Abstract
Objectives
This study aimed to determine temporal trends in the incidence of and mortality associated with heart failure (HF) and its subtypes (heart failure with reduced ejection fraction [HFrEF] and heart rate with preserved ejection fraction [HFpEF]) in the community.Background
Major shifts in cardiovascular disease risk factor prevalence and advances in therapies may have influenced HF incidence and mortality.Methods
In the FHS (Framingham Heart Study) and CHS (Cardiovascular Health Study), for participants who were ≥60 years of age and free of HF (n = 15,217; 60% women; 2,524 incident HF cases; 115,703 person-years of follow-up), we estimated adjusted incidence rate ratios of HF, HFrEF, and HFpEF from 1990 to 1999 and 2000 to 2009. We compared the cumulative incidence of and mortality associated with HFrEF versus HFpEF within and between decades.Results
Across the 2 decades, HF incidence rate ratio was similar (p = 0.13). The incidence rate ratio of HFrEF declined (p = 0.0029), whereas HFpEF increased (p < 0.001). Although HFrEF incidence declined more in men than in women, men had a higher incidence of HFrEF than women in each decade (p < 0.001). The incidence of HFpEF significantly increased over time in both men and women (p < 0.001 and p = 0.02, respectively). During follow-up after HF, 1,701 individuals died (67.4%; HFrEF, n = 557 [33%]; HFpEF, n = 474 [29%]). There were no significant differences in mortality rates (overall, cardiovascular disease, and noncardiovascular disease) across decades within HF subtypes or between HFrEF and HFpEF within decade.Conclusions
In several U.S. community-based samples from 1990 to 2009, we observed divergent trends of decreasing HFrEF and increasing HFpEF incidence, with stable overall HF incidence and high risk for mortality. Our findings highlight the need to elucidate factors contributing to these observations.Free full text

Temporal Trends in the Incidence of and Mortality Associated with Heart Failure with Preserved and Reduced Ejection Fraction
Abstract
Objectives
To determine temporal trends in the incidence of and mortality associated with HF and its subtypes (HF with reduced and preserved ejection fraction: HFREF, HFPEF) in the community.
Background
Major shifts in cardiovascular disease risk factor prevalence and advances in therapies may have influenced heart failure (HF) incidence and mortality.
Methods
In Framingham Heart and Cardiovascular Health Study participants ≥60 years and free of HF (n=15,217, 60%F, 2,524 incident HF, 115,703 person-years of follow-up), we estimated adjusted incidence rate ratios of HF, HFREF, and HFPEF for 1990–1999 and 2000–2009. We compared the cumulative incidence of and mortality associated with HFREF vs. HFPEF within and between decades.
Results
Across the two decades, HF incidence rate ratio was similar (p=0.13). Incidence rate ratio of HFREF declined (p=0.0029) of HFPEF increased (p<0.001). Though HFREF incidence declined more in men than women, men had a higher incidence of HFREF than women in each decade (p<0.001). The incidence of HFPEF significantly increased over time in both men and women (p<0.001 and p=0.02, respectively). During follow-up after HF, 1,701 individuals died [67.4%; HFREF, n=557 (33%), HFPEF, n=474 (29%)]. There were no significant differences in mortality rates (overall, CVD, and non-CVD) across decades within HF subtypes, nor between HFREF and HFPEF within decade.
Conclusions
In several U.S. community-based samples from 1990–2009, we observed divergent trends of decreasing HFREF and increasing HFPEF incidence, with stable overall HF incidence and high risk for mortality. Our findings highlight the need to elucidate factors contributing to these observations.
INTRODUCTION
Despite modern advances in the prevention and treatment of cardiovascular disease (CVD) risk factors, the estimated prevalence of heart failure (HF) in the U.S. exceeded 5 million in 2010.(1) However, changing trends in the burden of major HF risk factors over the last two decades may have impacted the incidence and the mortality rates of HF and its subtypes (reduced and preserved left ventricular ejection fraction [LVEF], HFREF and HFPEF). Examination of the temporal trends in the incidence of HF, HFPEF versus HFREF, and mortality after onset of each condition may guide our understanding of the changing epidemiology of HF. Over the past decade, reports have suggested a decline in HF incidence without change in mortality.(2,3) However, data on trends in a broad community sample in the U.S. are lacking.
We hypothesized that over 1990–2009, the incidence of overall HF and HFPEF in the community declined with improvements in treatment of cardiovascular risk factors. We also hypothesized that the incidence of HFREF increased and associated mortality decreased, given favorable trends in survival post-myocardial infarction (MI) and landmark trials for treatment of HFREF. We tested these hypotheses in the Framingham Heart Study (FHS) and Cardiovascular Health Study (CHS) cohorts, which have meticulous ascertainment of HF incidence and mortality, using standardized criteria for HF, HFPEF, and HFREF.
