigue and pain [35]. These scales were anchored on the left by “no fatigue/pain at all” and on the right by “the most intense fatigue/pain imaginable”. Resulting values were rescaled to 0-100 by multiplying participants’ ratings by 10. Although the HC subjects were required to be fatigue and pain free at enrollment, they were asked to provide ratings before and after the testing sessions to capture new onset symptoms (e.g. fatigue, back pain, headaches, etc.).
Similar to assessments of fatigue and pain, all subjects used mechanical VAS for ratings of anxiety and depression, which were anchored on the left by no anxiety/depression and on the right by most intense anxiety/depression imaginable.
2.4 Mental Fatigue Induction
Task-related fatigue was induced using the PASAT as reported recently [32]. Immediately before the PASAT the subjects rated their overall fatigue using an electronic VAS (eVAS) which ranged from 0 (‘no fatigue’) to 100 (‘most intense fatigue imaginable’). The VAS was presented throughout the scan at the center of a large monitor above the real-time display of PASAT calculation feed-back, i.e. correct or incorrect. During PASAT, they were asked to push one of two buttons of a response box with their right hand to indicate whether the most recent two numbers of the PASAT summed up to the pre-specified target value of 13. Originally developed by Gronwall [36] in 1977, the PASAT is a well-validated cognitive task of auditory information processing speed and flexibility, as well as calculation ability [34]. Additionally, the PASAT has good psychometric properties including high levels of internal consistency and test-retest reliability [34]. Moreover, it has been previously used as a cognitive challenge during functional brain imaging of fatigue [27,28].
A modified version of the PASAT was used for this study which was computer generated to ensure standardization of the rate of stimulus presentation. Auditory stimuli consisting of either single- or two-digit numbers were presented at two different frequencies via MR-compatible headphones (Avotec, Stuart, FL, USA) connected to a Dell Latitude laptop (Dell Inc., Round Rock, TX, USA) running PsychoPy v.1.77 [37,38]. During the first stage (Stage 1) each number was presented once every three seconds for 3 minutes (60 numbers). During Stage 2, numbers were presented once every two seconds over the next nine minutes (270 numbers). No breaks were provided during PASAT performance. Subjects were asked to add each new number to the preceding one and to determine whether the sum of these two numbers was 13 (target value). 35% of number combinations added up to 13. The subjects indicated their response (yes or no) to the mental task by pushing one of two buttons on an electronic response box with their right hand, with visual feedback (i.e., correct vs. incorrect) provided after each response on a monitor. After 12 minutes, PASAT performance ended and participants rested while rating their fatigue from the end of PASAT to the end of the resting period (6 minutes). Participants practiced performing the PASAT in the laboratory prior to scanning.
2.5 fMRI Image Acquisition and Analysis
2.5.1 Image Acquisition Parameters
A research dedicated whole body Phillips Achieva 3T MRI scanner and 32-channel phased array head coil were used to collect all imaging data. All subjects were placed into the scanner in the head first, supine position. During the scanning session, a high-resolution structural MRI and two pseudo-continuous ASL (pCASL) scans were performed. The first ASL scan (resting baseline) lasted 6-minutes and the duration of the second ASL scan (PASAT and subsequent recovery) was 18-minutes. Two dummy scans (not included in the total scan time) were collected prior to each ASL scan. The imaging protocol resulted in 45 and 135 pairs of control and tag images for the resting and task-related ASL scans, respectively.
On each subject, whole brain structural images were acquired using a three-dimensional (3D) T1-weighted magnetization-prepared rapid gradient-echo (MP-RAGE) sequence with a field-of-view (FOV) of 240 mm, in-plane resolution of 1mm × 1mm, 176 contiguous sagittal slices of 1mm thickness, and TR/TE/Flip angle = 7.2ms/3.2ms/8°. The total acquisition time was 4 min 34 seconds. ASL data were acquired using a two-dimensional (2D) pseudo-continuous arterial spin labeling (pCASL) [39] single shot echoplanar imaging (EPI) sequence with a field-of-view (FOV) of 230 mm, in-plane resolution of 3.2mm × 3.2mm, 20 axial slices of 6mm thickness, 1mm inter-slice gap, and TR/TE/Flip angle = 4s/11ms/90°. Background suppression was not employed. Arterial spin labeling was applied at a plane that was 30.5 mm inferior to the lowest imaging slice with a labeling during of 1500ms, and a post labeling delay time of 1800 ms.
2.5.2 Image Analysis
pCASL scans were corrected for subject motion using a rigid body 6 parameter algorithm implemented in the software Statistical Parametric Mapping 8 (SPM8). Label and control images were motion corrected independently as described by [40]. The images were coregistered to T1-weighted structural images registered to standard MNI space, resampled to 3×3×3mm voxel resolution (27mm3 voxel volume), spatially smoothed using a Gaussian filter with an 8 mm full-width-half-maximum (FWHM) kernel to improve the signal to noise ratio, and normalized to Montreal Neurological Institute (MNI) template space. Each tag and control pair was subtracted to create 135 perfusion-weighted images, which were then masked using the standard MNI template brain mask distributed with SPM8. In order to examine changes in CBF associated with fatigue resulting from PASAT performance, the 18-minute ASL scan was divided in to 6 discrete 3-minute segments (segment 1-4: PASAT task; segment 5-6: recovery period). Each of the 6 perfusion-weighted segments was then averaged to create 6 mean images of cerebral perfusion for each subject (i.e., one per 3-minute segment). All 45 perfusion-weighted images from the resting state scan were averaged together and CBF measures extracted using a whole brain mask in order to assess baseline global CBF.
