Abstract
Free full text

Clinical Trial Participants’ Views of the Risks and Benefits of Data Sharing
Associated Data
Abstract
BACKGROUND
Sharing of participant-level clinical trial data has potential benefits, but concerns about potential harms to research participants have led some pharmaceutical sponsors and investigators to urge caution. Little is known about clinical trial participants’ perceptions of the risks of data sharing.
METHODS
We conducted a structured survey of 771 current and recent participants from a diverse sample of clinical trials at three academic medical centers in the United States. Surveys were distributed by mail (350 completed surveys) and in clinic waiting rooms (421 completed surveys) (overall response rate, 79%).
RESULTS
Less than 8% of respondents felt that the potential negative consequences of data sharing outweighed the benefits. A total of 93% were very or somewhat likely to allow their own data to be shared with university scientists, and 82% were very or somewhat likely to share with scientists in for-profit companies. Willingness to share data did not vary appreciably with the purpose for which the data would be used, with the exception that fewer participants were willing to share their data for use in litigation. The respondents’ greatest concerns were that data sharing might make others less willing to enroll in clinical trials (37% very or somewhat concerned), that data would be used for marketing purposes (34%), or that data could be stolen (30%). Less concern was expressed about discrimination (22%) and exploitation of data for profit (20%).
CONCLUSIONS
In our study, few clinical trial participants had strong concerns about the risks of data sharing. Provided that adequate security safeguards were in place, most participants were willing to share their data for a wide range of uses. (Funded by the Greenwall Foundation.)
We are rapidly moving toward a world in which broad sharing of participant-level clinical trial data is the norm.1–4 The European Medicines Agency has implemented a policy to expand public access to data concerning products it approves,5,6 the Food and Drug Administration is considering how to expand access to data pooled within a product class,7 major research sponsors8–12 and journal editors13 have begun promoting data sharing, and lawmakers’ interest14 has resulted in legislation authorizing the National Institutes of Health to require all of its grantees to share data.15,16 Pharmaceutical industry associations have committed to making data more accessible,17 and several data platforms are now available.11,18–21
Previous work has identified diverse potential benefits of expanding access to participant-level data.1,4,22 These benefits include deterring inaccurate reporting of trial results,4,23,24 accelerating scientific discovery,25 and exploring questions that are not answerable within individual trials.4,26 In addition, data sharing helps fulfill the ethical obligation to make the most of research participants’ contributions to science.13,27–30
Yet some investigators and industry sponsors of clinical trials have expressed hesitancy about the swift move toward broad data sharing. These groups have shifted from opposing data sharing to supporting it31,32; however, several concerns have led them to urge caution, limit what they share, and resist some initiatives as going too far.32,33 Chief among these are concerns about potential harm to research participants.17,32,34,35 Sponsors and investigators express worries that participants’ privacy cannot be adequately protected, particularly in light of the fact that experts have demonstrated that it is possible to reidentify participant-level data.35–39 Some pharmaceutical company representatives warn that the threat to privacy posed by data sharing will chill willingness to participate in trials, thereby delaying the availability of new therapies.36,38
It is unclear to what extent participants in clinical trials share these concerns. There is a large body of empirical literature concerning people’s preferences related to biobanking40,41 but not about clinical trials. When patient advocacy groups have spoken about data sharing, they have sometimes been challenged as parroting the views of pharmaceutical companies that financially support them rather than conveying trial participants’ views.42 One commentator recently remarked that in debates about data sharing, “Both sides claim to have the patient’s and the public’s best interests at heart, but not many partisans of either camp have asked patients what those interests are.”43 To investigate this issue, we surveyed a large sample of participants in a diverse group of clinical trials.
METHODS
PARTICIPANTS
Survey participants had been enrolled, or were the parent or guardian of someone who had been enrolled, in an interventional clinical trial within the previous 2 years. We obtained agreement from nine principal investigators (PIs) in clinical trials at three academic medical centers to facilitate access to their trial participants, including one PI who provided access to all trials in the university’s Clinical and Translational Science Institute.
