Abstract
Free full text

Endocervical and vaginal microbiota in South African adolescents with asymptomatic Chlamydia trachomatis infection
Associated Data
Abstract
Adolescent girls and young women represent a key risk group for sexually transmitted infections (STIs). The vaginal microbiota is thought to play an important role in susceptibility to STIs such as Chlamydia trachomatis. We compared the microbiota of the lateral vaginal wall and endocervix, and assessed associations with C. trachomatis infection in South African adolescents. The endocervical and vaginal lateral wall microbiota were characterized by amplifying and sequencing the V4 region of the 16S rRNA gene and C. trachomatis diagnosed using molecular methods. Of the 72 girls included, 30 had asymptomatic C. trachomatis infections. Three major vaginal community types were identified; one Lactobacillus crispatus, one L. iners and one diverse, Gardnerella vaginalis dominant. The microbiota of the endocervix was significantly different from that of the lateral wall in terms of diversity. There were many differentially abundant taxa between the endocervix and lateral vaginal wall, including Achromobacter spanius and Enterococcus faecium. Women with C. trachomatis had higher relative abundance of G. vaginalis and other anaerobes. In this African adolescent cohort, significant differences between the lateral vaginal wall and endocervical microbiota diversity and composition were evident, although neither were strongly associated with C. trachomatis infection.
Introduction
Chlamydia trachomatis is an obligate intracellular bacterium and the leading cause of bacterial sexually transmitted infections (STIs) worldwide with young women being particularly at risk1. Up to 80% of C. trachomatis infections in women are asymptomatic. If not treated, C. trachomatis can cause serious complications such as pelvic inflammatory disease, ectopic pregnancy and infertility. Bacterial vaginosis (BV) is a condition characterized by the replacement of Lactobacillus species in the female genital tract (FGT) with various anaerobic and facultative bacteria, including Gardnerella vaginalis, Atopobium vaginae, Megasphaera, Prevotella, and BV-associated bacteria (BVAB) 1–3. Molecular-based studies of healthy, reproductive-age women have described a distinct group of common vaginal bacterial community types, which vary according to population2,3. In general, low-diversity communities dominated by a single Lactobacillus species (Lactobacillus crispatus, L. gasseri, L. iners, or L. jensenii) and one to two high-diversity communities, comprised of a mixture of anaerobic bacteria associated with BV, are described3–6. Lactobacillus species are thought to protect the lower genital tract from invading pathogenic microorganisms by competing for nutrients, secretion of bacteriostatic and microbicidal compounds and by lowering the pH (<4.5) through lactic acid production.
Several studies have demonstrated a positive association between BV and the incidence of STIs, including C. trachomatis, Neisseria gonorrhoeae and Human Immunodeficiency Virus (HIV)7–10. Recent molecular studies have shown that women with C. trachomatis infection are more likely to have L. iners-dominated or dysbiotic vaginal communities compared to those without11–13. Although less well studied, the composition of the endocervical microbiota may be of greater importance when looking at the relationship between commensal bacteria and STIs, as the cervix is the main site of infection for several pathogens including N. gonorrhoeae and C. trachomatis14. Whereas the vagina has stratified squamous epithelium, C. trachomatis infects the columnar epithelial cells of the cervix. The cervical transformation zone is enriched with T cells and antigen-presenting cells compared to the vagina15 and differing immune and physiological environments (including different oxygen levels) could impact the microbial composition in different regions of the lower FGT. Molecular studies characterizing bacterial communities at different anatomical sites throughout the FGT have generated varying results with regards to microbial homogeneity16–18. Distinct microbial communities in cervix differing from that of the vagina have been described19. It is therefore of great importance to characterize endocervical microbial communities when studying the role that commensal bacteria play in C. trachomatis infection. To our knowledge, few molecular studies have evaluated the relationship between prevalent C. trachomatis infections and the composition of cervicovaginal microbiota and none have focused on African adolescents, a group at extreme risk for STIs. Therefore, we compared the endocervical and vaginal microbiota and evaluated their association with C. trachomatis infection in young South African females from a high risk community.
Results
Study population characteristics
Of the 154 lateral vaginal wall and endocervical samples taken from 77 participants, 149 samples passed the sequencing and quality control (≥5000 reads/sample, 3 endocervical and 2 lateral wall samples failed). Downstream analyses were conducted on 72 participants for whom both the lateral wall and matched endocervical samples passed quality control measures. Of those 72 participants, 42 (58%) were C. trachomatis negative and 30 (42%) C. trachomatis positive. None of the 30 women infected with C. trachomatis had LGV serovar infections. Nugent scores were available for all 72 participants. Nearly half of the participants (49%, n =
= 35) were BV positive (Nugent score 7–10), 5% were BV intermediate (Nugent score 4–6, n
35) were BV positive (Nugent score 7–10), 5% were BV intermediate (Nugent score 4–6, n =
= 4) and the remaining 46% were BV negative (Nugent score 0–3, n
4) and the remaining 46% were BV negative (Nugent score 0–3, n =
= 33) (Table 1). The C. trachomatis positive and negative participants were similar in terms of most demographic and behavioral characteristics including age, body mass index (BMI), intravaginal practices and hormonal contraceptive use. Participants with BV were almost twice as likely to be infected with C. trachomatis compared to BV negative participants, but these observations were not statistically significant (OR
33) (Table 1). The C. trachomatis positive and negative participants were similar in terms of most demographic and behavioral characteristics including age, body mass index (BMI), intravaginal practices and hormonal contraceptive use. Participants with BV were almost twice as likely to be infected with C. trachomatis compared to BV negative participants, but these observations were not statistically significant (OR =
= 1.8; 95% CI: 0.71–4.98, p
1.8; 95% CI: 0.71–4.98, p =
= 0.21).