These mean perfusion weighted images were used to create a quantified map of cerebral blood flow (CBF) using the software ASLtbx [41]. In the current study, cerebral blood flow (CBF) was quantified as ml/100g/min using the equation described in [40]. Parameter values for this equation used in the current study have been previously published [31]. In this study, control images were used as the equilibrium magnetization signals (i.e., M0) in the quantification of CBF, as implemented in the ASLtbx software. With a TR=4s, and T1 (GM)=1200 ms, T1 relaxation would induce a negligible scaling factor in the CBF quantification. However, this factor was adjusted for in the CBF quantification function used in this study [see ref 31]. A separate proton density weighted scan was not acquired in this study for the following reasons: 1. Concerns regarding potential motion artifacts between the pCASL scan and the PD scan; 2. The added scanning time of the PD scan; and 3. Possible differences in image signal scaling factor between the PD and pCASL scans.. ASL images were visually inspected to ensure the absence of gross artifacts/abnormalities, resulting in the removal of one subject. ASL data have been shown to be less sensitive to motion effects than blood oxygenation level dependent (BOLD) fMRI [42], and the use of mean images across each segment reduces concerns regarding motion-related artifacts on CBF estimates. However, the Artifact Detection Toolbox [41] was used to identify 4D ASL volumes with excessive motion, resulting in the exclusion of one additional subject. Overall, quality checks resulted in the removal of one ME/CFS and HC participant each. Multivariate analysis of variance (MANOVA) indicated that mean x-axis (Mx-axis = .017 mm, SD = .005), y-axis (My-axis = .042 mm, SD = .019), and z-axis translation (Mz-axis = .064 mm, SD = .029) during the PASAT were minimal, and did not differ between HC and ME/CFS (F(3,26) = 1.39, p = .27; Cohen’s ds < .82). Similarly, pitch (Mpitch = .00092 rad, SD = .00039), roll (Mroll = .00035 rad, SD = .00011), and yaw (Myaw = .00032 rad, SD = .000099) during PASAT scans did not differ between groups (F(3,26) = 1.05, p = .39; Cohen’s ds < .56).
2.5.3 Analysis of Global CBF at Rest
In order to assess group differences in resting global (i.e., whole-brain) CBF [43], mean CBF values within the MNI whole-brain mask applied to the mn resting state CBF image were extracted for each subject. In addition, GM as well as WM masks were applied and voxel-wise analysis was used for analysis of resting CBF. Group differences were evaluated using an independent t-test.
2.5.4 Task Related ASL and a Priori Regions of Interest Analysis
A mask of bilateral regions of interest (ROIs) previously shown to be associated with ME/CFS [4,6,7,27,44–46] was constructed using the WFU pickatlas toolbox v3.0.5 [47] based on the Anatomical Automatic Labeling (AAL) atlas [48]. These ROIs included the insula, inferior frontal gyrus (IFG), middle frontal gyrus (MFG), parahippocampal gyrus, anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), angular gyrus, hippocampus, precuneus, caudate, pallidum, thalamus, cerebellum, and superior temporal gyrus. This ROI mask was used for all analyses in order to limit the overall number of voxel-wise comparisons. Further multiple comparisons correction for voxel-wise analyses was achieved through the use of weight-based false discovery rate (FDR) correction implemented in the NeuroElf toolbox v1.0 for MATLAB (t > 4.11; pFDR < .05).
Potential effects of group, segment, and their interaction on mean CBF values in regions within the a priori mask were assessed using repeated measures analysis of variance (rmANOVA) in SPSS 22 (IBM Corp., Armonk, NY, USA). In order to identify regions associated with the experience of fatigue, difference CBF maps were generated for each subject by subtracting CBF maps associated with segment 1 (consisting of the first 3 minutes of the PASAT) from those from segment 4 (the last 3 minutes of the PASAT). Difference maps generated by subtracting CBF maps from segment 4 from segment 6 (the last three minutes of the recovery period), in turn, were used to identify regions associated with the recovery from fatigue. Independent t-tests were used to assess group effects within the CBF difference maps. Then, analysis of covariance (ANCOVA) in SPM12 was used to identify voxels within the a priori ROI mask during both fatigue induction and recovery where the association between CBF and fatigue differed significantly between ME/CFS and HC. Where significant clusters were identified, CBF values were extracted from difference maps for each subject in order to decompose the detected interactions and for plotting purposes. Differences between ME/CFS and HC in the correlation between rCBF and fatigue were tested by comparing Fisher’s r-to-z transformed correlation coefficients.