We aimed for a broadly representative sample of trials that would be sufficient to provide at least 1200 potential survey participants. We selected the PIs we approached on the basis of personal contacts and stressed our interest in ensuring representation of racial and ethnic minority groups and persons with major health problems.
The final sample included both community-based trials (e.g., involving smoking cessation or diabetes prevention) and hospital-based trials (e.g., involving cancer or kidney disease). Within these trials, all the participants were eligible for the survey unless the trial team judged them as having cognitive impairment or being unable to respond to questions in English. The study was approved by the institutional review boards at Stanford and at the medical centers that provided access to the trial participants.
QUESTIONNAIRE DEVELOPMENT
A 10-page structured survey questionnaire was used to elicit clinical trial participants’ views on the sharing of data from clinical trials. Details of the survey development work, which included the use of focus groups, consultation with experts and community advisory boards, and pilot testing, are provided in Sections 2 and 5 in the Supplementary Appendix, available with the full text of this article at NEJM.org.
The questionnaire provided plain-English definitions of clinical trial, data sharing, and clinical trial data (Section 6 in the Supplementary Appendix). It included reminders that the survey was asking about sharing of individual-level information about trial participants, not research results, and that respondents should assume that the data were deidentified.
SURVEY ADMINISTRATION
Clinical trial PIs chose from among three methods of survey delivery: email, regular mail, or in-person distribution in study clinic waiting rooms. Four PIs chose regular mail, four chose the clinic, and one used both. All surveys were completed on paper, and the clinic staff’s interaction with respondents was limited to a receptionist or research assistant handing out and collecting the questionnaires (Section 1 in the Supplementary Appendix).
The surveys were accompanied by informed consent information and a $40 gift card. The responses were identified by participant identification number only.
STATISTICAL ANALYSIS
Responses were manually entered into a database in the Stanford University REDCap Survey system44 and analyzed with the use of Stata software, version 13 (StataCorp). In addition to univariate statistics and cross-tabulations, multivariable logistic-regression models were run to identify predictors of the expression of negative views of data sharing. The following outcomes were modeled: perceiving the potential negative consequences of data sharing to outweigh the benefits (either strongly, moderately, or a little); being somewhat or very unlikely to allow one’s own trial data to be shared with scientists in not-for-profit settings; and being somewhat or very unlikely to allow data to be shared with scientists in drug companies. To account for missing data, multiple imputation was performed with the Stata “mi” platform. Details of the model construction and regression results are provided in Sections 3 and 4 in the Supplementary Appendix.
RESULTS
SAMPLE CHARACTERISTICS
Completed surveys were received from 771 of the 978 invited trial participants (79%) and included 350 mailed surveys and 421 surveys completed in the clinic (Section 1 in the Supplementary Appendix). Respondents were fairly evenly distributed across the three academic medical centers (33%, 27%, and 40%) and were drawn from 119 different trials. Percentages based on the 771 respondents have a 95% confidence interval no wider than ±3.6 percentage points.
Table 1, and Table S6 in the Supplementary Appendix, show the characteristics of the sample. Within the previous 2 years, 42% of the respondents had participated in a clinical trial as a person with the health condition being studied, 55% as a healthy volunteer or person at risk for the studied health condition, and 3% as both. The two most common topics studied in the trials were diabetes and issues related to nutrition, weight, and vitamin supplementation. A total of 90% of respondents were trial participants themselves, and 7% were parents of participants. More than 94% of the respondents reported having had positive experiences as clinical trial participants. Half were motivated to participate in the trial by the prospect of a health benefit, 33% by altruism, and 16% by other factors.