0.21).
Table 1
Study population characteristics.
| C. trachomatis negative (n  = = 42)(58%) 42)(58%) | C. trachomatis positive (n  = = 30)(42%) 30)(42%) | P-value* | |
|---|---|---|---|
| Age, years (median) | 18.5 | 18 | 0.80 |
| Hormonal contraception | 0.73 | ||
| Injectable | 36 (86%) | 27 (90%) | |
| Non-injectable | 6 (14%) | 3 (10%) | |
| No (none) | 0 (0%) | 0 (0%) | |
| BMI (median)a | 25.39 | 25.15 | 0.35 |
| Intra-vaginal practices b | |||
| Douching | 3 (8%) | 0 (0%) | 0.31 |
| Washing w. water | 31 (97%) | 26 (93%) | 0.91 |
| Washing w. soap | 22 (67%) | 17 (63%) | 0.98 |
| Lifetime # partners (median) | 3 | 2 | 0.20 |
| Multiple partnersc | 22 (61%) | 10 (36%) | 0.32 |
| Regular condom used | 25 (76%) | 15 (56%) | 0.17 |
| BV prevalence | 0.10 | ||
| BV positive | 17 (40%) | 18 (60%) | |
| BV intermediate | 4 (10%) | 0 (0%) | |
| BV negative | 21 (50%) | 12 (40%) | |
| Endocervix Alpha Diversity (Shannon, median) | 1.1 | 1.8 | 0.28 |
| Vaginal pH (median) | 4.7 | 4.7 | 0.77 |
| N. gonorrhea positive | 2 (0.5%) | 7 (23%) | 0.02 |
| HPV positivee | 17 (49%) | 15 (75%) | 0.09 |
*Chi-squared test (Fisher’s exact test when expected values <
< 5) for the assessment of association of frequency among groups and the Mann–Whiney U-test for comparison of medians. Unpaired t-test for alpha diversity.
5) for the assessment of association of frequency among groups and the Mann–Whiney U-test for comparison of medians. Unpaired t-test for alpha diversity.
BV; bacterial vaginosis, STI; sexually transmitted infection, OCP; oral contraceptive pills.
aparticipants had missing information.
b11 participants had missing information.
c15 participants had missing information.
d12 participants had missing information.
e17 participants had missing information.
Adolescents infected with C. trachomatis were less likely to report multiple sexual partners and regular condom use (over the past year) and reported fewer lifetime sexual partners than C. trachomatis negative participants; although not significantly. C. trachomatis positive participants were, however, significantly more likely to be co-infected with N. gonorrhea than C. trachomatis negative participants (23% versus 0.5%, p =
= 0.03, Table 1).
0.03, Table 1).
Comparison of vaginal and endocervical microbial composition
A total of 365 OTUs were generated and allocated to 332 different taxa. Using fuzzy clustering with optimal k and data from both sample sites (lateral wall and endocervix), we identified three major community types (C1–3, Fig. 1) in agreement with previous data from this cohort20. These community types consisted either predominantly of diverse anaerobic bacteria (C1, n =
= 73; 51%), L. crispatus (C2, n
73; 51%), L. crispatus (C2, n =
= 27; 19%) or L. iners (C3, n
27; 19%) or L. iners (C3, n =
= 40; 28%), respectively. The most abundant species in C1 was G. vaginalis followed by BVAB1, BVAB2, Prevotella amnii, L. iners, Sneathia sanguinegens, Prevotella timonensis, A. vaginae, Dialister spp., Prevotella pallens and Megasphaera spp. The majority of participants with a C1 community type were BV positive according to Nugent scoring (82%) whereas participants with C2 and C3 community types were most commonly BV negative (89% and 73%, respectively, Fig. 1a–c).
40; 28%), respectively. The most abundant species in C1 was G. vaginalis followed by BVAB1, BVAB2, Prevotella amnii, L. iners, Sneathia sanguinegens, Prevotella timonensis, A. vaginae, Dialister spp., Prevotella pallens and Megasphaera spp. The majority of participants with a C1 community type were BV positive according to Nugent scoring (82%) whereas participants with C2 and C3 community types were most commonly BV negative (89% and 73%, respectively, Fig. 1a–c).
