Abstract
Free full text

Development of an Unrelated Donor Selection Score Predictive of Survival after HCT: Donor Age Matters Most
Abstract
Donor factors, in addition to HLA matching status, have been associated with recipient survival in unrelated donor (URD) hematopoietic cell transplantation (HCT), however there is no hierarchical algorithm that weights the characteristics of individual donors against each other in a quantitative manner to facilitate donor selection. The goal of this study was to develop and validate a donor selection score that prioritizes donor characteristics associated with better survival in 8/8 HLA-matched URDs. Two separate patient/donor cohorts, the first receiving HCT between 1999–2011 (n=5952, c1), and the second between 2012–2014 (n=4510, c2) were included in the analysis. Both cohorts were randomly spilt, 2:1, into training and testing sets. Despite studying over 10,000 URD transplants, we were unable to validate a donor selection score. The only donor characteristic associated with better survival was younger age, with 2-year survival being 3% better when a donor 10 years younger is selected. These results support previous studies suggesting prioritization of a younger 8/8 HLA-matched donor. This large data set also shows that none of the other donor clinical factors tested were reproducibly associated with survival and hence, flexibility in selecting URDs based on other characteristics is justified. These data support a simplified URD selection process and have significant implications for URD registries.
INTRODUCTION
The degree of HLA matching between donors and recipients is the key donor variable impacting overall survival (OS) in unrelated donor (URD) hematopoietic cell transplantation (HCT). Numerous studies show inferior OS when transplantation occurs from an HLA-mismatched URD, making 8/8 HLA matching (HLA-A, -B, -C, -DRB1) the gold standard1–4.
Due to increases in the size and representation of worldwide volunteer donor registries, many patients, particularly those of Caucasian background, can identify more than one 8/8 HLA matched donor (approximately 70% of Caucasian patients searches through NMDP, Kevin Tram, personal communication, July 2017). This situation raises the question of whether one donor might be better than another and if so, what factors should be used to select the optimal donor. Prior studies have suggested that a number of factors may impact outcome including: non-genetic factors (including age1, 5, sex6, parity1, race/ethnicity7, cytomegalovirus (CMV) serostatus3, 8, 9), other HLA factors including the low expression loci (including DRB3/4/510, DQB111 and DPB112–15), as well as non-HLA genetic factors (such as natural killer cell immunoglobulin-like receptors (KIR)16, 17 and ABO type1, 18).
Studies addressing donor selection have examined one or more of these ‘secondary selection characteristics’ as predictors for survival, but both positive and negative findings are presented in the literature. Interpretation of the impact of these factors is further hindered by heterogeneity of the study populations, lack of statistical power, failure by investigators to consider all donor factors in combination, and lack of validation in an independent cohort. Besides prioritizing HLA matching of the four key loci, it remains unclear how to weigh the significance of an individual donor characteristic against others, and thus which factors to prioritize in donor selection. A universally agreed upon selection algorithm would be an important advance for the transplant community.
A recent study from the Center for International Blood and Marrow Transplant Research (CIBMTR) 1 including >10,000 HLA matched and mismatched URD pairs (across two non-overlapping time periods, with the most recent transplants in 2011) analyzed the association between donor factors and transplant complications. This study showed HLA mismatching and older donor age were associated with a worse survival, while ABO mismatching had a significantly negative impact on survival in patients transplanted in the earlier (but not later) time period. Of note, HLA-DPB1 matching status was not included in this study, as the clinical significance of matching for this HLA allele was less well explored at that time, and thus fewer transplants had been done using HLA-DPB1 matching status as a factor important in donor selection.
The current study is restricted to 8/8 HLA-matched unrelated donor pairs, many with HLA-DPB1 typing. We devised statistical methods to assess donor-associated variables, with the goal of developing and validating a donor selection score that prioritizes and weights donor characteristics associated with better survival in the 8/8 HLA-matched URD setting. We envisioned that this score could be used to identify the best donor in the setting of multiple potential donors, based on his or her composite characteristic score.
RECIPIENTS, MATERIAL AND METHODS
Data collection
The CIBMTR is a voluntary network of >450 transplant centers worldwide that reports data on consecutive transplantations. Patient, disease, and transplantation characteristics and outcome data are submitted on standardized forms at the time of transplantation (baseline) and at 100 days, 6 months, and annually or biannually thereafter. All patients provided written informed consent and the Institutional Review Board of the National Marrow Donor Program (NMDP) approved this study.
Study population
We included two large patient cohorts in this study: 1999–2011 (n=5952, c1) and 2012–2014 (n=4510, c2). All donor recipient pairs were required to have high resolution typing available for HLA-A, -B, -C, -DRB1 and to be matched for both HLA assignments at these four loci. Non-permissive T-cell epitope (TCE) DPB1 mismatches were determined according to the TCE3 algorithm as described by Crivello et al 19. Patients were adults (>18), transplanted for acute myelogenous leukemia (AML), acute lymphocytic leukemia (ALL), chronic myelogenous leukemia (CML), or myelodysplastic syndrome (MDS) using an unrelated donor. The earlier cohort (c1) was drawn from two previously published populations 13, 20, and a percentage of pairs were also included in the Kollman et al analysis 1.