Table 1
Sample Characteristics as Reported in the Survey.*
| Characteristic | No. of Participants/Total No. (%) (N = 771) |
|---|---|
| Female sex | 380/762 (49.9) |
| Age | |
 <25 yr <25 yr | 63/762 (8.3) |
 25–44 yr 25–44 yr | 177/762 (23.2) |
 45–64 yr 45–64 yr | 286/762 (37.5) |
 ≥65 yr ≥65 yr | 236/762 (31.0) |
| Hispanic ethnic group | 101/759 (13.3) |
| Race | |
 White White | 518/768 (67.4) |
 Black or African American Black or African American | 113/768 (14.7) |
 American Indian or Alaskan Native American Indian or Alaskan Native | 51/768 (6.6) |
 Asian Asian | 25/768 (3.3) |
 Other Other | 61/768 (7.9) |
| Education | |
 Less than high school Less than high school | 40/752 (5.3) |
 High-school diploma High-school diploma | 125/752 (16.6) |
 Some college Some college | 206/752 (27.4) |
 College degree College degree | 238/752 (31.6) |
 Graduate degree Graduate degree | 143/752 (19.0) |
| Annual family income | |
 Less than $15,000 to $24,999 Less than $15,000 to $24,999 | 173/742 (23.3) |
 $25,000 to $54,999 $25,000 to $54,999 | 206/742 (27.8) |
 $55,000 to $99,999 $55,000 to $99,999 | 189/742 (25.5) |
 $100,000 or higher $100,000 or higher | 174/742 (23.5) |
| Health status | |
 Excellent Excellent | 168/757 (22.2) |
 Good Good | 420/757 (55.5) |
 Fair Fair | 156/757 (20.6) |
 Poor Poor | 13/757 (1.7) |
| Trial topic | |
 Nutrition, weight, or vitamins Nutrition, weight, or vitamins | 172/771 (22.3) |
 Diabetes Diabetes | 172/771 (22.3) |
 Cardiovascular disease Cardiovascular disease | 71/771 (9.2) |
 Aging, neurodegenerative disease, or memory Aging, neurodegenerative disease, or memory | 64/771 (8.3) |
 Tobacco use Tobacco use | 52/771 (6.7) |
 Liver disease Liver disease | 49/771 (6.4) |
 Mental illness Mental illness | 41/771 (5.3) |
 Cancer Cancer | 39/771 (5.1) |
 Kidney disease Kidney disease | 26/771 (3.4) |
 Other Other | 85/771 (11.0) |
| Overall experience as a trial participant | |
 Very positive Very positive | 573/752 (76.2) |
 Somewhat positive Somewhat positive | 136/752 (18.1) |
 Neither positive nor negative Neither positive nor negative | 34/752 (4.5) |
 Somewhat negative Somewhat negative | 9/752 (1.2) |
 Very negative Very negative | 0 |
PERCEIVED RISKS OF DATA SHARING
For 9 of 11 potential consequences of data sharing, less than 10% of the respondents said they were “very concerned” and less than one third were “very” or “somewhat” concerned about the risk (Fig. 1). A total of 20% to 26% of the respondents were very or somewhat concerned about discrimination, reidentification, and exploitation of data for profit. Respondents were more concerned that data sharing could deter people from enrolling in clinical trials (37%), that companies might use the information for marketing purposes (34%), or that their data could be stolen (30%). Asked to select the most important potential risk, respondents expressed divergent views, with the most common choices being that the information might be stolen (15%) or used for marketing purposes (11%) and that others might be more reluctant to enroll in clinical trials if they knew their data would be shared (10%) (Table S7 in the Supplementary Appendix).