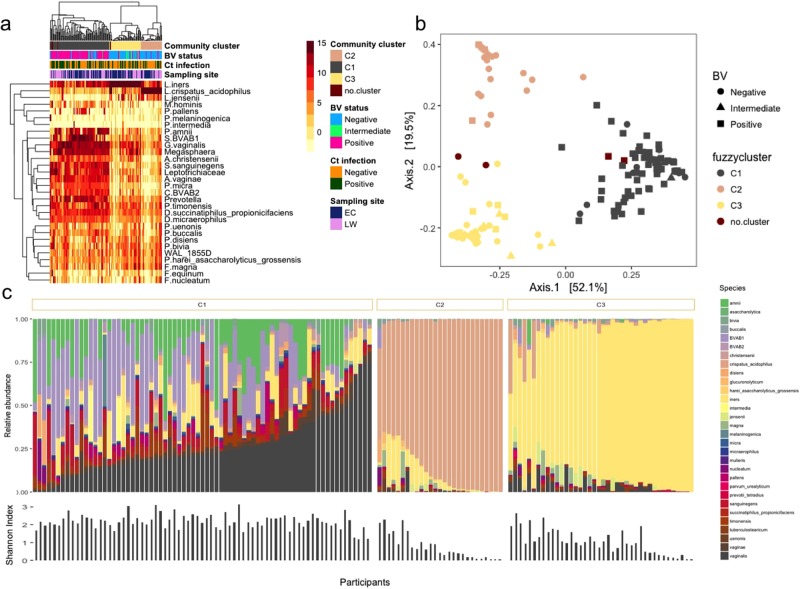
Composition of vaginal lateral wall and endocervical microbiota. (a) Heat map of the 30 most abundant taxa (rows) identified by 16S rRNA microbiome profiling using unsupervised hierarchical clustering with weighted UniFrac distances in all samples (columns). Unsupervised hierarchical clustering of 144 matched endocervial and vaginal lateral wall samples from 72 participants. The dendrogram was generated using average linkage clustering with weighted UniFrac distance, based on the relative abundance of taxa (merged at lowest taxonomic level) in each sample. Log2-transformed standardized read counts are illustrated by the colour key. Annotation bars above the heatmap depict community type cluster (top bar), BV status based on Nugent scoring (upper middle bar), C. trachomatis (Ct) infection status (lower middle bar) and anatomical sampling site (bottom bar). Samples that did not meet the minimum probability of ≥60% of belonging to any of the three clusters were excluded from downstream analyses (n =
= 4, “no.cluster” in figure). (b) Barplot of the 30 top most abundant taxa identified by 16S rRNA microbiome profiling. Samples are grouped by microbial compositional subtype (C1, C2, C3) established using Fuzzy clustering with weighted UniFrac distances and ordered based on the abundance of the most dominant species in each community type (G. vaginalis, L. crispatus and L. iners, respectively). Samples that did not meet the minimum probability of ≥60% of belonging to any of tree clusters (n
4, “no.cluster” in figure). (b) Barplot of the 30 top most abundant taxa identified by 16S rRNA microbiome profiling. Samples are grouped by microbial compositional subtype (C1, C2, C3) established using Fuzzy clustering with weighted UniFrac distances and ordered based on the abundance of the most dominant species in each community type (G. vaginalis, L. crispatus and L. iners, respectively). Samples that did not meet the minimum probability of ≥60% of belonging to any of tree clusters (n =
= 4) were excluded from the figure. Shannon diversity Index for each sample is depicted below the barplot. (c) Principal Coordinates Analysis (PCoA) of samples colored by compositional subtypes generated using Fuzzy clustering with weighted UniFrac distances. Samples are colored by compositional subtype (C1, C2, C3), with BV status displayed as shapes. Samples that did not meet the minimum probability of ≥60% of belonging to any of these clusters were excluded from downstream analyses (n
4) were excluded from the figure. Shannon diversity Index for each sample is depicted below the barplot. (c) Principal Coordinates Analysis (PCoA) of samples colored by compositional subtypes generated using Fuzzy clustering with weighted UniFrac distances. Samples are colored by compositional subtype (C1, C2, C3), with BV status displayed as shapes. Samples that did not meet the minimum probability of ≥60% of belonging to any of these clusters were excluded from downstream analyses (n =
= 4, “no.cluster” in figure).
4, “no.cluster” in figure).
To evaluate the differences in overall bacterial community composition between sampling sites, principal coordinates analysis (PCoA) using weighted UniFrac distances was performed (Fig. 1b). The samples clearly grouped into three distinct clusters corresponding to the three community types described above (Fig. 1b). However, within the three clusters the samples separated according to sampling site (Fig. 2a). (Adonis p =
= 0.002). Furthermore, lateral wall samples had significantly higher species richness and alpha diversity compared to endocervical samples (p
0.002). Furthermore, lateral wall samples had significantly higher species richness and alpha diversity compared to endocervical samples (p <
< 0.001, Fig. 2b). Yet, for 82% of the participants, the lateral wall and endocervical samples were assigned to the same community cluster and the within-participant variability was significantly less than the variability of samples from same site between participants based on mean weighted Unifrac distances (p
0.001, Fig. 2b). Yet, for 82% of the participants, the lateral wall and endocervical samples were assigned to the same community cluster and the within-participant variability was significantly less than the variability of samples from same site between participants based on mean weighted Unifrac distances (p <
< 0.001 for endocervical and lateral wall, Student’s paired t-test, Fig. 2e).
0.001 for endocervical and lateral wall, Student’s paired t-test, Fig. 2e).
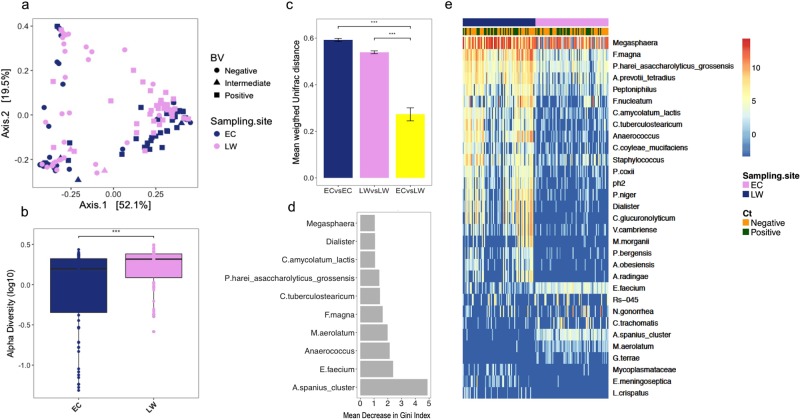
Taxa significantly different between vaginal lateral wall versus endocervical microbiota. (a) Principal Coordinates Analysis (PCoA) of the vaginal and endocervical microbiota using weighted UniFrac distances. Individual samples are colored by anatomical sampling site (endocervical:EC and lateral vaginal wall:LW) with BV status displayed as shapes. (b) Boxplot depicting the alpha diversity of the vaginal lateral wall (LW) and endocervical (EC) microbiota. (c) Taxa significantly differentially abundant and/or frequent by anatomical site category by metagenomeSeq (FDR ≤0.05, coefficient ≥1.25, taxa present in ≥20% of samples in at least one of the two groups being compared). Unsupervised clustering of samples (columns) by Bray-Curtis distance; heat map scale: log2-transformed standardized counts. (d) The top ten most influential taxa by random forests analysis. The x-axis indicates the mean decrease in Gini Index (length of bar represents predictive ability of each taxon). (e) Barplot depicting within-subject pair-wise distances (weigthed UniFrac) between sampling sites (ECvsLW) and the mean between-subject pair-wise distances (weighted UniFrac) between either endocervical samples (ECvsEC) or lateral wall samples (LWvsLW).