2.5.5 Demographic and Behavioral Analyses
Analyses of demographic, affective, and task-related variables (i.e., fatigue ratings and performance) were conducted using SPSS 22. Potential differences in demographic and affective variables between ME/CFS and HC were assessed using independent t-tests. Group and time effects on in-scanner fatigue ratings were assessed using rmANOVA.
The sample size for this study was determined based on previous studies of functional brain abnormalities in ME/CFS [22,23,27,28]. These studies have indicated statistically large differences between HC and ME/CFS patients (e.g., Cohen’s d of ~1 in CBF between affected and unaffected individuals). Thus, a sample size of 30 participants was expected to provide ~80% power to detect effects of this magnitude at alpha = .05, assuming two-tailed hypothesis tests.
3. Results
3.1 Study Subjects
One ME/CFS and one HC participant were excluded from the analysis due to poor image quality, resulting in a final sample of 16 ME/CFS (15 women) and 15 HC. An independent t-test revealed no significant age differences between the groups (t(29) = 0.09, p = 0.93; Cohen’s d = .03). Mean (SD) duration of illness for ME/CFS subjects was 12.46 (9.27) years.
3.1.1 Baseline Fatigue, Pain, Anxiety, and Depression
Mean (SD) ratings of baseline fatigue, pain, anxiety and depression for HC and ME/CFS subjects are listed in Table 1. Independent t-tests revealed significantly higher baseline scores of ME/CFS subjects compared to HC on all measures (p ≤ .001).
Table 1
Average VAS Ratings of Study Subjects at Baseline
| Fatigue (SD) (0-100) | Pain (SD) (0-100) | Anxiety (SD) (0-100) | Depression (SD) (0-100) | |
|---|---|---|---|---|
| Healthy Controls | 7.73 (9.48) | 1.87 (5.46) | 2.67 (6.11) | 4.47 (15.67) |
| ME/CFS Subjects | 53.31 (18.74) | 43.94 (22.71) | 41.19 (28.79) | 36.06 (25.76) |
| p-value | < .001 | < .001 | < .001 | < .001 |
3.2 Task Related Fatigue
During the ASL scan all subjects performed the modified PASAT to induce task related fatigue. In addition, they also provided ratings of their fatigue before and after the PASAT using the eVAS (0 - 100) (see Figure 1). After the PASAT all subjects indicated that they had no or only minor problems comprehending the numbers provided via headphones. A repeated measures ANOVA of task related fatigue with time as within and group as between subjects’ factors revealed a significant quadratic effect of time (F(1,29) = 93.09.; p < .0001; η2p = .76), as well a significant group difference, with ME/CFS reporting greater fatigue than HC (F(1,29) = 13.12; p = .001; η2p = .31). The quadratic time by group interaction effect did not achieve significance (F(1,29) = 2.95, p > .09; η2p = .09), indicating the change of task-related fatigue did not differ significantly between groups. Individual fatigue ratings of ME/CFS and HC participants during PASAT, and subsequent rest period are shown in Supplemental Figure 1.
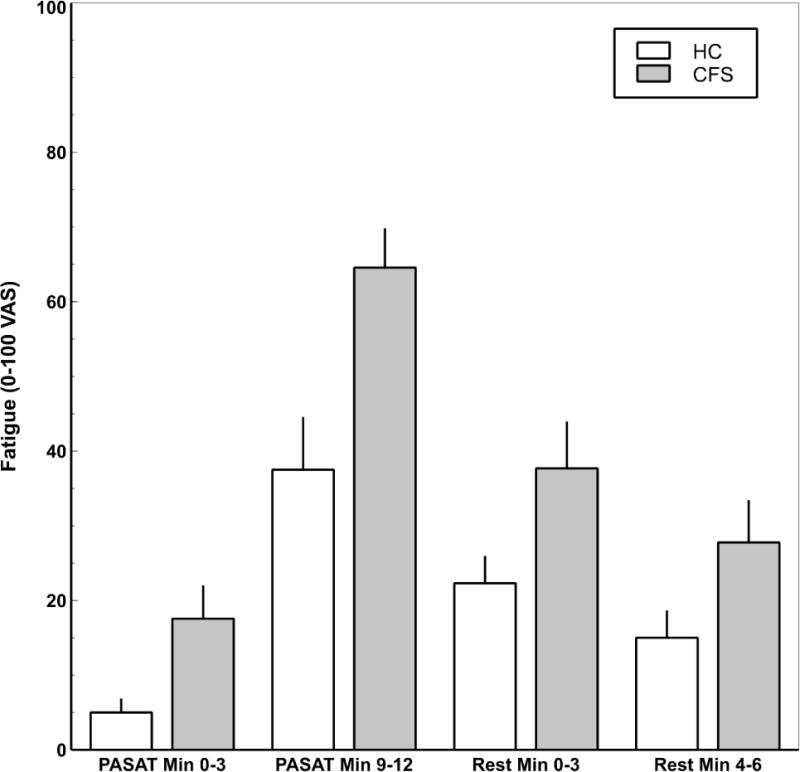
Mean (SEM) fatigue ratings by group and segment. Repeated measures ANOVA with time as within and group as between subjects factors revealed a significant quadratic main effect for segment (F(1,29) = 93.09.; p < .0001; η2p = .76). ME/CFS patients reported greater fatigue than HC (F(1,29) = 13.12; p = .001; η2p = .31). The segment by group interaction effect did not achieve significance (F(1,29) = 2.95, p > .09; η2p = .09).