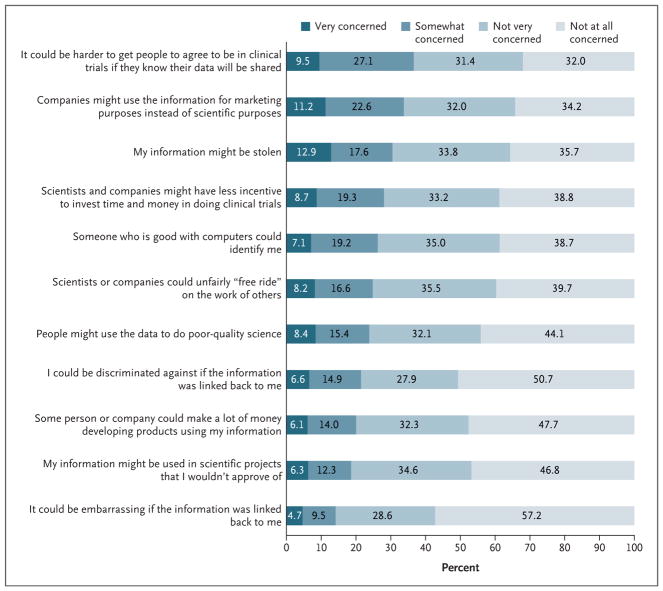
Shown are the responses to an item worded as “How concerned are you about the following potential consequences of sharing anonymous, individual clinical trial data?” Numbers were rounded to the nearest tenth. The accuracy (95% confidence interval) of the percentages close to 50% is ±3.6 percentage points, diminishing to ±2.2 percentage points for percentages close to 10%.
PERCEIVED BENEFITS OF DATA SHARING
Strong majorities of respondents (67% to 82%) believed that data sharing would yield “a great deal” or “a lot” of several benefits (Fig. 2). In contrast, 43% believed it would help lawyers prove their case in product liability lawsuits. When respondents were asked to choose the most important benefit of data sharing, the most popular choices were making sure people’s participation in clinical trials leads to the most scientific benefit possible (18%) and helping to get answers to scientific questions faster (17%) (Table S8 in the Supplementary Appendix). More than 85% of respondents expected that scientists in universities and other not-for-profit settings would benefit “a great deal” or “a lot” from data sharing; 81% of respondents had this expectation for physicians taking care of patients, 79% for companies developing medical products, and 72% for patients (Table S9 in the Supplementary Appendix)
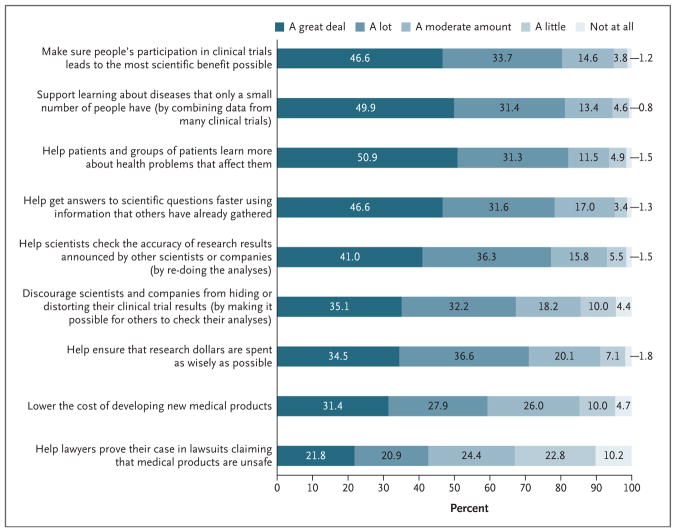
Shown are the responses to an item worded as “How much do you think sharing anonymous, individual clinical trial data can . . . .” Numbers were rounded to the nearest tenth. The accuracy (95% confidence interval) of percentages close to 50% is ±3.6 percentage points, diminishing to ±2.2 percentage points for percentages close to 10%.
OVERALL SUPPORT FOR DATA SHARING
In response to a question at the end of the survey, 82% of respondents indicated that they perceived that the benefits of data sharing outweighed the negative aspects, 8% felt the negative aspects outweighed the benefits, and 10% considered them equal (Table S10 in the Supplementary Appendix).
A total of 93% of respondents said they were very (69%) or moderately (24%) likely to allow their clinical trial data to be shared with scientists in universities and other not-for-profit organizations (Table 2), and 4% were very or somewhat unlikely to share. Although respondents had less trust in drug companies (18% trusted them a great deal or a lot) and health insurance companies (15%) than in universities (63%), 82% reported that they would be very or somewhat willing to share data with for-profit companies, whereas 8% were very or somewhat unwilling to share (Table 2, and Table S11 in the Supplementary Appendix).