In order to identify differentially abundant bacterial species by anatomical sites, metagenomeSeq analysis was applied. The relative abundances of 31 taxa (OTUs merged at lowest taxonomic level) were significantly different between endocervical and lateral wall samples (Fig. 2c). These included Achromobacter spanius_cluster, Gordonia terrae, Methylobacterium aerolatum, Enterococcus faecium, C. trachomatis and N. gonorrhea, which were all more abundant in the endocervical samples, and Peptoniphilus spp., Anaerococcus spp., Corynebacterium ssp., Megasphaera, Morganella morganii, Finegoldia magna, Elizabethkingia meningoseptica, Staphylococcus, Actinomyces radingae, Prevotella bergensis, Dialister, Fusobacterium nucleatum, Peptococcus niger, Varibaculum cambriense and Lactobacillus crispatus which were more abundant in lateral wall samples. Random forest analysis was additionally used to identify species predictive of sampling site. In concordance with the metagenomeSeq results, the most influencial taxa in differentiating endocervical from lateral wall included A. spanius_cluster, E. faecium, M. aerolatum, Peptoniphilus harei_asaccharolyticus_grossensis and Megasphaera which were more prominent in endocervical samples, and Anaerococcus, F. magna, Dialister and Corynebacterium ssp., which were more common in lateral wall samples (Fig. 2d, AUC =
= 0.98, PPV
0.98, PPV =
= 0.96, NPV
0.96, NPV =
= 0.90 for the test set and a validation predicted error rate of 6.25%).
0.90 for the test set and a validation predicted error rate of 6.25%).
Relationship between genital tract microbiota with C. trachomatis infection
The association between C. trachomatis infection and microbiota was considered separately for lateral wall and endocervical samples. The alpha diversity of endocervical microbiota tended to be higher in C. trachomatis positive participants compared to C. trachomatis negative participants, although not significantly (1.8 versus 1.1, p =
= 0.28, Table 1); this was also the case in lateral wall samples (p
0.28, Table 1); this was also the case in lateral wall samples (p =
= 0.32, data not shown). In univariate analysis, participants with a community type dominated by diverse anaerobic bacteria (C1) or L. iners (C3) were more likely to be infected with C. trachomatis compared to those having an L. crispatus-dominated community, although not significantly so (OR
0.32, data not shown). In univariate analysis, participants with a community type dominated by diverse anaerobic bacteria (C1) or L. iners (C3) were more likely to be infected with C. trachomatis compared to those having an L. crispatus-dominated community, although not significantly so (OR =
= 2.98; 95% confidence interval: 0.76–15.0 and OR
2.98; 95% confidence interval: 0.76–15.0 and OR =
= 2.50; 95% CI: 0.56–13.6, respectively). None of the study participant demographic or behavioral factors listed in Table 1 were associated with C. trachomatis in univariate or multivariate analyses.
2.50; 95% CI: 0.56–13.6, respectively). None of the study participant demographic or behavioral factors listed in Table 1 were associated with C. trachomatis in univariate or multivariate analyses.
On the other hand, differential abundance testing (using metagenomeSeq) revealed many significantly differentially abundant cervical taxa between C. trachomatis positive versus negative women, including as expected higher relative abundance of N. gonorrhea, but also Rs-045, Sutterella sanguinus_morbirenis, Porphyromonas somerae and Corynebacterium urealyticum (Fig. 3b). Using random forest analysis, these same species, as well as G. vaginalis, Aerococcus christensenii, Dialister spp., Megasphaera, A. vaginae and Prevotella disiens were found to be predictive of C. trachomatis infection (Fig. 3b). However, the predictive model had an AUC of 0.46 and predicted error rate of 54.17%. Many of these same genera were also predictive of C. trachomatis infection status in lateral wall samples, with Prevotella spp. and Megasphaera being the strongest predictors (Supplementary Figure 1); however this predictive model was very weak with an predicted error rate of 66.67% and AUC of 0.29 for the test set. In concordance with this, no overall differences in microbiota composition (β-diversity) were found between C. trachomatis positive and C. trachomatis negative participants (Fig. 3c, Adonis p =
= 0.27 and p
0.27 and p =
= 0.58 for endocervical and lateral wall, respectively).
0.58 for endocervical and lateral wall, respectively).