3.3 Resting Global CBF
Using a whole brain mask, independent t-tests indicated that resting global CBF did not differ significantly between ME/CFS subjects and HC (MCFS = 46.06, SD = 8.38; MHC = 46.95, SD =7.78; t(29) = .31, p = .76; Cohen’s d = .11). Resting CBF in GM was also not significantly different (MCFS = 49.15, SD = 8.79; MHC = 49.92, SD = 8.65; t(29) = .24, p = .81; Cohen’s d = .09). Similarly, when a WM mask and even voxelwise analysis of CBF were used, no significant group difference (p = .71) and voxelwise difference (p> .05 after FDR corrections) were found, respectively.
3.4 PASAT Related rCBF Changes
3.4.1 CBF Within the a priori ROI mask
Repeated measures ANOVA of CBF with regions encompassing the a priori ROI mask indicated there was a significant main effect of time (F(6,174) = 4.68, p < .0001; η2p = .14) such that CBF appeared to increase from baseline to segment 1 of the PASAT, then tended to decline over the course of the 18-minute ASL scan (Figure 2). Subsequent pairwise comparisons indicated the increase in rCBF from baseline to the start of the PASAT (t(30) = 3.51, p = .001; Cohen’s d = .68) was statistically significant. Furthermore, rCBF at the beginning of the PASAT was significantly greater than at 75% completion, (t(30) = 2.26, p = .03; Cohen’s d = .42), the end of the PASAT (t(30) = 3.44, p = .002; Cohen’s d = .72), the beginning of the rest period (t(30) = 3.42, p = .002; Cohen’s d = .62), and the end of the rest (t(30) = 3.28, p = .003; Cohen’s d = .58). rCBF 50% of the way through the PASAT was significantly greater than at the beginning of rest (t(30) = 2.86, p = .008; Cohen’s d = .53) and the end of rest (t(30) = 2.98, p = .006; Cohen’s d = .57), and rCBF 75% of the way through the PASAT was significantly greater than at the beginning of the rest period (t(30) = 2.15, p = .04; Cohen’s d = .38). rCBF of baseline, the end of the PASAT, the beginning of rest, and the end of the rest period did not differ significantly from one another (ps > .22). The main effect of participant group (F1,29 = .12, p = .73; η2p = .004) and segment by group interaction (F(6,174) = .41; p = .87; η2p = .01) did not achieve significance. Individual CBF changes of ME/CFS and HC participants during baseline, PASAT, and subsequent rest period are demonstrated in Supplemental Figure 2. Supplemental Figure 3 shows average axial ASL images of CBF (ml/100g/min) of ME/CFS participants and healthy controls during baseline, PASAT and rest.
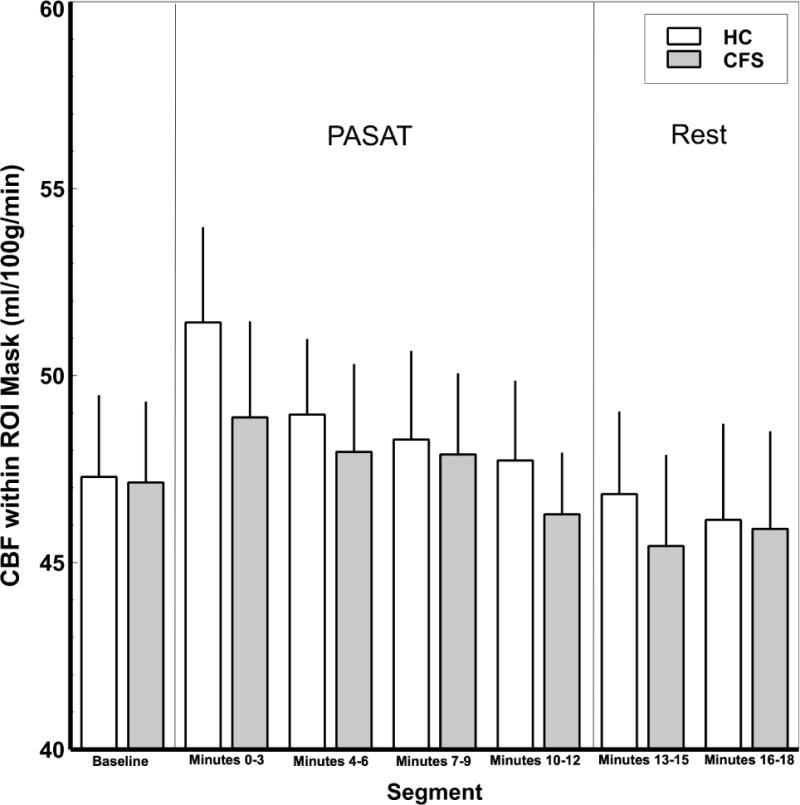
Mean (SEM) CBF within the a priori mask of fatigue-related regions in ME/CFS patients and HC. A main effect of segment was detected such that the mean rCBF increased from resting baseline to PASAT segment 1 (minutes 0-3), and then declined over the course of the 18-minute ASL scan to baseline levels (F(6,174) = 4.68, p < .0001; η2p = .14). Pairwise comparisons indicated a significant increase in rCBF from baseline to PASAT segment 1 (minutes 0-3) (p = .001). rCBF at PASAT segment 1 was significantly greater than during PASAT segment 3 (minutes 6-9; p = .03), PASAT Segment 4 (minutes 9-12; p = .002), minutes 0-3 of the rest period (p = .002), and minutes 4-6 of the rest period (p = .003). rCBF during PASAT segment 2 (minutes 4-6) was significantly greater than during both rest periods (p < .008), and rCBF during PASAT segment 3 (minutes 6-9) was significantly greater than minutes 0-3 of the rest period (p = .04). rCBF of baseline, PASAT segment 4 (minutes 9-12), and both rest periods did not differ significantly from one another (ps > .22).
