Abstract
Introduction
The incidence of blindness due to methanol intoxication is higher in males of productive age. The management of methanol-induced toxic optic neuropathy is yet to produce satisfactory results. Antioxidant therapy is now used as an alternative method of preventing methanol intoxication. The aim of this study was to observe the effect of TEMPOL (4-hydroxy-2,2,6,6-tetramethylpiperidinyl-1-oxyl), a superoxide dismutase (SOD) mimetic, on retinal ganglion cells in methanol-intoxicated rats.Methods
This experimental study was conducted with 20 male Wistar rats that were 10-12 weeks old and weighed 300-350 g. The rats were divided into four groups that each received a different treatment: a negative control group, a positive control group, a methanol group, and a methanol + TEMPOL group. Enucleated eyes from all groups were sliced and stained using hematoxylin-eosin (HE). Retinal layer and ganglion cells were assessed based on cellular structure, cellular swelling, and vacuole formation in the ganglion cell layer as observed at × 200 magnification. The Kruskal-Wallis test and the Mann-Whitney test were used, with significance taken to correspond to p < 0.05.Results
Retinal ganglion cells of the control group had fewer vacuoles and a more well-organized cellular structure compared to those of the methanol group. The histopathologic scores of the methanol-intoxicated group were lower than those of the TEMPOL therapy group; p = 0.011 (i.e., p < 0.05).Conclusions
TEMPOL had a positive impact on the cellular structure of retinal ganglion cells in methanol-intoxicated rats.Free full text

The Superoxide Dismutase Mimetic TEMPOL and Its Effect on Retinal Ganglion Cells in Experimental Methanol-Intoxicated Rats
Abstract
Introduction
The incidence of blindness due to methanol intoxication is higher in males of productive age. The management of methanol-induced toxic optic neuropathy is yet to produce satisfactory results. Antioxidant therapy is now used as an alternative method of preventing methanol intoxication. The aim of this study was to observe the effect of TEMPOL (4-hydroxy-2,2,6,6-tetramethylpiperidinyl-1-oxyl), a superoxide dismutase (SOD) mimetic, on retinal ganglion cells in methanol-intoxicated rats.
Methods
This experimental study was conducted with 20 male Wistar rats that were 10–12 weeks old and weighed 300–350 g. The rats were divided into four groups that each received a different treatment: a negative control group, a positive control group, a methanol group, and a methanol +
+ TEMPOL group. Enucleated eyes from all groups were sliced and stained using hematoxylin–eosin (HE). Retinal layer and ganglion cells were assessed based on cellular structure, cellular swelling, and vacuole formation in the ganglion cell layer as observed at × 200 magnification. The Kruskal–Wallis test and the Mann–Whitney test were used, with significance taken to correspond to p
TEMPOL group. Enucleated eyes from all groups were sliced and stained using hematoxylin–eosin (HE). Retinal layer and ganglion cells were assessed based on cellular structure, cellular swelling, and vacuole formation in the ganglion cell layer as observed at × 200 magnification. The Kruskal–Wallis test and the Mann–Whitney test were used, with significance taken to correspond to p <
< 0.05.
0.05.
Results
Retinal ganglion cells of the control group had fewer vacuoles and a more well-organized cellular structure compared to those of the methanol group. The histopathologic scores of the methanol-intoxicated group were lower than those of the TEMPOL therapy group; p =
= 0.011 (i.e., p
0.011 (i.e., p <
< 0.05).
0.05).
Conclusions
TEMPOL had a positive impact on the cellular structure of retinal ganglion cells in methanol-intoxicated rats.
Introduction
Methanol intoxication can cause various health problems such as optic neuropathy, gastrointestinal problems, and metabolic acidosis leading to death. The abuse of methanol (as a substitute for alcohol) was reported for the first time in 1904, and its incidence is rising. Blindness is a common outcome of methanol intoxication [1, 2].
The incidence of optic neuropathy due to methanol intoxication is increasing in developing countries such as Indonesia and Tunisia [3, 4]. Studies have found that males of productive age are the population most affected by blindness due to methanol intoxication [5].
As yet, therapy for methanol-induced toxic optic neuropathy has not yielded satisfactory results. Steroid therapy and vitamin supplementation have been found to have no significant effect. Different outcomes are observed for different individuals, and a therapeutic mechanism has not been identified. Methanol intoxication leads to oxidative stress and mitochondrial dysfunction, which in turn results in ganglion cell and optic nerve necrosis.