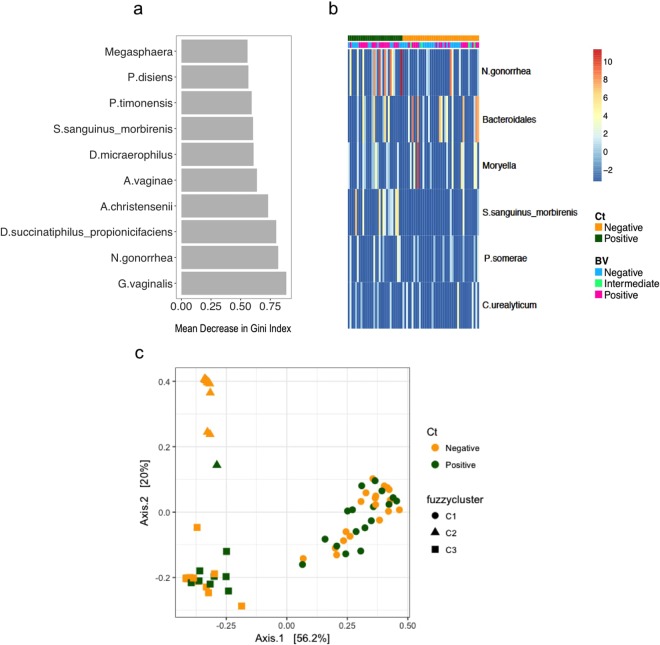
Taxa significantly different in C. trachomatis infected versus uninfected individuals in the endocervical microbiota. (a) Taxa significantly differentially abundant and/or frequent by C. trachomatis category in the endocervical (EC) microbiota by metagenomeSeq (FDR ≤0.05, coefficient ≥1.25, taxa present in ≥20% of samples in at least one of the two groups being compared). Unsupervised clustering of samples (columns) by Bray-Curtis distance; heat map scale: log2-transformed standardized counts. The OTU assigned C. trachomatis taxonomy was excluded for this analysis. (b) The top 20 most influential taxa by random forests analysis. The x-axis indicates the mean decrease in Gini Index (length of bar represents predictive ability of each taxon), where a larger index indicates greater predictive power. Taxa that were significantly differentially abundant and/or frequent in C. trachomatis infected versus uninfected individuals (FDR ≤0.05, coefficient ≥1.25, taxa present in ≥20% of samples in at least one of the two groups being compared); hierarchical clustering (Bray-curtis distance); heat map scale: log2-transformed standardized counts. The OTU assigned C. trachomatis taxonomy was excluded for this analysis. (c) Principal Coordinates Analysis (PCoA) of the endocervical microbiota using weighted UniFrac distances. Individual samples are colored by C. trachomatis infection status with fuzzycluster displayed as shapes.
Discussion
Adolescent girls and young women in sub-Saharan Africa are at high risk for STIs and frequently have vaginal dysbiosis20. To our knowledge, this is the first study to describe the relationship between genital tract microbial communities and C. trachomatis in adolescents. Adolescence is the period of greatest risk for STI acquisition. Furthermore, few studies have examined this relationship in African women, who have very different vaginal microbiota than their European counterparts. Finally, most if not all, studies interrogating the relationship between C. trachomatis and genital tract microbiota have focused only the vagina, which is not the site of C. trachomatis infection. Here, we find significant differences between endocervical and vaginal microbiota, and identify associations between cervical bacterial taxa and C. trachomatis infection in African adolescents.
Although the overall bacterial community compositions were found to be more similar between the endocervix and vagina within each participant than between participants, we found significantly different β-diversity between endocervical and vaginal samples within clusters. Furthermore, consistent with the results of others21 we found the within sample diversity in the vagina to be higher than in the ectocervix. Although previous studies have suggested that the microbiota in different anatomical sites within the FGT of adults is similar in composition16–18, we identified multiple taxa that were significantly more abundant in the vagina. These included anaerobes, such as Peptoniphilus, Anaerococcus and Fusobacterium spp. The endocervix also had significantly more E. faecium, the source of which is likely the gastrointestinal tract. These findings are surprising, since the endocervix is further from the external (aerobic) environment and the rectum, than the vagina. In Chinese women19, L. crispatus was notably more abundant in the vagina compared to the endocervix, which may be due to epithelial cell characteristics. L. crispatus expresses an adhesion molecule which mediates adherence to stratified squamous epithelium (found in the vagina) but not to columnar epithelium which constitutes the cervical epithelium22. Therefore, factors other than geography likely play a role in microbial ecosystems in the FGT. Since adolescence is a time of change in the cervix, it is possible that the differences we see here are unique to this age group. Alternatively, differences in sample collection technique between lateral vaginal wall and endocervical swabs may have occurred.
No studies have explored the relationship between specific taxa in the endocervix and C. trachomatis in adolescents. Our findings suggest the endocervical microbiota diversity is not grossly altered in women with C. trachomatis, although the study was small. However, we found some taxa differentially abundant between C. trachomatis infected and uninfected women, including G. vaginalis, Megasphaera, A. vaginae, Dialister ssp., Prevotella spp. and Megasphaera, all BV-associated bacteria. Several studies have reported an increased risk of C. trachomatis infection among women with BV2,5,23. In a longitudinal study among Dutch women, microbiota dominated by L. iners was an independent risk factor for later C. trachomatis acquisition12, and L. crispatus may have been protective11–14. The correlation between lactobacilli and vaginal health is often attributed to the production of lactic acid resulting in low vaginal pH. Importantly, L. crispatus produces significantly more D-lactate than L. iners, and this difference has been shown to be important in trapping of pathogens24. Indeed, L. crispatus has been shown to inhibit growth, adhesion and infectivity of C. trachomatis in vitro, attributed to the production of D-lactate25–27. Of note, L. iners is far more prevalent than other lactobacilli in African women, often co-exists with BV9,20, and may be one of the reasons why women of African descent are at high risk for STIs.
One limitation of this study is its cross-sectional nature, which precludes definitive conclusions regarding whether specific taxa directly increase risk of C. trachomatis infection. Prospective longitudinal cohort studies will allow comparison of microbial communities prior to and after infection with C. trachomatis, which will allow us to make conclusions regarding the role of microbiota in modifying C. trachomatis risk in adolescents. None-the-less, the potential for altered functional profiles of C. trachomatis susceptible bacterial communities between different anatomical sites exists, despite overall compositional similarities between women with and without C. trachomatis infections.