Endpoint
Overall survival (OS) was the primary endpoint. Death from any cause was considered an event.
Statistical Methods
A total of 10,462 patients were available for analysis. First, the earlier cohort (c1) was randomly split into 2/3 (n=3969) for modeling/score development (training data set) and 1/3 (n=1983) for testing. Second, the more recent cohort (c2) was randomly split into a training (2/3; n=3051) and testing set (1/3; n=1459). In each analysis, Cox models were fit to assess the impact of donor characteristics while adjusting for significant recipient, disease, and transplant characteristics; p-values less than 0.05 were considered significant. All variables were checked for nonproportional hazards using time-dependent covariate and graphical approaches; stratification of the Cox model was used to account for identified nonproportional hazards. Interactions between donor and recipient characteristics were tested for, as were interactions between donor characteristics. Missing data were placed in a separate category for model building. Continuous donor variables such as donor age were evaluated using splines to examine the functional form of the relationship with survival visually, and then formally tested against a linear model using a likelihood ratio test. When more than one donor characteristic was associated with survival, donor score weights were assigned based on 1 point per approximately 4–5% increased relative risk (RR=1.04–1.05) of mortality. The donor score derived in the training sets was applied directly to the testing set using a Cox proportional hazards model.
The following recipient/disease/transplant characteristics were considered in the adjustment model: Age, self-identified race/ethnicity, Karnofsky performance score (KPS), disease/status, comorbidity, interval from diagnosis to transplant, conditioning intensity, use of total body irradiation (TBI), graft-versus-host disease (GVHD) prophylaxis, use of in vivo T-cell depletion (TCD), graft type, year of transplant, CMV status. The following donor characteristics were considered for the donor score: HLA-DQB1 matching, HLA-DPB1 matching (fully (allele)-matched, permissive TCE and non-permissive TCE matching status (combining graft-versus-host (GvH) and host-versus-graft (HvG) direction)), age, sex matching, parity, CMV matching, ABO matching, race/ethnicity matching.
RESULTS
Patient, donor and transplant characteristics of the training and testing sets from the two cohorts are shown in Table 1. HLA-DQB1 typing was available in over 95% of the population, while DPB1 typing was available for approximately 60% of pairs. There were no clinically or statistically significant differences in characteristics or outcomes between the training and testing sets within each cohort.
Table 1
Patient, donor and transplant characteristics for the two cohorts, 1999–2011 and 2012–2014, divided by training and testing sets
| Variable | 1999–2011 (c1) | 2012–2014 (c2) | ||
|---|---|---|---|---|
| Training N (%) | Testing N (%) | Training N (%) | Testing N (%) | |
| Number of patients | 3969 | 1983 | 3051 | 1459 |
| Number of centers | 174 | 143 | 128 | 122 |
| Recipient age at transplant | ||||
 20–29 years 20–29 years | 735 (19) | 334 (17) | 288 (9) | 141 (10) |
 30–39 years 30–39 years | 642 (16) | 327 (16) | 313 (10) | 134 (9) |
 40–49 years 40–49 years | 817 (21) | 449 (23) | 400 (13) | 201 (14) |
 50–59 years 50–59 years | 1015 (26) | 510 (26) | 680 (22) | 338 (23) |
 60 years and older 60 years and older | 759 (19) | 362 (18) | 1351 (45) | 636 (44) |
 Unknown Unknown | 1 (N/A) | 1 (N/A) | 19 (N/A) | 9 (N/A) |
| Recipient race | ||||
 Caucasian Caucasian | 3687 (95) | 1864 (95) | 2854 (96) | 1377 (96) |
 African-American African-American | 95 (2) | 35 (2) | 55 (2) | 19 (1) |
 Asian Asian | 60 (2) | 36 (2) | 62 (2) | 24 (2) |
 Pacific Islander Pacific Islander | 41 (1) | 24 (1) | 6 (<1) | 3 (<1) |
 Native American Native American | 16 (<1) | 3 (<1) | 10 (<1) | 4 (<1) |
 Other Other | 1 (<1) | 0 | 0 | 0 |
 Unknown Unknown | 69 (N/A) | 21 (N/A) | 64 (N/A) | 32 (N/A) |
| Recipient ethnicity | ||||
 Hispanic or Latino Hispanic or Latino | 174 (4) | 102 (5) | 151 (5) | 67 (5) |
 Non-Hispanic or non-Latino Non-Hispanic or non-Latino | 3309 (83) | 1652 (83) | 2837 (93) | 1358 (93) |