3.4.2 Contrast 1: rCBF at Beginning to rCBF at End of Cognitive Task
Analyses indicated no clusters within the a priori ROI mask where CBF changes from the beginning to the end of the PASAT differed between ME/CFS subjects and HC (pFDR > .05).
3.4.3 Contrast 2: rCBF at End of Cognitive Task to rCBF at End of Recovery Period
During the recovery period, a small cluster within left inferior frontal gyrus (−20, 15, −19; k = 2; Peak t = 4.45; Mean t = 4.31) was detected where rCBF increased in ME/CFS subjects but not controls.
3.5 Group Effects on the Relationship between CBF and Fatigue Changes
3.5.1 Analysis of Task Related Fatigue
ANCOVA revealed several clusters/voxels within the ROI analysis mask where the association between change in rCBF from the beginning to the end of the PASAT and change in fatigue ratings differed significantly between ME/CFS and HC. These regions included bilateral STG and left cerebellum (Table 2). Characterization of these interaction effects by group indicated that larger rCBF increases in the right STG was negatively associated with fatigue in HC (r = −.74, p = .002; Figure 3a). In contrast, rCBF in left STG, right STG, and left cerebellum was positively correlated with fatigue over the course of the PASAT in ME/CFS (rs > .64; ps < .007; Figure 3b). rCBF in Left STG and cerebellum was not associated with fatigue in HC (rs > −.32, ps > .24). Comparisons of Fisher’s r to z-transformed correlation coefficients indicated that each correlation differed significantly between ME/CFS and HC (p < .0004).
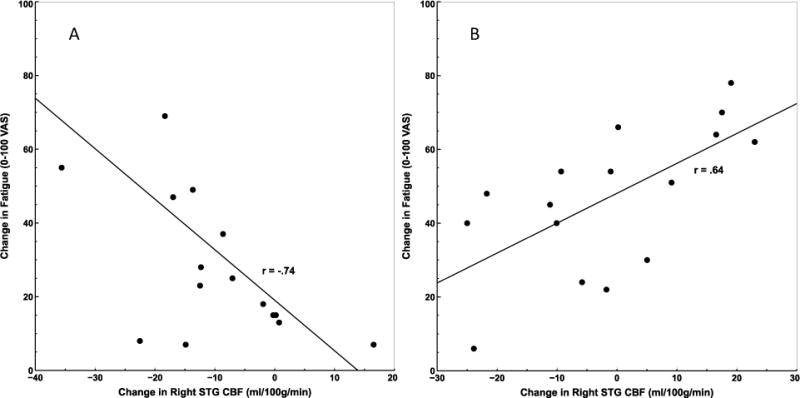
Correlation between change in rCBF at right STG and change in fatigue from the beginning to the end of the PASAT in HC (A) and ME/CFS patients (B). For HC, increased blood flow to STG was associated with less fatigue from PASAT performance, while the opposite was true for ME/CFS patients.
Table 2
Regions demonstrating group differences in the relationship between rCBF and fatigue changes from the beginning (segment 1) to the end of the PASAT (segment 4)
| Region | MNI Coordinates (x,y,z) | Cluster extent (mm3) | Peak/mean t-value | Correlation with change in fatigue (HC) | Correlation with change in fatigue (ME/CFS) |
|---|---|---|---|---|---|
| Left Superior Temporal Gyrus | −62, −9, 1 | 54 | 4.57/4.39 | −.32 | .77 |
| Right Superior Temporal Gyrus | 64, −52, 19 | 81 | 4.41/4.28 | −.74 | .64 |
| Left Cerebellum | −42, −48, −30 | 54 | 4.80/4.62 | −.32 | .84 |
3.5.2 Analysis of Post-Task Related rCBF and Fatigue
Participant group moderated the relationship between rCBF and fatigue changes for several regions during the 6-minute recovery period, including left fusiform gyrus, right STG, and left precuneus (Table 3; Figure 4). Correlations conducted by group indicated that reductions in rCBF in these regions from the end of the PASAT to the end of the recovery period were associated with better recovery of fatigue for ME/CFS patients (rs > .69, ps < .002; Figure 5b). In contrast, negative correlations were detected for HC (rs < −.52, ps < .05; Figure 5a), suggesting increases in rCBF in these regions during the recovery period were associated with better recovery of fatigue for this group (i.e., fatigue reduction). Comparisons of Fisher’s r to z transformed correlation coefficients indicated each correlation differed significantly between ME/CFS and HC (p < .0001).