Table 2
Willingness of Clinical Trial Participants to Share Their Data, According to Type of Use and Recipient.*
| Type of Use or Recipient | Very Likely | Somewhat Likely | Neither Likely nor Unlikely | Somewhat Unlikely | Very Unlikely |
|---|---|---|---|---|---|
| percent of respondents | |||||
| Type of use | |||||
|
| |||||
 To help patients and groups of patients learn more about health problems that affect them To help patients and groups of patients learn more about health problems that affect them | 77.8 | 18.8 | 2.6 | 0.4 | 0.4 |
|
| |||||
 To do research on health problems that affect my family or me To do research on health problems that affect my family or me | 78.3 | 17.1 | 3.2 | 1.1 | 0.5 |
|
| |||||
 To do research that will help others To do research that will help others | 79.9 | 17.1 | 2.0 | 0.4 | 0.7 |
|
| |||||
 To help get answers to scientific questions faster using information that others have already gathered To help get answers to scientific questions faster using information that others have already gathered | 72.2 | 22.6 | 3.4 | 1.2 | 0.5 |
|
| |||||
 To help scientists check the accuracy of research results announced by other scientists or companies (by re-doing the analyses) To help scientists check the accuracy of research results announced by other scientists or companies (by re-doing the analyses) | 70.9 | 22.6 | 3.8 | 1.5 | 1.2 |
|
| |||||
 To learn more about diseases that only a small number of people have (by combining data from many clinical trials) To learn more about diseases that only a small number of people have (by combining data from many clinical trials) | 69.1 | 22.1 | 5.9 | 1.7 | 1.2 |
|
| |||||
 To help lawyers prove their case in lawsuits claiming that medical products are unsafe To help lawyers prove their case in lawsuits claiming that medical products are unsafe | 27.9 | 24.5 | 26.9 | 12.7 | 8.0 |
|
| |||||
| Recipient | |||||
|
| |||||
 Scientists in universities and other not-for-profit organizations Scientists in universities and other not-for-profit organizations | 69.2 | 24.0 | 3.3 | 1.7 | 1.8 |
|
| |||||
 Scientists in companies developing medical products, such as prescription drugs Scientists in companies developing medical products, such as prescription drugs | 53.4 | 28.5 | 10.6 | 5.4 | 2.1 |
Willingness to share data varied little according to the purpose for which it would be used — with the exception of its use in lawsuits, although a majority of respondents were still willing to share even for that purpose (Table 2). No appreciable differences were found between uses that did and uses that did not benefit the participant directly or between uses for verifying previous research results and uses for making new discoveries.
Among the write-in comments, the most dominant theme was the need to help others as much as possible. Many commenters expressed confidence in the deidentification of data. Several urged greater cooperation and less competition among scientists.
PREDICTORS OF ATTITUDES
In multivariable modeling, the likelihood that a respondent would feel that the negative aspects of data sharing outweighed the benefits was significantly higher among those who felt that other people generally could not be trusted (odds ratio, 2.3; 95% confidence interval [CI], 1.2 to 4.6) and among those who were concerned about the risk of reidentification (odds ratio, 2.4; 95% CI, 1.2 to 4.5) or about information theft (odds ratio, 2.2; 95% CI, 1.2 to 4.1) (Section 4 in the Supplementary Appendix). The only other significant predictor was having a college degree, which was associated with a lower likelihood of feeling that the negative aspects of data sharing outweighed the benefits (odds ratio, 0.27; 95% CI, 0.2 to 0.5).
A low level of trust in people was also a significant predictor of being somewhat or very unlikely to share one’s own data with scientists in not-for-profit contexts (odds ratio, 3.7; 95% CI, 1.6 to 8.3) or drug companies (odds ratio, 2.5; 95% CI, 1.3 to 4.8). Low trust in drug companies was a significant predictor of unwillingness to share data with drug-company scientists (odds ratio, 2.4; 95% CI, 1.4 to 4.2). Having a college degree was associated with a significantly lower likelihood of refusing to share data with not-for-profit scientists (odds ratio, 0.28; 95% CI, 0.10 to 0.78).