 Not answered Not answered | 486 (12) | 229 (12) | 63 (2) | 34 (2) |
| Recipient sex | ||||
 Male Male | 2211 (56) | 1066 (54) | 1752 (58) | 801 (55) |
 Female Female | 1757 (44) | 916 (46) | 1280 (42) | 649 (45) |
 Unknown Unknown | 1 (N/A) | 1 (N/A) | 19 (N/A) | 9 (N/A) |
| Karnofsky score | ||||
 10–80 10–80 | 1205 (30) | 614 (31) | 1254 (41) | 556 (38) |
 90–100 90–100 | 2493 (63) | 1220 (62) | 1744 (57) | 875 (60) |
 Missing Missing | 271 (7) | 149 (8) | 53 (2) | 28 (2) |
| HCT-comorbidity index score | ||||
 0 0 | 698 (18) | 337 (17) | 684 (23) | 302 (21) |
 1 1 | 288 (7) | 138 (7) | 438 (14) | 195 (13) |
 2 2 | 266 (7) | 135 (7) | 465 (15) | 228 (16) |
 3+ 3+ | 649 (16) | 331 (17) | 1442 (48) | 725 (50) |
 N/A, earlier than 2007 N/A, earlier than 2007 | 2042 (52) | 1036 (52) | 0 | 0 |
 Unknown Unknown | 26 (N/A) | 6 (N/A) | 22 (N/A) | 9 (N/A) |
| Time from diagnosis to donation | ||||
 0 ≤ 6 months 0 ≤ 6 months | 1593 (40) | 769 (39) | 1564 (52) | 736 (51) |
 6 months to < 1 year 6 months to < 1 year | 1081 (27) | 522 (26) | 749 (25) | 383 (26) |
 1 year or more 1 year or more | 1284 (32) | 680 (35) | 718 (24) | 331 (23) |
 Unknown Unknown | 11 (N/A) | 12 (N/A) | 20 (N/A) | 9 (N/A) |
 Median (range) Median (range) | 7 (−6–296) | 8 (−2–607) | 6 (0–308) | 6 (1–239) |
| Disease at transplant | ||||
 Acute myeloid leukemia Acute myeloid leukemia | 2181 (55) | 1108 (56) | 1854 (61) | 873 (60) |
 Acute lymphoblastic leukemia Acute lymphoblastic leukemia | 746 (19) | 340 (17) | 492 (16) | 245 (17) |
 Chronic myeloid leukemia Chronic myeloid leukemia | 474 (12) | 248 (13) | 44 (1) | 27 (2) |
 Myelodysplastic syndrome Myelodysplastic syndrome | 567 (14) | 286 (14) | 642 (21) | 305 (21) |
 Unknown Unknown | 1 (N/A) | 1 (N/A) | 19 (N/A) | 9 (N/A) |
| Disease status at transplant | ||||
 Early Early | 1924 (48) | 951 (48) | 1794 (59) | 890 (61) |
 Intermediate Intermediate | 851 (21) | 447 (23) | 441 (15) | 216 (15) |
 Advanced Advanced | 1193 (30) | 584 (29) | 797 (26) | 344 (24) |
 Unknown Unknown | 1 (N/A) | 1 (N/A) | 19 (N/A) | 9 (N/A) |
| Conditioning regimen | ||||
 Myeloablative Myeloablative | 2922 (74) | 1430 (72) | 1648 (54) | 765 (52) |
 Reduced intensity Reduced intensity | 1046 (26) | 552 (28) | 1384 (45) | 685 (47) |
 Other Other | 1 (<1) | 1 (<1) | 19 (1) | 9 (1) |
| GvHD prophylaxis | ||||
 Tacrolimus ± others Tacrolimus ± others | 2921 (74) | 1475 (74) | 2575 (84) | 1196 (82) |
 Cyclosporine ± others Cyclosporine ± others | 907 (23) | 442 (22) | 297 (10) | 164 (11) |
 Others Others | 141 (4) | 66 (3) | 179 (6) | 99 (7) |
| In vivo T cell depletion GvHD prophylaxis | ||||
 No No | 2763 (70) | 1344 (68) | 1949 (64) | 922 (64) |
 Yes Yes | 1205 (30) | 638 (32) | 1083 (36) | 528 (36) |
 Unknown Unknown | 1 (N/A) | 1 (N/A) | 19 (N/A) | 9 (N/A) |
| Total body irradiation | ||||
 No No | 2457 (62) | 1232 (63) | 2322 (77) | 1125 (78) |
 Yes Yes | 1490 (38) | 736 (37) | 710 (23) | 325 (22) |
 Unknown Unknown | 22 (N/A) | 15 (N/A) | 19 (N/A) | 9 (N/A) |
| Graft type | ||||
 Bone marrow Bone marrow | 1165 (29) | 593 (30) | 433 (14) | 216 (15) |
 Peripheral blood Peripheral blood | 2803 (71) | 1389 (70) | 2599 (86) | 1234 (85) |
 Unknown Unknown | 1 (N/A) | 1 (N/A) | 19 (N/A) | 9 (N/A) |
| Number of high-resolution HLA - DQB1 matches | ||||
 Double allele mismatch Double allele mismatch | 7 (<1) | 3 (<1) | 1 (<1) | 1 (<1) |
 Single allele mismatch Single allele mismatch | 258 (7) | 128 (7) | 160 (5) | 61 (4) |
 Fully matched Fully matched | 3484 (93) | 1762 (93) | 2791 (95) | 1355 (96) |
 Unknown Unknown | 220 (N/A) | 90 (N/A) | 99 (N/A) | 42 (N/A) |
| High-resolution HLA - DPB1 matches | ||||
 Fully matched Fully matched | 383 (17) | 204 (18) | 335 (18) | 144 (16) |
 Permissive Permissive | 1040 (45) | 520 (45) | 917 (49) | 478 (53) |
 Graft-vs.-host non-permissive Graft-vs.-host non-permissive | 448 (19) | 222 (19) | 336 (18) | 136 (15) |
 Host-vs.-graft non-permissive Host-vs.-graft non-permissive | 449 (19) | 219 (19) | 289 (15) | 139 (15) |
 Unknown Unknown | 1649 (N/A) | 818 (N/A) | 1174 (N/A) | 562 (N/A) |
| Donor race | ||||
 Caucasian Caucasian | 3464 (92) | 1725 (93) | 2175 (90) | 996 (90) |
 African-American African-American | 79 (2) | 28 (2) | 36 (1) | 12 (1) |
 Asian Asian | 60 (2) | 32 (2) | 61 (3) | 27 (2) |