Antioxidant therapy that inhibits the oxidative stress reaction is now being used as an alternative method of preventing methanol intoxication. The enzyme superoxide dismutase (SOD) is an antioxidant that is used to treat nerve cell degeneration. TEMPOL (4-hydroxy-2,2,6,6-tetramethylpiperidinyl-1-oxyl) is a SOD mimetic that has been proven to have a neuroprotective effect. The study reported in the present paper investigated the antioxidant effect of this SOD mimetic on the histopathological profile of retinal ganglion cells in methanol-intoxicated rats.
Methods
This experimental study was conducted with 20 Wistar rats that were 10–12 weeks old and weighed 300–350 g, so clinical trial registration was not conducted given that no human subjects were involved in the study. The Ethical Committee of the Faculty of Medicine at Universitas Padjadjaran approved the study. All institutional and national guidelines for the care and use of laboratory animals were followed. The rats were divided into four groups: a negative control group (no exposure), a positive control group (exposed to N2O:O2 =
= 1:1 flowing at 2 L/min for 16 h), a methanol group (exposed to N2O:O2
1:1 flowing at 2 L/min for 16 h), a methanol group (exposed to N2O:O2 =
= 1:1 and methanol 3 g/kg-BW orally after 4 h), and a methanol
1:1 and methanol 3 g/kg-BW orally after 4 h), and a methanol +
+ TEMPOL group (exposed to N2O:O2
TEMPOL group (exposed to N2O:O2 =
= 1:1, methanol 3 g/kg-BW orally 4 h later, and TEMPOL 30 mg/kg-BW 12 h after that). Rats receiving the gas mixture were placed inside a glass box and given gas as per their group. The control group was enucleated 16 h after the start of the experiment, whereas the methanol only and the methanol
1:1, methanol 3 g/kg-BW orally 4 h later, and TEMPOL 30 mg/kg-BW 12 h after that). Rats receiving the gas mixture were placed inside a glass box and given gas as per their group. The control group was enucleated 16 h after the start of the experiment, whereas the methanol only and the methanol +
+ TEMPOL group were enucleated 22 h after the start of treatment. This method was based on that used in a previous study in which TEMPOL was applied in cases of optic nerve trauma and tests were performed 22 h after the start of treatment [6, 7]. All animal models were sacrificed after enucleation. Enucleated eyes were sectioned in paraffin blocks with the cornea facing upward. The blocks were sliced to a thickness of 4 µm into sagittal sections with a keratome. The slices were placed in a water bath and mounted on a microscope slide. Specimens were dried and stained with hematoxylin–eosin (HE). One certified anatomical pathologist examined the retinal layer and retinal ganglion cells using an image-multiplier light microscope (Olympus® BX21) at
TEMPOL group were enucleated 22 h after the start of treatment. This method was based on that used in a previous study in which TEMPOL was applied in cases of optic nerve trauma and tests were performed 22 h after the start of treatment [6, 7]. All animal models were sacrificed after enucleation. Enucleated eyes were sectioned in paraffin blocks with the cornea facing upward. The blocks were sliced to a thickness of 4 µm into sagittal sections with a keratome. The slices were placed in a water bath and mounted on a microscope slide. Specimens were dried and stained with hematoxylin–eosin (HE). One certified anatomical pathologist examined the retinal layer and retinal ganglion cells using an image-multiplier light microscope (Olympus® BX21) at × 200 magnification. Retinal ganglion cells were assessed based on the regularity, swelling, and vacuolation of ganglion cells observed at × 200 magnification, as explained elsewhere. Each of these parameters was scored based on a scale of 0–5, as shown in Table 1 [8].
× 200 magnification. Retinal ganglion cells were assessed based on the regularity, swelling, and vacuolation of ganglion cells observed at × 200 magnification, as explained elsewhere. Each of these parameters was scored based on a scale of 0–5, as shown in Table 1 [8].