DISCUSSION
In this study assessing the views of clinical trial participants on the sharing of participant-level clinical trial data beyond genomic information, several key messages emerged. First, most of the clinical trial participants in our study believed that the benefits of data sharing outweighed the potential negative aspects and were willing to share their data. Their willingness to share was high regardless of the way in which the data would be used, with the exception of litigation, and it extended to uses that involved no prospect of direct benefit to themselves or their family members. Despite low levels of trust in pharmaceutical companies, most trial participants were willing to share their data with them.
The respondents’ lack of differentiation among different data users and uses contrasts with previous study findings related to biobank participation. Those studies consistently showed substantially less willingness to share biospecimens with researchers in for-profit companies than with university researchers.45–53 One study showed the same effect for sharing information from electronic health records (EHRs) for research purposes.54
The willingness of the respondents in our study to share clinical trial data was greater than that found in many previous studies that involved participants’ attitudes toward research use of biospecimens or EHR data.40,41,54–56 Expanding access to clinical trial data shares some ethical complexities with biobanking, such as how to obtain meaningful informed consent, but genetic information raises special concerns.45,57 On the other hand, clinical trial data include information from medical records and questionnaires that reveals much more about participants than biospecimens. Some such information — for example, sexual orientation or substance use — may carry serious social risks.38 A further consideration is that with rare exceptions,58 biobanking studies presume that an institutional review board will approve future uses of the data — a safeguard that may not be present for sharing of clinical trial data. Finally, biobanking and EHR studies have generally presumed that the data would be used by qualified researchers, but some proposals for “open access” data sharing are not so limited.1,4
The values and concerns of clinical trial participants may differ from those of the general public, patients in general, or other populations surveyed in biobanking and EHR studies. Clinical trial participants typically constitute a small proportion of the people who are eligible for participation and may represent those who are least bothered by data sharing and most enthusiastic about contributing to science. Their familiarity with physician-researchers may impart especially high trust in research and researchers.59 Indeed, nearly all of our respondents reported very positive experiences as trial participants.
Our findings are broadly consistent with other literature on engagement in clinical trials in underscoring the idea that altruism as well as self-regarding motivations influence participation decisions.60,61 In write-in comments, many respondents expressed the view that agreeing to broad use of their data was inherent in agreeing to participate in research.
A second finding of our study is that even when presented with a list of negative potential consequences, most trial participants do not express substantial concern about the risks of data sharing. On average, across the negative consequences they considered, approximately 8% of respondents were very concerned and 17% somewhat concerned. However, a substantial minority of respondents did express some concern, especially about discouraging others from volunteering for trials (37% somewhat or very concerned), having information used for marketing (34%), and having information stolen (31%). Many potential harms that trial sponsors and investigators worry a great deal about, such as reidentification and discrimination, were not of great concern to a sizable majority of participants, a finding that differs from surveys about biobanking that highlight these issues as leading concerns.62
Third, multivariable analysis revealed few differences in views across participant subgroups. Despite concern that distrust in research among African Americans may extend to data sharing,1,46,58,63 we found no significant differences according to race. Because few of our respondents expressed negative views of data sharing, only large subgroup differences were detectable.