 Pacific Islander Pacific Islander | 1 (<1) | 0 | 1 (<1) | 0 |
 Native American Native American | 40 (1) | 19 (1) | 19 (1) | 8 (1) |
 Other Other | 129 (3) | 60 (3) | 127 (5) | 58 (5) |
 Unknown Unknown | 196 (N/A) | 119 (N/A) | 632 (N/A) | 358 (N/A) |
| Donor/recipient race matching | ||||
 No No | 250 (7) | 110 (6) | 181 (8) | 84 (8) |
 Yes Yes | 3460 (93) | 1734 (94) | 2199 (92) | 1005 (92) |
 Unknown Unknown | 259 (N/A) | 139 (N/A) | 671 (N/A) | 370 (N/A) |
| Donor/recipient cytomegalovirus serostatus | ||||
 Negative/negative Negative/negative | 1141 (29) | 604 (30) | 784 (26) | 369 (25) |
 Negative/positive Negative/positive | 1424 (36) | 726 (37) | 1072 (35) | 529 (36) |
 Positive/negative Positive/negative | 424 (11) | 180 (9) | 287 (9) | 151 (10) |
 Positive/positive Positive/positive | 912 (23) | 444 (22) | 866 (29) | 384 (26) |
 Unknown Unknown | 68 (2) | 29 (1) | 42 (1) | 26 (2) |
| Donor/recipient sex match | ||||
 Male/male Male/male | 1616 (41) | 781 (39) | 1348 (44) | 619 (43) |
 Male/female Male/female | 1141 (29) | 565 (29) | 845 (28) | 423 (29) |
 Female/male Female/male | 595 (15) | 285 (14) | 404 (13) | 182 (13) |
 Female/female Female/female | 616 (16) | 350 (18) | 435 (14) | 226 (16) |
 Unknown Unknown | 1 (N/A) | 2 (N/A) | 19 (N/A) | 9 (N/A) |
| Donor age at donation | ||||
 18–32 years 18–32 years | 2060 (52) | 999 (50) | 2097 (69) | 1018 (70) |
 33–49 years 33–49 years | 1633 (41) | 850 (43) | 765 (25) | 354 (24) |
 50+ years 50+ years | 209 (5) | 101 (5) | 141 (5) | 68 (5) |
 Unknown Unknown | 67 (2) | 33 (2) | 48 (2) | 19 (1) |
 Median (range) Median (range) | 32 (18–61) | 32 (18–60) | 27 (18–66) | 27 (18–63) |
| Donor prior pregnancies | ||||
 No No | 3283 (85) | 1621 (85) | 2685 (90) | 1284 (89) |
 Yes Yes | 570 (15) | 294 (15) | 308 (10) | 153 (11) |
 Unknown Unknown | 116 (N/A) | 68 (N/A) | 58 (N/A) | 22 (N/A) |
| Donor/recipient ABO matching | ||||
 ABO matched ABO matched | 1478 (44) | 729 (44) | 979 (48) | 482 (49) |
 ABO minor mismatch ABO minor mismatch | 853 (26) | 404 (24) | 519 (25) | 227 (23) |
 ABO major mismatch ABO major mismatch | 760 (23) | 403 (24) | 418 (20) | 213 (22) |
 ABO bidirectional mismatch ABO bidirectional mismatch | 244 (7) | 133 (8) | 130 (6) | 65 (7) |
 Unknown Unknown | 634 (N/A) | 314 (N/A) | 1005 (N/A) | 472 (N/A) |
| Year of transplant by group | ||||
 1999–2002 1999–2002 | 477 (12) | 230 (12) | 0 | 0 |
 2003–2006 2003–2006 | 1145 (29) | 585 (30) | 0 | 0 |
 2007–2011 2007–2011 | 2346 (59) | 1167 (59) | 0 | 0 |
 2012–2014 2012–2014 | 0 | 0 | 3032 (100) | 1450 (100) |
 Unknown Unknown | 1 (N/A) | 1 (N/A) | 19 (N/A) | 9 (N/A) |
| Follow-up among survivors, months | ||||
 N Eval N Eval | 1717 | 846 | 1634 | 810 |
 Median (range) Median (range) | 71 (3–198) | 72 (4–195) | 24 (3–52) | 24 (4–53) |
Abbreviation: HCT, hematopoietic cell transplant comorbidity index; GVHD, graft versus host disease; HLA, human leukocyte antigen; TCE, T-cell epitope; N/A, not applicable; N Eval, number evaluable
Table 2: Final multivariate model for overall survival and possible weighting of donor characteristics in the 1999–2011 training set (c1)
Overall survival in the 1999–2011 cohort (cohort 1)
Development of the model in the 1999–2011 training set
The final survival model from the training datasets is shown in Table 2. We found significant negative effects on survival for three donor factors: non-permissive DPB1 mismatching (HR 1.13; 95% CI 1.01, 1.26; p-value=0.032), older donor age (as a linear effect, HR 1.07 per decade increase in age; 95% CI 1.02, 1.12, p-value=0.004), and CMV mismatching for CMV positive recipients (HR 1.14; 95% CI 1.02, 1.27; p-value=0.022). For CMV- recipients, a CMV+ donor was not significantly associated with an increase in mortality (HR=1.03; 95% CI 0.89–1.20; p-value=0.68), so this was not included in the score. ABO mismatching (any type: major, minor or bidirectional) was associated with mortality in initial modelling, but the effect was diminished in more recent transplants (HR for ABO mismatch among patients transplanted since 2007: 1.04; 95% CI 0.91–1.19; p-value=0.638), so it was not included in the final model and donor score. Other donor characteristics were investigated but were not significantly associated with survival.