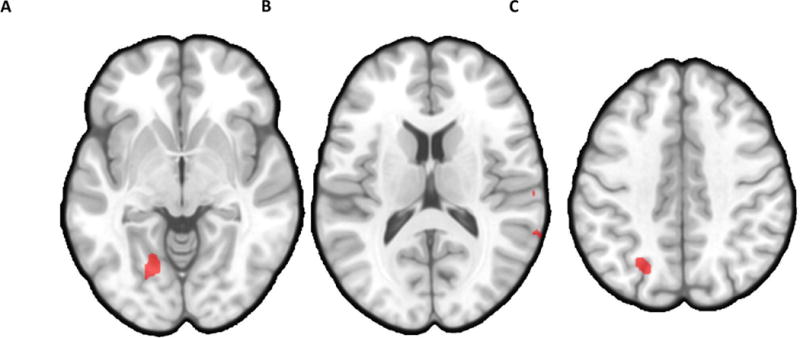
Axial views illustrating clusters where decreased blood flow over the course of the 6-minute recovery period was associated with larger reductions in fatigue for ME/CFS patients, but not HC, in A) left fusiform gyrus (−22x, −62y, −7z; 1242 mm3); B) right superior temporal gyrus (61x, −30y, 18z; 216 mm3; 64x, −65y, 13z; 135 mm3) ; and C) left precuneus (−25x, −67y, 38z; 540 mm3).
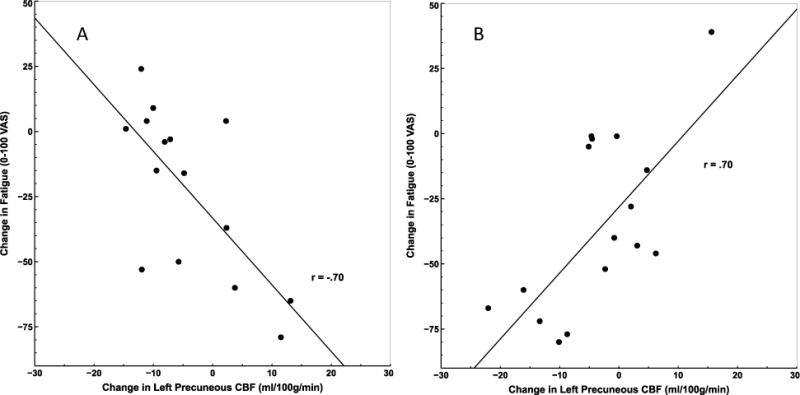
Correlation between change in rCBF at left precuneus and change in fatigue from the end of the PASAT to the end of the rest period in HC (A) and ME/CFS (B). ME/CFS patients who declined in CBF in left precuneus during recovery showed a greater reduction in fatigue. HC who declined showed a smaller reduction.
Table 3
Regions demonstrating group differences in the relationship between rCBF and fatigue changes from the end of the PASAT (segment 4) to the end of the recovery period (segment 6)
| Region | MNI Coordinates (x,y,z) | Cluster extent (mm3) | Peak/mean t-value | Correlation with change in fatigue (HC) | Correlation with change in fatigue (ME/CFS) |
|---|---|---|---|---|---|
| Left Fusiform Gyrus | −22, −62, −7 | 1242 | 5.40/4.45 | −.64 | .76 |
| Right Superior Temporal Gyrus | 61, −30, 18 | 216 | 4.66/4.31 | −.59 | .69 |
| Right Superior Temporal Gyrus | 64, −52, 13 | 135 | 4.95/4.59 | −.52 | .76 |
| Left Precuneus | −25, −67, 38 | 540 | 4.89/4.43 | −.52 | .70 |
4. Discussion
Despite high levels of clinical fatigue, our fMRI study of ME/CFS patients did not demonstrate cerebral blood flow abnormalities at rest, a result supported by several other investigations [25,49]. Several previous ME/CFS studies, using different techniques for CBF measurements, demonstrated whole brain hypoperfusion of ME/CFS patients at rest [20,21,23]. However, the temporal and spatial resolution of ASL functional brain imaging as used in the current study is superior in those aspects to previous methods including PET and SPECT imaging. As expected, the PASAT resulted in significant fatigue increases in both ME/CFS and HC subjects, which were associated with similar global as well as regional CBF changes in both groups. Interestingly, CBF increased over the first 3 min of the PASAT and then gradually returned to baseline over the next 9 min. This was particularly remarkable as task related fatigue steadily increased during the same time period. This discrepancy between fatigue and CBF changes seems to suggest that there were sufficient cerebral resources available in both groups to deal with the metabolic demands of this difficult task. This result, however, does not necessarily suggest that interactions between brain regions were similar between HC and ME/CFS patients. Of particular interest are the different correlation of rCBF with fatigue in brain regions associated with memory and cognition in HC and ME/CFS patients. These different association between rCBF and fatigue were apparent both during fatigue induction and the 6 min recovery period.