METHODS
Study Participants
We included participants in the Framingham Heart Study (FHS) Original and Offspring Cohorts and the Cardiovascular Health Study (CHS), as these studies are representative of community-based samples in the U.S. with surveillance for and phenotyping of HF and detailed follow-up. Cohort study designs, recruitment and surveillance have been detailed previously.(4–7) Cardiovascular physical examinations occur for the FHS Original cohort every two years (since 1948) and for the Offspring Cohort approximately every four years (since 1971). The CHS includes adults ≥65 years of age, with an original cohort of 5,201 participants in 1989–1990 recruited from 4 U.S. communities: Forsyth County, NC, Sacramento County, CA, Washington County, MD, and Pittsburgh, PA. Additional participants (n=687), predominantly African-Americans, were additionally enrolled in 1992–1993. Both FHS and CHS evaluations included medical history, anthropometry and blood pressure, phlebotomy, electrocardiography, and echocardiography. We conducted analyses of HF incidence in older adults between 1990–2009 and mortality up to 5 years following HF diagnosis (n=2,524). Our samples of FHS and CHS participants were adults at least 60 years of age at the start of each decade 1990–1999 and 2000–2009. We excluded participants with prevalent HF or lacking follow up data (n=831 (5%), n=554 (8%) from FHS and n=277 (3%) from CHS).
Clinical Characteristics and Follow Up
All FHS and CHS cohort participants are under continuous surveillance for CVD events and mortality. In addition to in-person examinations, FHS participants complete regular health history updates and questionnaire-based surveys by phone. A panel of three physicians reviews all pertinent medical records to adjudicate CVD outcomes. CHS participants receive similar clinic visits, telephone contacts, and medical record review to adjudicate outcomes.(8) Clinical covariates at or at the closest examination to the incident HF event were recorded in each cohort. In both cohorts, blood pressure was recorded as the average of two measurements obtained in a seated position on resting participants separated by 5 minutes. The occurrence of MI was assessed by integrating the clinical presentation, cardiac biomarkers, and ECG. Diabetes mellitus was defined as fasting blood glucose ≥126 mg/dl, random blood glucose >200 mg/dl, or the use of hypoglycemic agents. Current smoking was considered ≥1 cigarette daily over the past year prior to the cohort examination. Obesity was defined as body mass index ≥30 kg/m2.
In the FHS, HF was diagnosed by a physician adjudication panel that reviewed all pertinent inpatient and outpatient medical records, applying FHS criteria that have been consistent over several decades.(9) The CHS Events Committee similarly adjudicated HF events by reviewing documented signs, symptoms, diagnostic test results, and/or medical treatment.(8,10,11) For the events in this analysis, either probable or definite heart failure were considered. The sensitivity and specificity of criteria for HF used in FHS and CHS have been previously reported and found to be comparable with other HF criteria(12) and the FHS and CHS definitions have similar associations with mortality.(13)
We considered the date of onset of HF as any of the following: the first episode of HF symptoms, physician visit documenting HF, or HF hospitalization in both FHS and CHS. For additional analyses, we defined HFREF and HFPEF as HF with LVEF <50% and ≥50%, respectively. This cutoff, utilized by other studies, has been considered by the American Heart Association and European Society of Cardiology reasonable to differentiate the two groups.(2,14–17) LVEF was obtained from medical record review of imaging studies, including transthoracic echocardiograms, radionuclide ventriculograms, and invasive angiocardiograms performed within a year of HF diagnosis for FHS and 30 days for CHS. Participants without an assessment of LVEF performed in proximity to the HF episode were included in the analyses of all-HF but excluded in analyses of HF subtype. We evaluated the incidence of HF (and the two subtypes HFREF vs. HFPEF) during the time period of interest, and considered 5-year follow-up for the outcome of death due to all causes among HF cases. To provide further granularity regarding cause of death, in secondary analysis, we compared the prevalence of CVD- vs. non-CVD death by HF subtype and decade. To understand the specific entities that may have contributed to CVD death, we also examined the prevalence of interim MI and sudden cardiac death within between onset of HF and death.
Statistical Analysis
We standardized HF incidence to the U.S. population aged 60–95 years in 2010. Considering differences in HF incidence by age and sex, we examined standardized age-and sex-adjusted incidence rates of HF overall, and of the subtypes HFPEF and HFREF, in each of the two decades: 1990–1999, and 2000–2009.(18) Additionally, to account for possible cohort effects, we utilized age-, sex-, and cohort-adjusted Poisson regression models which estimated the incidence rate ratio of HF and HF subtypes over time (ratio of incidence rate of HF in 2nd to 1st decade).(18) In a secondary analysis to evaluate pertinent trends in HF incidence by sex over the two decades, we evaluated patterns in incidence of these outcomes in men and women.