Table 2
Effect of Donor selection score and individual donor components on overall survival in the 1999–2011 training set. Value in proposed score is in the right-hand column
| Variable | N | Log (HR) | HR (95% CI) | p-value | Score |
|---|---|---|---|---|---|
| DPB1 Matching | |||||
 Matched/permissive Matched/permissive | 1485 | 1 | 0 | ||
 Non-permissive Non-permissive | 836 | 0.12 | 1.13 (1.01–1.26) | 0.03 | 2 |
 Unknown Unknown | 1648 | 0.04 | 1.04 (0.94–1.15) | 0.41 | 1 |
| Donor age (decades)* | |||||
 Linear effect Linear effect | 0.07 | 1.07 (1.02–1.12) | <0.01 | Age in decades (Age/10) | |
| CMV matching (recipient positive only) | |||||
 Matched Matched | 912 | 1 | 0 | ||
 Mismatched Mismatched | 1424 | 0.13 | 1.14 (1.02–1.27) | 0.02 | 2 |
The final model was adjusted for age, race, KPS, disease/status, use of TBI, recipient CMV and was stratified on conditioning intensity, recipient sex, graft type, and GVHD prophylaxis due to non-proportional hazards.
A donor selection score was constructed (right hand column in Table 2), where donor score weights were assigned based on 1 point per approximately 4–7% increased relative risk (RR=1.04–1.07) of mortality. The impact of increasing donor selection score in a Cox model was assessed by removing the individual donor factors and replacing them with the donor selection score in the model for the training dataset. The resulting HR for the donor score in the training dataset is RR=1.06 (95% CI 1.03–1.10, p<0.001), suggesting that a 1 unit increase in the donor selection score is associated with a 6% increased risk of mortality.
Performance in the 1999–2011 testing set
The final survival model from the full testing dataset is shown in Table 3. None of the donor variables were significantly associated with survival in the independent testing set, nor was the donor selection score confirmed (HR=1.01; 95% CI 0.96–1.06; p=0.71).
Table 3
Effect of Donor selection score and individual donor components on overall survival in the 1999–2011 testing set. Overall donor selection score was not significantly associated with survival (HR=1.01; 95% CI 0.96–1.06; p-value=0.712)
| Variable | N | Log (HR) | HR (95% CI) | p-value |
|---|---|---|---|---|
| DPB1 Matching | ||||
 Matched/permissive Matched/permissive | 761 | 1 | ||
 Non-permissive Non-permissive | 405 | 0.01 | 1.01 (0.86–1.18) | 0.93 |
 Unknown Unknown | 817 | 0.08 | 1.08 (0.94–1.25) | 0.28 |
| Donor age (decades)* | ||||
 Linear effect Linear effect | 0.03 | 1.03 (0.96–1.10) | 0.42 | |
| CMV matching (recipient positive only) | ||||
 Matched Matched | 444 | 1 | ||
 Mismatched Mismatched | 726 | −0.03 | 0.97 (0.82–1.13) | 0.68 |
Overall survival in the 2012–2014 cohort (cohort 2)
In the second attempt, a model was developed in a randomly assigned training subset from the 2012–2014 patient cohort. Only donor age was associated with survival after adjustment for recipient age, KPS, disease/status, HCT- comorbidity index (CI) and in vivo TCD. Since only one donor characteristic was significantly associated with recipient survival, no donor selection score was developed. Instead, donor age was investigated in the testing subset and was also found to be significantly associated with survival (Table 4).