Overall, these data shed doubt on the essential role of widespread cerebral hypoperfusion in the etiology of ME/CFS. Rather, as we have previously demonstrated, abnormalities in functional interactions between fatigue-related brain regions may be better explanations for ME/CFS symptomatology. Supportive of this hypothesis was the finding that ME/CFS patients and HC demonstrated discrepancies in the association between task-related CBF changes and fatigue in several regions within an a priori ROI mask that was based on previous structural and functional neuroimaging studies of ME/CFS patients. These relationships should be interpreted within the context of the PASAT, which is an auditory cognition task. Coordinates within the STG clusters correspond to Brodmann area 22, or auditory association cortex. Greater CBF to this area during the auditory PASAT is consistent with greater metabolic demand and neuronal activity. rCBF decreases in the STG following extended performance of a fatiguing psychomotor vigilance task have been previously documented in healthy subjects [50], although the association between STG blood flow and fatigue was not specifically examined. Furthermore, one study found that ME/CFS patients had significantly greater BOLD activation of STG during PASAT performance than HC [27]. The cerebellum has also been implicated as important for verbal working memory, as well as broader aspects of cognitive function germane to PASAT performance, including motor response, regulation of attention, and performance monitoring [14,51]. Compared to HC, greater functional activation in the left cerebellum during the PASAT has been previously demonstrated in ME/CFS patients [27].
In addition, given that alterations in rCBF can result in changes of functional brain networks [52], it is possible that individual differences in rCBF response to PASAT performance in critical nodes resulted in alterations of fatigue-related networks in a manner moderated by ME/CFS status. Indeed, our group has recently published results indicating perturbations in functional connectivity related to both resting and task-related fatigue [31,32] between brain regions where structural or functional alterations have been previously noted in ME/CFS patients (e.g., [7,43,45,46,53,54]. Further interrogation of such nodes/networks by directly modulating their function through pharmacological means or via transcranial magnetic stimulation may help elucidate the neural underpinnings of fatigue, as well as potential treatments for ME/CFS.
Our results demonstrated some commonalities between PASAT performance and subsequent recovery with regard to brain regions where ME/CFS status moderated the relationship between changes in CBF and fatigue. Consistent with PASAT performance, decreasing CBF in the right STG, precuneus, and left fusiform gyrus over the recovery period was associated with greater reductions in fatigue ratings for ME/CFS patients, suggesting that those patients who demonstrated the greatest reductions of rCBF to regions associated with PASAT experienced the most effective recovery from fatigue. The precuneus, located in the medial posterior parietal lobe, is thought to be critical for a number of neurocognitive functions, including attention, motor coordination, and sense of self/self-reflection [55]. We have previously reported significant alterations in functional connectivity of the precuneus in ME/CFS patients to brain regions associated with movement planning and motor function (supplementary motor area, precentral gyrus, basal ganglia), cognitive control (superior frontal gyrus), and sensory function (thalamus) both during resting state and task performance [32] [31]. The divergent relationship between rCBF and fatigue noted in the fusiform gyrus for ME/CFS and HC participants builds on existing evidence that metabolic [54], structural [46], and functional perturbations [30–32] in occipital lobe structures may at least partially underlie ME/CFS symptomatology.
Although regions identified as having discrepant associations between blood flow and fatigue during PASAT performance and subsequent recovery in this study are broadly consistent with those showing functional abnormalities in previous work, the mechanism(s) underlying this effect are unclear. In essence, our results indicated that, in ME/CFS, fatigue and CBF to regions associated with task performance are strongly correlated. We speculate that ME/CFS patients may recruit more resources within the STG and cerebellum in order to maintain PASAT performance, a process associated with greater fatigue. When the PASAT ended and participants were allowed to recover, the same association between CBF and fatigue was detected, suggesting re-allocation of resources from areas involved in task performance correlated with better recovery. In contrast, HC participants showed a negative association between CBF and fatigue, suggesting greater blood flow to relevant areas may have produced more efficient, and therefore less effortful/fatiguing, task performance. This inverse association between CBF and fatigue was maintained during recovery, which may be suggestive of an active recovery process.
4.4 Study Strengths and Limitations
The results of our study provide evidence for abnormal associations between CBF changes and fatigue in ME/CFS. Moreover, such abnormalities may also underlie ME/CFS symptomatology. As expected, ME/CFS patients reported greater fatigue throughout the mental task than HC. However, PASAT performance increased fatigue levels at a similar rate for both HC and ME/CFS patients. The use of ASL represents an additional strength of our study because of its a) lower sensitivity to movement artifacts compared to BOLD fMRI; and b) direct measurement of CBF, which better reflects underlying neuronal activity [42].