Our study had limitations. The respondents were relatively healthy: approximately a quarter characterized their health status as fair or poor. Although health status was not a significant predictor of attitudes in our models, a less healthy group of respondents might have reported different views. Our response rate was high, but we cannot exclude the possibility of nonresponse bias. Some people may decline to enroll in clinical trials out of concern that their data might be shared, and they are not represented in our sample. The survey concepts were complex, and although we conducted pilot work to clarify questions, some respondents may have had comprehension difficulties or lacked sufficient understanding of data sharing to meaningfully assess the potential consequences. Finally, respondents’ actual willingness to share their data might be lower than their hypothetical willingness. Previous research on genomic data, however, has shown the reverse.59,62
Our findings suggest that concerns about trial participants’ attitudes toward data sharing invoked by companies and investigators who caution against it may be exaggerated. Participants perceive data sharing to have many benefits, and most are willing to share their data. Finally, participants’ concern about the use of their data for marketing is worth addressing. Data repositories could require data requesters to attest that no marketing use will occur, and consent documents could offer assurances about this requirement.
Reaching a world in which the sharing of clinical trial data is routine requires surmounting several challenges — financial, technical, and operational. But in this survey, participants’ objections to data sharing did not appear to be a sizable barrier.
Acknowledgments
Supported by a grant from the Greenwall Foundation. Research information technology used in the study was funded by a grant from the National Center for Research Resources, National Institutes of Health (Clinical and Translational Sciences Award UL1 TR001085, to Stanford University).
We thank the clinical trial principal investigators and teams for their generosity in facilitating access to the trial participants and administering the survey; Harry Selker, Edward Kuczynski, Anantha Shekhar, Laurie Trevino, and Brenda Hudson for connecting us with the trial teams; Sayeh Fattahi, Cynthia Rinaldo, and Quinn Walker for research assistance; and Jon Krosnick, Eric Campbell, Joe Ross, Jennifer Miller, Randy Stafford, and members of Dr. Stafford’s American Indian Community Action Board for feedback on earlier drafts of the survey questionnaire.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
Full text links
Read article at publisher's site: https://doi.org/10.1056/nejmsa1713258
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc6057615?pdf=render
Citations & impact
Impact metrics
Article citations
Reporting on data sharing: executive position of the EQUATOR Network.
BMJ, 386:e079694, 13 Aug 2024
Cited by: 0 articles | PMID: 39137944 | PMCID: PMC11320295
Unleashing the power of clinical trial data: a proposal for enhancing informed consent and data sharing.
Oncologist, 29(8):651-652, 01 Aug 2024
Cited by: 0 articles | PMID: 38856720 | PMCID: PMC11299935
Evaluating the discoverability of supporting research materials in ClinicalTrials.gov for US federally funded COVID-19 clinical studies.
J Med Libr Assoc, 112(3):250-260, 29 Jul 2024
Cited by: 0 articles | PMID: 39308913 | PMCID: PMC11412123
Description of subgroup reporting in clinical trials of chronic diseases: a meta-epidemiological study.
BMJ Open, 14(6):e081315, 21 Jun 2024
Cited by: 0 articles | PMID: 38908852 | PMCID: PMC11328666
Breaking the silence of sharing data in medical research.
PLoS One, 19(5):e0301917, 29 May 2024
Cited by: 0 articles | PMID: 38809894 | PMCID: PMC11135734
Go to all (93) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A two-site survey of medical center personnel's willingness to share clinical data for research: implications for reproducible health NLP research.
BMC Med Inform Decis Mak, 19(suppl 3):70, 04 Apr 2019
Cited by: 5 articles | PMID: 30943963 | PMCID: PMC6448185
The benefits, risks and costs of privacy: patient preferences and willingness to pay.
Curr Med Res Opin, 33(5):845-851, 12 Mar 2017
Cited by: 3 articles | PMID: 28166481
The project data sphere initiative: accelerating cancer research by sharing data.
Oncologist, 20(5):464-e20, 15 Apr 2015
Cited by: 50 articles | PMID: 25876994 | PMCID: PMC4425388
Attitudes of research participants and the general public towards genomic data sharing: a systematic literature review.
Expert Rev Mol Diagn, 14(8):1053-1065, 26 Sep 2014
Cited by: 57 articles | PMID: 25260013
Review
Funding
Funders who supported this work.
Greenwall Foundation (1)
Grant ID: None
NCATS NIH HHS (1)
Grant ID: UL1 TR001085