Table 4
Final multivariate model for overall survival in the 2012–2014 training and testing set (c2)
| Variable | N | Log (HR) | HR (95% CI) | p-value |
|---|---|---|---|---|
| Donor age (decades)* | ||||
 Linear effect Linear effect | 3051 | 0.12 | 1.13 (1.07–1.19) | <0.01 |
| Donor age (decades)* | ||||
 Linear effect Linear effect | 1459 | 0.10 | 1.11 (1.02–1.20) | 0.01 |
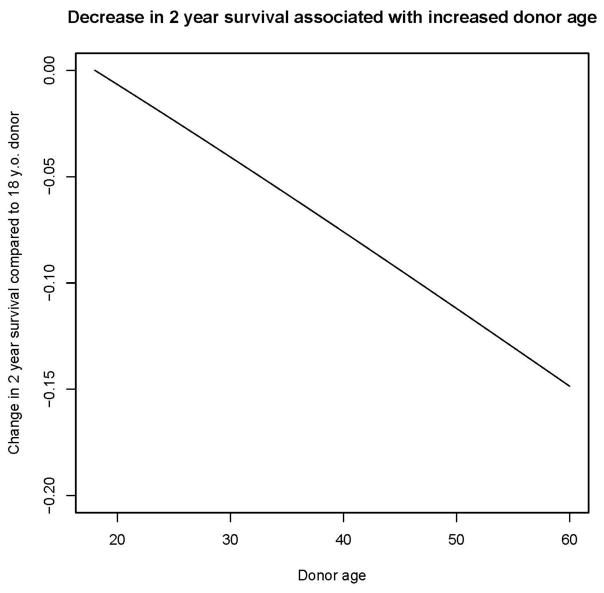
The effect of increasing donor age on 2-year survival outcomes for the testing cohort from 2012–2014 was predicted based on the fitted Cox model, and absolute decreases in overall survival at 2 years associated with increasing donor age above age 18 are shown in Figure 1. We assumed a 2-year OS of 54%, similar to the 2-year survival of the testing cohort. We also considered different baseline 2-year OS probabilities for low, intermediate, and high disease risk groups, but the impact of increasing donor age on these probabilities were similar and so are not shown. In this cohort, choosing a donor 2 years older is associated with a 1% decrease in 2-year OS, choosing a donor 5 years older is associated with a 2% decrease in 2-year OS, choosing a donor 10 years older is associated with a 3% decrease in 2-year OS, and choosing a donor 20 years older is associated with a 7% decrease in 2-year OS.
DISCUSSION
Our aim in this study was to develop an URD selection score, based on the weight of individual donor characteristics, which would allow for identification of the ‘optimal’ donor (i.e. associated with the best recipient survival) for a transplant recipient who has multiple 8/8 HLA matched donors available. We report the important finding that younger donor age is the only tested donor characteristic consistently associated with better survival across both transplant cohorts studied. Although we were able to generate a donor score in the first training set, this was not validated in the testing set. In the most recent cohort, we were not able to develop a score because the only donor factor associated with recipient survival was donor age. We believe that these findings explain the variability in the literature with regards to the impact of donor factors on survival. In addition, these findings provide a significant clinical advance to the field in simplifying donor selection and providing clear priorities to URD registries.
Previous URD studies have reported a survival advantage for recipients receiving a transplant from a younger donor1, 3, 5, 21–23. These studies searched for a threshold for donor age above which recipient survival was worse, and reported cut-offs of 30–39. In our study, we found a linear effect of age, such that for every additional year of donor age the recipient survival was worse. An important difference between our study and several others1, 3, 5, is that we included only 8/8 HLA-matched donors. Although other studies did adjust or control for HLA matching status (with a persistent impact of donor age on survival), it is known that patients receiving cells from older donors have less common HLA genotypes1, and that there is a correlation between older donor age and higher rates of HLA mismatch5. Here we have removed any unmeasurable impact due to this variable.
Questions about the quality of ‘aging’ hematopoietic stem cells (HSC) have been raised24, and mechanisms have been extrapolated from mouse models. There is evidence that the HSC pool in fact expands as aging occurs, with normal reconstitution of the HSC compartment when these cells are transplanted into lethally irradiated mice, although individual cellular function is compromised/reduced with increased DNA damage. Additionally, these cells show an increasing myeloid bias25, 26 and clonal haematopoiesis has been shown to increase with age27. Genome-wide expression studies show upregulation of multiple genes involved in inflammatory pathways25. Finally, through microenvironment-driven reduction in pre-B cells and curtailment of naive T-cell development following thymic involution, the lymphocyte compartment numbers can only be maintained by homeostatic proliferation with antigen-experienced cells leading to a reduction in tolerance to recipient antigens26, 28.
A clear difference in the causes of death for recipients based on their donors’ ages has not been reported. Some studies29, 30 have shown an increase in graft failure after transplantation from older donors, even after accounting for any differences in cell counts, but this is not true in all studies1. Despite some evidence that older donors may mobilize less well than younger donors, the lower threshold of cells necessary for engraftment is almost always achieved24, supporting the notion that the defect is qualitative rather than quantitative.
Studies have also shown a higher incidence of GVHD after transplantation from an older donor1, 5, although again this is not a consistent finding31. A higher incidence of GVHD could be explained by the predominance of antigen-experienced cells, as mentioned above, as well as the fact that aging itself is characterized by a state of low-level inflammation32.