Several limitations should be kept in mind when interpreting the results of this study. First, the cross-sectional nature of the study precludes conclusions whether the disparate relationships between CBF changes and fatigue of ME/CFS patients and HC during cognitive performance are cause or effect of ME/CFS status. In addition, only small differences in rCBF change within the a priori ROI mask over the course of the PASAT recovery were detected between HC and ME/CFS. These data suggest that rCBF responses to sustained cognitive effort are largely similar between ME/CFS and HC. Finally, technical refinements of the pCASL imaging method used in this study, including use of B0-map distortion correction and background suppression, may help to improve sensitivity of the method to perturbations in CBF associated with ME/CFS and related disorders in future studies.
Conclusions
The results of this study add to existing data regarding functional brain abnormalities of ME/CFS by showing that the relationship between CBF in fatigue-related brain regions and self-reported fatigue differs significantly between ME/CFS patients and HC. Such functional abnormalities of ME/CFS patients were most pronounced during the recovery period from a challenging mental task (i.e., the PASAT). The positive linear relationship between rCBF and fatigue ratings in STG, cerebellum, precuneus, and fusiform gyrus of ME/CFS patients suggests that these structures play a key role in modulating the fatigue associated with cognitive effort, which is one of the hallmarks of ME/CFS.
Supplementary Material
1
Supplemental Figure 1. Individual fatigue ratings (0-100) during PASAT and subsequent rest-period in ME/CFS participants (A) and healthy controls (B).
2
Supplemental Figure 2. Individual CBF changes (ml/100g/min) during baseline, PASAT and rest in ME/CFS participants (A) and healthy controls (B).
3
Supplemental Figure 3. Average ASL CBF (ml/100g/min) during baseline, PASAT and rest in ME/CFS participants and healthy controls.
Acknowledgments
The expert assistance Ricky Madhavan, and Yesenia M. Lucas is gratefully acknowledged. We also would like to thank Donald D. Price, Ph.D. (deceased) for his valuable input.
Funding
This study was supported by NIH Nursing Research grant R01 NR014049 (PI: R. Staud) and NIH/Center for Advancing Translational Science grant UL1 TR000064
Footnotes
Ethics approval and consent to participate
Ethics approval was granted by the University of Florida Institutional Research Ethics Board (IRB-01). All participants in this study provided informed consent to participate.
Competing interests
The authors declare that they have no competing interests.
Reference List
Full text links
Read article at publisher's site: https://doi.org/10.1080/21641846.2018.1453919
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc5914525?pdf=render
Citations & impact
Impact metrics
Article citations
Herpesvirus Infection of Endothelial Cells as a Systemic Pathological Axis in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome.
Viruses, 16(4):572, 08 Apr 2024
Cited by: 0 articles | PMID: 38675914 | PMCID: PMC11053605
Review Free full text in Europe PMC
Subcortical and default mode network connectivity is impaired in myalgic encephalomyelitis/chronic fatigue syndrome.
Front Neurosci, 17:1318094, 29 Jan 2024
Cited by: 1 article | PMID: 38347875 | PMCID: PMC10859529
Hypothalamus volumes in adolescent Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): impact of self-reported fatigue and illness duration.
Brain Struct Funct, 228(7):1741-1754, 03 Aug 2023
Cited by: 0 articles | PMID: 37537279 | PMCID: PMC10471696
ME/CFS and Long COVID share similar symptoms and biological abnormalities: road map to the literature.
Front Med (Lausanne), 10:1187163, 02 Jun 2023
Cited by: 55 articles | PMID: 37342500 | PMCID: PMC10278546
Review Free full text in Europe PMC
Cardiovascular and haematological pathology in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): A role for viruses.
Blood Rev, 60:101075, 20 Mar 2023
Cited by: 13 articles | PMID: 36963989 | PMCID: PMC10027292
Review Free full text in Europe PMC
Go to all (15) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Abnormal resting state functional connectivity in patients with chronic fatigue syndrome: an arterial spin-labeling fMRI study.
Magn Reson Imaging, 34(4):603-608, 18 Dec 2015
Cited by: 56 articles | PMID: 26708036 | PMCID: PMC4801728
Limbic Perfusion Is Reduced in Patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS).
Tomography, 7(4):675-687, 01 Nov 2021
Cited by: 11 articles | PMID: 34842817 | PMCID: PMC8628916
Reductions in Cerebral Blood Flow Can Be Provoked by Sitting in Severe Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients.
Healthcare (Basel), 8(4):E394, 11 Oct 2020
Cited by: 19 articles | PMID: 33050553 | PMCID: PMC7712289
Single-photon emission computerized tomography and neurocognitive function in patients with chronic fatigue syndrome.
Psychosom Med, 65(1):129-136, 01 Jan 2003
Cited by: 40 articles | PMID: 12554824
Funding
Funders who supported this work.
NCATS NIH HHS (1)
Grant ID: UL1 TR001427
NIH (1)
Grant ID: R01 NR014049
NINR NIH HHS (1)
Grant ID: R01 NR014049