Table 1
Possible scores in retinal ganglion cell assessment
| Histopathological observations for retinal ganglion cells | Score |
|---|---|
| Normal structurea and no swellingb | 5 |
Structural abnormality in < in < 50% of cells, 20% of cells show swelling, vacuolation 50% of cells, 20% of cells show swelling, vacuolation < < 50%c 50%c | 4 |
Structural abnormality in 50–80% of cells, < < 50% of cells show swelling, vacuolation 50% of cells show swelling, vacuolation < < 50% 50% | 3 |
Structural abnormality in > in > 80% of cells, 80% of cells, < < 50% of cells show swelling, vacuolation 50% of cells show swelling, vacuolation < < 50% 50% | 2 |
Structural abnormality in > in > 80% of cells, 80% of cells, > > 50% of cells show swelling, vacuolation 50% of cells show swelling, vacuolation > > 50% 50% | 1 |
aNormal structure: ganglion cells occur in a regular linear arrangement with no aberrant cells apparent
bSwelling: ganglion cells appear significantly edematous
cAll percentages are estimated from the mean of three high-power fields of each slide
In this study, the sample size was calculated using Federer’s formula: (t − 1)(n − 1) >
> 15 (t
15 (t =
= number of experimental groups; n
number of experimental groups; n = number of
= number of samples in each group). Feeding the appropriate values into the formula results in (3 – 1)(n – 1)
samples in each group). Feeding the appropriate values into the formula results in (3 – 1)(n – 1) >
> 15, i.e., n
15, i.e., n =
= 8. However, given the ethical principles for animal experiments outlined in the Helsinki Declaration, the sample size was reduced to five per group [9, 10]. Statistical analysis was performed using the Kruskal–Wallis test and the Mann–Whitney test; a p value
8. However, given the ethical principles for animal experiments outlined in the Helsinki Declaration, the sample size was reduced to five per group [9, 10]. Statistical analysis was performed using the Kruskal–Wallis test and the Mann–Whitney test; a p value <
< 0.05 was considered to indicate significance.
0.05 was considered to indicate significance.
Results
Layers of retina near the optic disc in sagittal sections of rat eyeballs were examined histopathologically. Assessment was based on the structure, swelling, and vacuolation of retinal ganglion cells. The examiner observed less vacuole formation in the K0 (negative control) group and the K1 (N2O: O2) group than in the K2 (methanol only) and K3 (methanol +
+ TEMPOL) groups. The retinal ganglion cell layer (GCL), internal nuclear layer (INL), and outer nuclear layer (ONL) were found to be well ordered in the non-methanol (K0 and K1) groups and the TEMPOL (K3) group, as shown in Fig. 1.
TEMPOL) groups. The retinal ganglion cell layer (GCL), internal nuclear layer (INL), and outer nuclear layer (ONL) were found to be well ordered in the non-methanol (K0 and K1) groups and the TEMPOL (K3) group, as shown in Fig. 1.
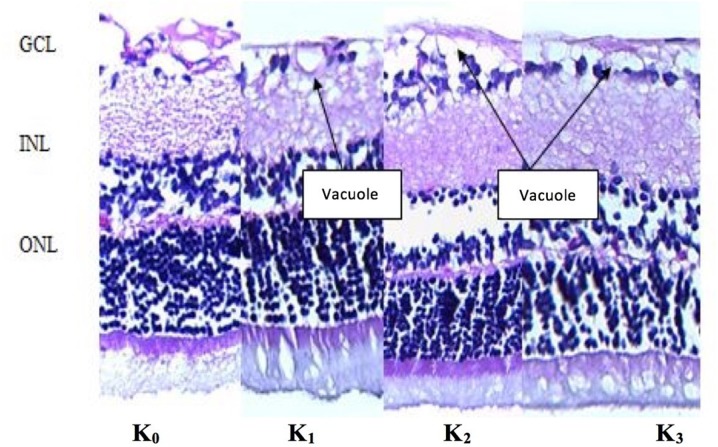
HE staining of the retinal ganglion cell layer. Retinal ganglion cell layers of the K0 and K1 groups show less vacuolation than those layers in the K2 and K3 groups. Better cellular structures and cell layers were seen for the GCL, INL, and ONL in the K0, K1, and K3 groups. GCL ganglion cell layer, INL internal nuclear layer, ONL outer nuclear layer
The histopathological examination yielded a lower score for the treated group (K3) than for the negative control group (K0, the normal score reference) and the positive control group (K1, the acidosis score reference), as shown in Fig. 2.