The variability in results in the literature of the association of individual donor factors with survival could be explained by the interactions between donor and recipient factors that become apparent when large numbers of patients are studied. For example, in this study, we found that a negative impact of ABO matching was more pronounced in the patients transplanted prior to 2011 compared to transplants after that time, possibly due to the higher percentage of bone marrow grafts used in that era. We believe that differences such as these are the main reason that we were unable to validate our original score despite multiple attempts to understand and/or address any bias or obvious interactions within the population (despite the random assignments we performed). As can be seen from the results obtained from the initial training set (c1), the statistical impact associated with all donor characteristics was relatively modest (hazard ratios 1.08–1.10) and statistical significance was lost when the derived donor selection score was applied to the independent testing set (c1). It is clear from table 1, that there are significant differences in the patient, donor and transplant characteristics found between the two cohorts. For example, the increase in the age of patients at transplant, related to the advent of reduced intensity conditioning and careful attention to co-morbidities as a more discriminatory factor than age itself. No donor factors, besides age, were significant in the more recent patients (c2) suggesting that these dramatic changes in clinical transplant practice over recent years33 further complicate attempts to identify a universal donor selection profile.
Consistent with our aim of developing a score to simplify donor selection, we addressed only the outcome of survival in this study. It is possible that separate scores could be generated to predict the risk of other important outcomes such as graft failure or GVHD that might help distinguish among several potential donors. Unless the adverse donor characteristics are identical for these outcomes, however, centers will still have to prioritize the various donor characteristics to select from a pool of potential donors.
This study has limitations. It is impossible to account for the variability in individual transplant center donor selection practices, or to fully adjust for the systematic changes that have occurred over time. As the patient population is almost exclusively Caucasian, the power to detect an impact of mismatched race/ethnicity on survival is likely to be low, and these data may not be translatable to other races/ethnicities. We also focused on 8/8 matched unrelated donor procedures, to eliminate the effects of HLA-mismatching. However, we plan a similar study in 7/8 matched pairs, to try to identify robust donor factors that can help prioritize donors in this situation. We also did not have comprehensive data on DP typing (40% of pairs without these data), or on newer donor factors that might prove important such the presence or absence of specific KIR loci or variation in HLA expression levels34, 35. As additional data accumulate, these factors can be evaluated within the CIBMTR database for their prognostic importance.
In general, the field has changed to support selection of younger donors. The percentage of selected donors under the age of 30 years was 69% in 2012–2014 compared to 51% in 1999–2011 (and 36% in 1988–2006 from a previous CIBMTR study1). This change is driven by earlier results showing an impact of younger donor age on survival5 as well as many practical and logistic issues for selection by transplant centers (better HLA typed donors at recruitment, increased worldwide registry size and annual recruitment of new donors), and faced by donor registries which have driven them to prioritize the recruitment of young donors (value of longer subsequent time on the registry, fewer medical concerns). This effort by the registries to enroll younger people has been highly successful with the proportion of volunteers under 35 in the world-wide registries now being 44% compared to 35% in 2006 (personal communication, WMDA annual report). Many registries have expanded the younger age to recruitment below 18 and no longer recruit donors above a certain age [https://bethematch.org/support-the-cause/donate-bone-marrow/join-the-marrow-registry/; https://www.anthonynolan.org/8-ways-you-could-save-life/donate-your-stem-cells/who-can-join-register; https://blood.ca/en/stem-cell/onematch-information-new-registrants]. Finally, there are donor factors which are often considered by transplant centers when selecting a donor, but which we were not able to address in our analysis. These include donor weight36–38, donor center/registry preferences (including financial restrictions and distance from the transplant center)39 and donor availability40.
In conclusion, we set out to develop a composite score to identify the best unrelated donor, using two large patient cohorts from the CIBMTR. However, the only donor factor consistently associated with patient survival in the testing sets was donor age. This finding significantly simplifies the donor selection process, while clearly highlighting the priority for URD registry recruitment and retention strategies. Future studies to better understand the biologic basis for this finding are encouraged, as is the testing of biomarkers that can refine donor selection algorithms. Novel statistical techniques for a personalized approach to donor selection, considering each of these specific donor characteristics in the context of an individual transplant recipient’s disease and demographic profile may further refine our ability to select the best donor.
Acknowledgments
The CIBMTR is supported primarily by Public Health Service Grant/Cooperative Agreement 5U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 4U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-17-1-2388 and N0014-17-1-2850 from the Office of Naval Research; and grants from * Actinium Pharmaceuticals, Inc.; * Amgen, Inc.; * Amneal Biosciences; * Angiocrine Bioscience, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; Atara Biotherapeutics, Inc.; Be the Match Foundation; * bluebird bio, Inc.; * Bristol Myers Squibb Oncology; * Celgene Corporation; Cerus Corporation; * Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Gilead Sciences, Inc.; HistoGenetics, Inc.; Immucor; * Incyte Corporation; Janssen Scientific Affairs, LLC; * Jazz Pharmaceuticals, Inc.; Juno Therapeutics; Karyopharm Therapeutics, Inc.; Kite Pharma, Inc.; Medac, GmbH; MedImmune; The Medical College of Wisconsin; * Mediware; * Merck & Co, Inc.; * Mesoblast; MesoScale Diagnostics, Inc.; Millennium, the Takeda Oncology Co.; * Miltenyi Biotec, Inc.; National Marrow Donor Program; * Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Otsuka Pharmaceutical Co, Ltd. – Japan; PCORI; * Pfizer, Inc; * Pharmacyclics, LLC; PIRCHE AG; * Sanofi Genzyme; * Seattle Genetics; Shire; Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; * Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Takeda Oncology; Telomere Diagnostics, Inc.; and University of Minnesota. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
Footnotes
*Corporate Members
AUTHORSHIP CONTRIBUTIONS
BES, SJL, BRL and SRS designed the research, analyzed and interpreted the data, wrote the manuscript, and had responsibility for the entire manuscript; HW and MH prepared the data file and analyzed the data; B.R.L. performed the statistical analysis; all other authors actively reviewed the results during analysis and assisted with interpretation. All authors reviewed and approved the final manuscript.DISCLOSURES OF CONFLICT OF INTEREST
None of the authors has any conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.bbmt.2018.02.006
Read article for free, from open access legal sources, via Unpaywall:
http://www.bbmt.org/article/S1083879118300843/pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.bbmt.2018.02.006
Article citations
Haploidentical vs HLA-matched sibling donor HCT with PTCy prophylaxis: HLA factors and donor age considerations.