Methods
Study participants and sample collection
The study population consisted of 149 16–22-year-old women from a low income, high population density community in Cape Town, South Africa. Participants were enrolled in the Women’s Initiative in Sexual Health (WISH) study between November 2013 and December 2014. Detailed procedures and characteristics of the cohort have previously been described28. Approval for the study was obtained from the Human Research Ethics Committee of the University of Cape Town and all study procedures were conducted in accordance with the International Conference on Harmonization (ICH) and the South African Good Clinical Practice Guidelines29. All participants 18 years or older provided informed consent, while informed parental consent and participant assent were obtained for those younger than 18 years. Females were enrolled if they were HIV-negative, in good health, not pregnant or menstruating at the time of sampling, if they had not had unprotected sex or douched in the last 48 hours, nor taken antibiotics in the prior two weeks. The women were followed longitudinally every two months if they were using Norethisterone enanthate (NET-EN) injectable contraceptive, combined oral contraceptives or barrier contraception only, or every three months if they were using depot medroxyprogesterone acetate (DMPA) injectable contraceptive, for a total of three visits. Study visits were scheduled two weeks after injection for participants on injectable contraceptives, or otherwise during the luteal phase of their menstrual cycles (between day 14–28) if they were not using hormonal contraceptives or if they were using oral contraceptives. Prior to any specimen collection, an HIV rapid test (Alere Determine™ HIV-1/2 Ag/Ab Combo, Alere, Waltham, MA), a pregnancy test (U-test Pregnancy strip, Humor Diagnostica, Pretoria, South Africa) and a general physical examination was performed. At each visit, vulvovaginal swabs for STI testing and Nugent scoring, as well as swabs from the lateral vaginal wall and endocervix for microbiome analyses, were obtained.
hours, nor taken antibiotics in the prior two weeks. The women were followed longitudinally every two months if they were using Norethisterone enanthate (NET-EN) injectable contraceptive, combined oral contraceptives or barrier contraception only, or every three months if they were using depot medroxyprogesterone acetate (DMPA) injectable contraceptive, for a total of three visits. Study visits were scheduled two weeks after injection for participants on injectable contraceptives, or otherwise during the luteal phase of their menstrual cycles (between day 14–28) if they were not using hormonal contraceptives or if they were using oral contraceptives. Prior to any specimen collection, an HIV rapid test (Alere Determine™ HIV-1/2 Ag/Ab Combo, Alere, Waltham, MA), a pregnancy test (U-test Pregnancy strip, Humor Diagnostica, Pretoria, South Africa) and a general physical examination was performed. At each visit, vulvovaginal swabs for STI testing and Nugent scoring, as well as swabs from the lateral vaginal wall and endocervix for microbiome analyses, were obtained.
STI and BV testing
Vulvovaginal swabs were tested with a variety of in-house multiplex and commercial PCR assays (Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, Mycoplasma genitalium, herpes simplex virus type 1 (HSV-1) and HSV-2, Haemophilus ducreyi, Treponema pallidum, for positive C. trachomatis DNA extracts, additional testing for Lymphogranuloma venereum-associated serovars) as previously described21. Chlamydial infections were further differentiated into lymphogranuloma venereum (LGV)-associated serovars or non-LGV serovars, using an LGV-specific PCR. Blood was obtained for HIV rapid testing and HSV-2 serology. Endocervical swabs were collected for human papillomavirus (HPV) detection and genotyping by Roche Linear Array30. Posterior fornix swabs were collected for Nugent scoring to classify samples as BV negative (Nugent 0–3), intermediate (Nugent 4–6) or positive (Nugent 7–10); and vaginal pH was measured using colour-fixed indicator strips (Macherey-Nagel, Düren, Germany).
16S rRNA gene amplification and Illumina MiSeq sequencing
Vaginal lateral wall and endocervical swabs were collected for microbiome analysis by 16S rRNA gene sequencing. Matched lateral wall and endocervical swabs from the first sample visit were thawed and treated with an enzyme cocktail consisting of mutanolysin (25kU/ml, Sigma Aldrich, Modderfontein, RSA), lysozyme (450kU/ml, Sigma Aldrich), and lysostaphin (4kU, Sigma Aldrich) for 1 hour at 37
hour at 37 °C. This was followed by mechanical disruption using the Thermo Savant FastPrep 120 Cell Disrupter system for 3
°C. This was followed by mechanical disruption using the Thermo Savant FastPrep 120 Cell Disrupter system for 3 ×
× 30
30 seconds at speed setting 5.5
seconds at speed setting 5.5 m/s. Microbial DNA was extracted using the PowerSoil DNA Isolation kit (Mo Bio Laboratories Inc., Germantown, MD, USA) following the manufacturer’s protocol. The V4 hypervariable region of the bacterial 16
m/s. Microbial DNA was extracted using the PowerSoil DNA Isolation kit (Mo Bio Laboratories Inc., Germantown, MD, USA) following the manufacturer’s protocol. The V4 hypervariable region of the bacterial 16 S rRNA gene was amplified using modified universal primers31. Pooled duplicate samples were purified using Agencourt AMPure XP beads (Beckman Coulter, Brea, CA, United States) and quantified using the Qubit dsDNA HS Assay (Life Technologies, Carlsbad, CA, USA). Illumina sequencing adapters and dual-index barcodes were added to the purified amplicon products using limited cycle PCR and the Nextera XT Index Kit (Illumina, San Diego, CA, USA). Amplicons from 96 samples and controls were pooled in equimolar amounts and the resultant libraries purified by gel extraction (Qiagen, Hilden, Germany) and quantified. The libraries were sequenced on an Illumina MiSeq platform (300
S rRNA gene was amplified using modified universal primers31. Pooled duplicate samples were purified using Agencourt AMPure XP beads (Beckman Coulter, Brea, CA, United States) and quantified using the Qubit dsDNA HS Assay (Life Technologies, Carlsbad, CA, USA). Illumina sequencing adapters and dual-index barcodes were added to the purified amplicon products using limited cycle PCR and the Nextera XT Index Kit (Illumina, San Diego, CA, USA). Amplicons from 96 samples and controls were pooled in equimolar amounts and the resultant libraries purified by gel extraction (Qiagen, Hilden, Germany) and quantified. The libraries were sequenced on an Illumina MiSeq platform (300 bp paired-end) with v3 chemistry.
bp paired-end) with v3 chemistry.