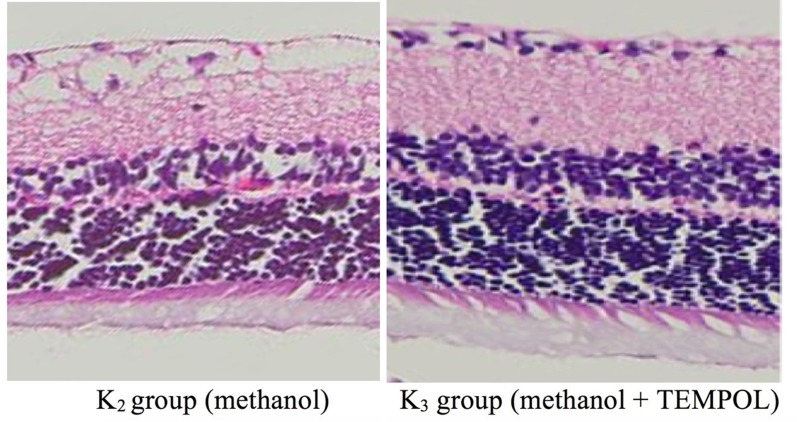
Comparison of the results of histopathological examinations of retinal ganglion cells from two of the groups. The cellular structure, internal cell layer, and outer cell layer of the K3 group were better than those of the K2 group
Low scores were obtained upon histopathological examination of the methanol-intoxicated group (K2) and the methanol +
+ TEMPOL group (K3), although the score for K3 was higher than that for K2. Statistical analysis of these scores using the Kruskal–Wallis test suggested that the scores for the groups were significantly different (p
TEMPOL group (K3), although the score for K3 was higher than that for K2. Statistical analysis of these scores using the Kruskal–Wallis test suggested that the scores for the groups were significantly different (p =
= 0.011), as shown in Table 2.
0.011), as shown in Table 2.
Table 2
Results of histopathological analyses of ganglion cell layers from the four groups
| Variable |
K
0
(n  = = 5) 5) |
K
1
(n  = = 5) 5) |
K
2
(n  = = 5) 5) |
K
3
(n  = = 5) 5) |
X
2
K-W | p value |
|---|---|---|---|---|---|---|
| Minimum | 8 | 6 | 3 | 4 | 11.102 | 0.011 |
| Maximum | 9 | 8 | 8 | 8 | ||
| Median | 9 | 6 | 4 | 7 |
K0 negative control group, K1 N2O group, K2 methanol group, K3 methanol +
+ TEMPOL group
TEMPOL group
 =
= Kruskal–Wallis test
Kruskal–Wallis test
Discussion
Methanol intoxication causes acidosis. HCO2 can easily pass through the ganglion cell wall, leading to the following formate oxidation reaction in the mitochondria and lysosome:
In rats, the oxidative reaction of formate is twice as fast as it is in humans. Thus, formate has a greater tendency to accumulate in the human body than in rats. During this formate oxidation reaction, O–H bonds are more likely to break when the body is in a state of methanol intoxication. Problems with electron transport lead to inefficient ATP synthesis and a higher concentration of reactive oxygen species (ROS). This in turn results in a higher rate of oxidative destruction of mitochondrial molecules. The electron transport chain in the inner mitochondrial membrane is involved in ATP synthesis through the intracellular respiration system. Decreased ATP and ADP in the mitochondria of retinal ganglion cells causes photoreceptor structure and function impairment and thus weakens the attachment of the photoreceptors to the retinal pigment epithelium [11].
Formate accumulation causes the formation of CO2 +
+ H2O, and this H2O causes the retina to swell. The degree of vacuolation in the methanol group (K2) was significantly higher than that in the methanol plus antioxidant group (K3). Regular cellular structure was seen in the samples from group K3 (Fig. 1). There was no significant difference between the thicknesses of the INL and ENL in those groups; this was assumed to be due to balanced COX and SDH activity in both layers [11].
H2O, and this H2O causes the retina to swell. The degree of vacuolation in the methanol group (K2) was significantly higher than that in the methanol plus antioxidant group (K3). Regular cellular structure was seen in the samples from group K3 (Fig. 1). There was no significant difference between the thicknesses of the INL and ENL in those groups; this was assumed to be due to balanced COX and SDH activity in both layers [11].