Blood Adv, 8(20):5306-5314, 01 Oct 2024
Cited by: 0 articles | PMID: 39167765 | PMCID: PMC11497453
Histocompatibility assessment in hematopoietic stem cell transplantation: recommendations from the Italian Society for Immunogenetics and Transplantation Biology (Associazione Italiana di Immunogenetica e Biologia dei Trapianti - AIBT).
Blood Transfus, 22(4):338-349, 01 Jul 2024
Cited by: 1 article | PMID: 37458719 | PMCID: PMC11251828
Review Free full text in Europe PMC
Young (<35 years) haploidentical versus old (≥35 years) mismatched unrelated donors and vice versa for allogeneic stem cell transplantation with post-transplant cyclophosphamide in patients with acute myeloid leukemia in first remission: a study on behalf of the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation.
Bone Marrow Transplant, 59(11):1552-1562, 18 Aug 2024
Cited by: 0 articles | PMID: 39155338
Unrelated hematopoietic stem cell donor registries: present reality and future prospects.
Curr Opin Hematol, 31(6):251-260, 19 Jul 2024
Cited by: 0 articles | PMID: 39046928 | PMCID: PMC11426981
Review Free full text in Europe PMC
Combined effect of unrelated donor age and HLA peptide-binding motif match status on HCT outcomes.
Blood Adv, 8(9):2235-2242, 01 May 2024
Cited by: 1 article | PMID: 38467032 | PMCID: PMC11061210
Go to all (52) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Comparing outcomes of matched related donor and matched unrelated donor hematopoietic cell transplants in adults with B-Cell acute lymphoblastic leukemia.
Cancer, 123(17):3346-3355, 27 Apr 2017
Cited by: 12 articles | PMID: 28452054 | PMCID: PMC5568918
Donor-Recipient Matching for KIR Genotypes Reduces Chronic GVHD and Missing Inhibitory KIR Ligands Protect against Relapse after Myeloablative, HLA Matched Hematopoietic Cell Transplantation.
PLoS One, 11(6):e0158242, 24 Jun 2016
Cited by: 16 articles | PMID: 27341514 | PMCID: PMC4920429
Graft-versus-host disease in recipients of male unrelated donor compared with parous female sibling donor transplants.
Blood Adv, 2(9):1022-1031, 01 May 2018
Cited by: 7 articles | PMID: 29739773 | PMCID: PMC5941995
Selection of matched unrelated donors moving forward: from HLA allele counting to functional matching.
Hematology Am Soc Hematol Educ Program, 2019(1):532-538, 01 Dec 2019
Cited by: 2 articles | PMID: 31808865 | PMCID: PMC6913495
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Actinium Pharmaceuticals, Inc.
Amgen, Inc.
Amneal Biosciences
Angiocrine Bioscience, Inc.
Astellas Pharma US
Atara Biotherapeutics, Inc.
Be The Match Foundation
Bristol Myers Squibb Oncology
Celgene Corporation
Cerus Corporation
Chimerix, Inc.
Fred Hutchinson Cancer Research Center
Gamida Cell Ltd.
Gilead Sciences, Inc.
Health Resources and Services Administration (1)
Grant ID: HHSH250201200016C
HistoGenetics, Inc.
Immucor
Incyte Corporation
Janssen Scientific Affairs, LLC
Jazz Pharmaceuticals, Inc.
Juno Therapeutics
Karyopharm Therapeutics, Inc.
Kite Pharma, Inc.
MedImmune
Medac, GmbH
Mediware
Merck & Co, Inc.
Mesoblast; MesoScale Diagnostics, Inc.
Millennium
Miltenyi Biotec, Inc.
NCI NIH HHS (2)
Grant ID: U24 CA076518
Grant ID: P30 CA008748
NHLBI NIH HHS (1)
Grant ID: U10 HL069294
National Cancer Institute
National Heart, Lung and Blood Institute (1)
Grant ID: 4U10HL069294
National Institute of Allergy and Infectious Diseases
National Marrow Donor Program
Neovii Biotech NA, Inc.
Novartis Pharmaceuticals Corporation
Grant ID: N00014-17-1-2388
Grant ID: N0014-17-1-2850
Otsuka Pharmaceutical Co, Ltd. – Japan
PCORI
PIRCHE AG
Pfizer, Inc
Pharmacyclics, LLC
Public Health Service Grant/Cooperative Agreement (1)
Grant ID: 5U24CA076518