16S rRNA gene sequencing analysis
Following demultiplexing, raw reads were preprocessed as follows: forward and reverse reads were merged using usearch732, allowing a maximum of three mismatches and the resultant merged reads were quality filtered using usearch7 (reads with E scores larger than 0.1 were discarded). Next, primer sequences were removed using a custom python script and the reads truncated at 250 bp. Sequences were then de-replicated whilst recording the level of replication for each sequence using usearch7. De-replicated sequences were sorted by abundance (highest to lowest) and clustered de novo into operational taxonomic units (OTUs) at 97% similarity using usearch7. Chimeric sequences were detected (against the Gold database) using UCHIME33 and removed. Individual sequences were assigned to the specific identifiers using a 97% similarity threshold. Taxonomic assignment was performed in QIIME 1.8.034 using the RDP classifier (using the default confidence level of 0.5) against the GreenGenes 13.8 reference taxonomy for 97% identity. To increase species-level resolution, we used the usearch_global command implemented in VSEARCH35 to search the de novo picked OTUs’ representative sequences against a Custom Vaginal 16
bp. Sequences were then de-replicated whilst recording the level of replication for each sequence using usearch7. De-replicated sequences were sorted by abundance (highest to lowest) and clustered de novo into operational taxonomic units (OTUs) at 97% similarity using usearch7. Chimeric sequences were detected (against the Gold database) using UCHIME33 and removed. Individual sequences were assigned to the specific identifiers using a 97% similarity threshold. Taxonomic assignment was performed in QIIME 1.8.034 using the RDP classifier (using the default confidence level of 0.5) against the GreenGenes 13.8 reference taxonomy for 97% identity. To increase species-level resolution, we used the usearch_global command implemented in VSEARCH35 to search the de novo picked OTUs’ representative sequences against a Custom Vaginal 16 S Reference Database (described previously20). All hits with ≥97% identity were accepted. The remaining OTUs (n
S Reference Database (described previously20). All hits with ≥97% identity were accepted. The remaining OTUs (n =
= 109) were manually curated using both BLAST on NCBI’s nucleotide database (excluding uncultured organisms) and with usearch_global against the Vaginal 16S rDNA Reference Database36. Samples with ≥5000 reads were selected for downstream analyses. The OTU table was normalized (i.e. transformed to relative abundance * median sample read depth), and filtered to include OTUs with at least 10 counts in at least 20% of samples or a relative abundance of at least 0.001%.
109) were manually curated using both BLAST on NCBI’s nucleotide database (excluding uncultured organisms) and with usearch_global against the Vaginal 16S rDNA Reference Database36. Samples with ≥5000 reads were selected for downstream analyses. The OTU table was normalized (i.e. transformed to relative abundance * median sample read depth), and filtered to include OTUs with at least 10 counts in at least 20% of samples or a relative abundance of at least 0.001%.
Statistical analyses
All downstream statistical analyses were performed in RStudio37 using the packages phyloseq38 for beta diversity analyses, metagenomeSeq39 for differential abundance testing, vegan40 for ordinations and redundancy analysis, and pheatmap41 and NMF42 for annotated heat maps. Microbiota subtypes were established by fuzzy clustering using the R package ‘cluster’43 with k =
= 3 (optimal k), a membership exponent of 1.25 and weighted Unifrac as the dissimilarity measure. Members with a probability of less than 60% of belonging to any of the three clusters were excluded from downstream analyses (n
3 (optimal k), a membership exponent of 1.25 and weighted Unifrac as the dissimilarity measure. Members with a probability of less than 60% of belonging to any of the three clusters were excluded from downstream analyses (n =
= 4).
4).
Differences in study population characteristics were tested using the Chi-square, Fisher’s exact (when the expected value was <
< 5) or Mann-Whitney U test, accordingly. To examine whether the community type (C1–3) or any demographic or behavioral characteristic were associated with C. trachomatis infection we performed univariate and multivariate logistic regression analyses. Variation partitioning to investigate factors influencing microbial composition was performed by analysis of variance using distance matrices (adonis) with 999 permutations using weighted UniFrac distance measures. Specific differences in microbial composition between groups were assessed using metagenomeSeq’s MRfulltable function with a custom filter to determine significance. Merged taxa were deemed significantly different if they exhibited a fold change (beta coefficient) of ≥1.25, had an adjusted p-value of ≤0.05 and if at least one of the two groups being compared had ≥20% of samples with the given OTU/taxa OR the Fisher’s exact test result was significant (after multiple testing correction (MTC) by Benjamini-Hochberg method. OTUs were first merged at the lowest available taxonomic level using a custom script20. Random forests analyses were conducted on merged OTUs using the R package randomForest44. For randomForest, the data were randomly divided into training and test sets, comprising two thirds and one third of the data, respectively. Ecologic distances between lateral wall and endocervical samples from the same participants were compared with distances between samples from the same site from different particpants by comparing within-subject pair-wise distances (weighted Unifrac) with the mean of between-subject pair-wise distances using paired t-tests.