The antioxidant TEMPOL is a stable nitrite oxide radical that resembles SOD [12]. This substance passes through the blood–brain barrier and ocular tissues easily. In this study, TEMPOL was given 12 h after methanol administration to the Wistar rats in group K3. This study found that the histologic structure of retinal tissue improved after the administration of TEMPOL (Fig. 2), showing that the antioxidant TEMPOL can decrease the concentration of free radicals while acting as a neuroprotector in methanol intoxication, leading to better cellular structure. Thaler et al. [13] found TEMPOL to be an effective neuroprotector in cases of brain trauma, ischemic stroke, and Parkinson disease. TEMPOL was also found to act as a neuroprotector in rats with optic neuron impairment [7]; that observation agrees with the results of this study, in which we found that, in cases of toxic methanol optic neuropathy, the cellular structure of the retinal ganglion cells improved after the administration of TEMPOL [13].
Limitations of this study include the short observational period and the small sample size. Also, the slides were examined by only one certified anatomical pathologist, so there was no test/retest variability in this study. This study could be improved by including more samples, expanding the duration of TEMPOL administration, lengthening the observational period, and incorporating slide examination variability.
Conclusions
In conclusion, the administration of the SOD mimetic TEMPOL had a significant positive effect on the structure of retinal ganglion cells in methanol-intoxicated rats. Antioxidant therapy shows considerable potential as a possible future therapy for methanol-induced toxic optic neuropathy.
Acknowledgements
Funding
The authors hereby acknowledge the receipt of a Universitas Padjadjaran Research Internal Grant for this study. No funding was received for the publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Category 1: (a) concept and design: all authors; (b) acquisition of data: BS; (c) analysis and interpretation of data: BS. Category 2: (a) drafting of manuscript: II, RV, MR; (b) revision of manuscript for intellectual content: all authors. Category 3: (a) final approval: AK.
Non-Author Contributions
The authors hereby acknowledge Bethy S. Hernowo, who served as a participating investigator, and Adinda C.D. Nugraheni, who participated in the writing and technical editing of the manuscript.
Disclosures
Bambang Setiohadji, Irawati Irfani, Arief S. Kartasasmita, Maula Rifada, and Rova Virgana have nothing to disclose.
Compliance with Ethics Guidelines
The ethics committee of the Medical Faculty of the Universitas Padjadjaran approved this study. All institutional and national guidelines for the care and use of laboratory animals were followed during the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced digital features
To view enhanced digital features for this article go to 10.6084/m9.figshare.6159821.
References
Articles from Ophthalmology and Therapy are provided here courtesy of Springer
Full text links
Read article at publisher's site: https://doi.org/10.1007/s40123-018-0132-z
Read article for free, from open access legal sources, via Unpaywall:
https://link.springer.com/content/pdf/10.1007/s40123-018-0132-z.pdf
Citations & impact
Impact metrics
Article citations
Methanol-induced optic neuropathy: a still-present problem.
Arch Toxicol, 96(2):431-451, 06 Jan 2022
Cited by: 17 articles | PMID: 34988610 | PMCID: PMC8731680
Review Free full text in Europe PMC
Superoxide Dismutase Administration: A Review of Proposed Human Uses.
Molecules, 26(7):1844, 25 Mar 2021
Cited by: 65 articles | PMID: 33805942 | PMCID: PMC8037464
Review Free full text in Europe PMC
Proteomic insight into the pathogenesis of CAPN5-vitreoretinopathy.
Sci Rep, 9(1):7608, 20 May 2019
Cited by: 5 articles | PMID: 31110225 | PMCID: PMC6527583
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Neuroprotective Effects of Citicoline on Methanol-Intoxicated Retina Model in Rats.
J Ocul Pharmacol Ther, 37(9):534-541, 08 Sep 2021
Cited by: 2 articles | PMID: 34495749
Neuroprotective effects of tempol on retinal ganglion cells in a partial optic nerve crush rat model with and without iron load.
Exp Eye Res, 90(2):254-260, 04 Nov 2009
Cited by: 22 articles | PMID: 19883642
Neuroprotective effects of tempol acyl esters against retinal ganglion cell death in a rat partial optic nerve crush model.
Acta Ophthalmol, 89(7):e555-60, 03 Jun 2011
Cited by: 8 articles | PMID: 21645284
[Aiming for zero blindness].
Nippon Ganka Gakkai Zasshi, 119(3):168-93; discussion 194, 01 Mar 2015
Cited by: 2 articles | PMID: 25854109
Review

 1,2
1,2