5) or Mann-Whitney U test, accordingly. To examine whether the community type (C1–3) or any demographic or behavioral characteristic were associated with C. trachomatis infection we performed univariate and multivariate logistic regression analyses. Variation partitioning to investigate factors influencing microbial composition was performed by analysis of variance using distance matrices (adonis) with 999 permutations using weighted UniFrac distance measures. Specific differences in microbial composition between groups were assessed using metagenomeSeq’s MRfulltable function with a custom filter to determine significance. Merged taxa were deemed significantly different if they exhibited a fold change (beta coefficient) of ≥1.25, had an adjusted p-value of ≤0.05 and if at least one of the two groups being compared had ≥20% of samples with the given OTU/taxa OR the Fisher’s exact test result was significant (after multiple testing correction (MTC) by Benjamini-Hochberg method. OTUs were first merged at the lowest available taxonomic level using a custom script20. Random forests analyses were conducted on merged OTUs using the R package randomForest44. For randomForest, the data were randomly divided into training and test sets, comprising two thirds and one third of the data, respectively. Ecologic distances between lateral wall and endocervical samples from the same participants were compared with distances between samples from the same site from different particpants by comparing within-subject pair-wise distances (weighted Unifrac) with the mean of between-subject pair-wise distances using paired t-tests.
Acknowledgements
The DTHF also recognizes the support from ViiV health care in their YouthShield program. PHRU was supported through funding from the South African Medical Research Council. ALW’s work is based upon research supported by the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation. We thank the WISH Study Teams, particularly Ms Pinky Ngobo, Sr Nozipho Hadebe, Sr Janine Nixon, and all the young women who kindly participated in the study. Computations were performed using facilities provided by the University of Cape Town’s ICTS High Performance Computing team: http://hpc.uct.ac.za. This study was supported by grants from the European and Developing Countries Clinical Trials Partnership (EDCTP) Strategic Primer grant [SP·2011·41304·038] and the South African Department of Science and Technology [DST/CON 0260/2012]. HBJ was supported in part by K08HD069201. KL was supported by the Harry Crossley Foundation and the Suid-Afrikaanse Akademie vir Wetenskap en Kuns. SLB was supported by the HIV Vaccine Trials Network SHAPe Program, the Fogarty Foundation and the South African Medical Research Council (MRC). SD was supported by the National Research Foundation of South Africa. MAG was supported by the University of Washington STI CRC DRP (NIH U19 AI11317).
Author Contributions
Conceived and designed the experiments: H.B.J., J.A.P., C.B., M.A.G. Recruited the cohort: S.B., L.G.B. Performed wet lab experiments: C.B., S.D., S.B., S.J. Performed S.T.I. and B.V. testing Z.M., A.L.W., D.A.L., V.M. Analyzed the data: C.B., K.L. Wrote the manuscript: C.B., K.L., J.A.P., H.B.J., S.B., S.D., D.A.L., L.G.B., S.Z.J.
Notes
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-29320-x.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Articles from Scientific Reports are provided here courtesy of Nature Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1038/s41598-018-29320-x
Read article for free, from open access legal sources, via Unpaywall:
https://www.nature.com/articles/s41598-018-29320-x.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1038/s41598-018-29320-x
Article citations
The vaginal microbiome of South African pregnant women living with human immunodeficiency virus (HIV) with and without Chlamydia trachomatis infection.
BMC Womens Health, 24(1):410, 18 Jul 2024
Cited by: 0 articles | PMID: 39026297 | PMCID: PMC11256509
Human papillomavirus and cervical cancer in the microbial world: exploring the vaginal microecology.
Front Cell Infect Microbiol, 14:1325500, 25 Jan 2024
Cited by: 1 article | PMID: 38333037 | PMCID: PMC10850380
Review Free full text in Europe PMC
Unique microbial diversity, community composition, and networks among Pacific Islander endocervical and vaginal microbiomes with and without Chlamydia trachomatis infection in Fiji.
mBio, 15(1):e0306323, 20 Dec 2023
Cited by: 0 articles | PMID: 38117091 | PMCID: PMC10790706
<i>Amygdalobacter indicium</i> gen. nov., sp. nov., and <i>Amygdalobacter nucleatus</i> sp. nov., gen. nov.: novel bacteria from the family <i>Oscillospiraceae</i> isolated from the female genital tract.
Int J Syst Evol Microbiol, 73(10), 01 Oct 2023
Cited by: 3 articles | PMID: 37787404 | PMCID: PMC11318147
Possible Therapeutic Mechanisms and Future Perspectives of Vaginal Microbiota Transplantation.
Microorganisms, 11(6):1427, 29 May 2023
Cited by: 8 articles | PMID: 37374929 | PMCID: PMC10305445
Review Free full text in Europe PMC
Go to all (30) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Metagenomic Shotgun Sequencing of Endocervical, Vaginal, and Rectal Samples among Fijian Women with and without Chlamydia trachomatis Reveals Disparate Microbial Populations and Function across Anatomic Sites: a Pilot Study.
Microbiol Spectr, 10(3):e0010522, 17 May 2022
Cited by: 10 articles | PMID: 35579443 | PMCID: PMC9241848
The Cervicovaginal Microbiota in Women Notified for Chlamydia trachomatis Infection: A Case-Control Study at the Sexually Transmitted Infection Outpatient Clinic in Amsterdam, The Netherlands.
Clin Infect Dis, 64(1):24-31, 27 Aug 2016
Cited by: 50 articles | PMID: 27567124
<i>Lactobacillus iners</i>-dominated vaginal microbiota is associated with increased susceptibility to <i>Chlamydia trachomatis</i> infection in Dutch women: a case-control study.
Sex Transm Infect, 94(2):117-123, 25 Sep 2017
Cited by: 68 articles | PMID: 28947665 | PMCID: PMC6083440
Cervicovaginal Microbiota Composition in Chlamydia trachomatis Infection: A Systematic Review and Meta-Analysis.
Int J Mol Sci, 23(17):9554, 23 Aug 2022
Cited by: 7 articles | PMID: 36076948 | PMCID: PMC9455926
Review Free full text in Europe PMC

 1,5,13
1,5,13