We followed participants with incident HF for death within five years of diagnosis (up to 2014). After confirming the proportionality of hazards assumption, we examined the age- and sex-adjusted risk for mortality within HFPEF and HFREF subtypes across the two decades using Cox regression models. Additionally, we compared age- and sex-adjusted mortality for HFPEF versus HFREF and tested whether relative risks for death by HF subtype changed between these decades. To evaluate for possible differences in characteristics of FHS and CHS participants that may have affected the results, we assessed for effect modification by cohort in mortality analyses. Furthermore, because HF is a heterogeneous disorder with multiple extra-cardiovascular comorbidities, in tertiary analysis, we determined the risks of HF and HF subtype for CVD as compared with non-CVD mortality over 1990–1999 to 2000–2009. We considered a two-tailed p≤0.05 as statistically significant. All analyses were performed using SAS v.9.4 (SAS Institute, Cary, NC).
RESULTS
Characteristics of the Sample
Characteristics of individuals with HF in each decade are presented in Table 1. We studied older adults, with slightly greater prevalence of women in both decades. Participants in the second decade had a similar to slightly higher prevalence of hypertension but greater treatment, with lower mean blood pressure. The use of antihypertensive medications was similar among HF subgroups, with similar mean blood pressure levels within each decade. Despite a slightly higher prevalence of obesity, body mass index was similar between decades and the prevalence of diabetes slightly lower in the second decade. The prevalence of smoking and myocardial infarction were lower in the second decade. In both decades, the majority of participants with HFREF had LVEF <35% (nearly 70% in FHS; 47% in CHS). Over the two decades, the use of cardiovascular medications after HF varied by class of medication, with large increases in the use of aspirin, beta blockers, and lipid-lowering agents, stable and similar use of angiotensin-converting enzyme (ACE) inhibitors and diuretics, and a decline in use of digoxin (Supplemental Table 1).
Table 1
Characteristics of FHS and CHS Participants with HF
| Characteristic | 1990–1999 | 2000–2009 | ||||
|---|---|---|---|---|---|---|
| HFREF (n=491) | HFPEF (n=309) | Unclassified HF (n=567) | HFREF (n=353) | HFPEF (n=431) | Unclassified HF (n=373) | |
| Age, years | 75±6 | 76±7 | 76±7 | 80±6 | 81±5 | 81±6 |
| Male, % | 57 | 38 | 48 | 52 | 38 | 35 |
| Race, % | ||||||
| White | 91 | 91 | 87 | 90 | 92 | 82 |
| Black | 8 | 9 | 12 | 10 | 8 | 17 |
| Hispanic | 0 | 3 | 0 | 1 | 1 | 0.3 |
| American Indian/Alaskan native | 0 | 0 | 0 | 0 | 0 | 1 |
| Asian/ Pacific Islander | 0 | 0 | 0.4 | 0 | 0 | 0.3 |
| Other | 1 | 0 | 0.4 | 0 | 0 | 0 |
| BMI, kg/m2 | 27.4±5.0 | 27.5±5.0 | 27.4±5.3 | 27.4±4.4 | 28.5±5.3 | 27.5±5.3 |
| SBP, mm Hg | 146±24 | 146±25 | 142±22 | 138±21 | 139±22 | 139±22 |
| DBP, mm Hg | 73±13 | 71±11 | 71±13 | 69±12 | 68±11 | 70±10 |
| Prevalent MI, % | 40 | 29 | 29 | 40 | 21 | 25 |
| Hypertension, % | 75 | 75 | 70 | 74 | 75 | 79 |
| Hypertension treatment, % | 61 | 61 | 60 | 66 | 68 | 73 |
| Diabetes, % | 24 | 24 | 25 | 29 | 20 | 21 |
| Smoking, % | 13 | 7 | 14 | 7 | 5 | 10 |
| Obesity, % | 20 | 24 | 24 | 24 | 26 | 24 |
BMI = body mass index. CVD = cardiovascular disease (coronary artery disease, myocardial infarction, angina, CVA, or TIA). DBP = Diastolic blood pressure. HFREF and HFPEF= heart failure with reduced (<50%) and preserved (≥50%) left ventricular ejection fraction, respectively. MI = myocardial infarction. SBP = Systolic blood pressure. Unclassified HF= HF cases in whom left ventricular ejection fraction was unavailable. Data are presented as mean±SD for continuous variables.
Incidence of HF, HFREF, and HFPEF during 1990–2009
Overall, 2,524 incident HF events occurred (1,367 in the first decade), of which 844 (33%; 491 in the first decade) were HFREF, 704 (29%; 309 in the first decade) were HFPEF, and 940 (37%, 567 in the first decade) were unclassified HF. The age- and sex-adjusted standardized incidence rates for 1990–1999 and 2000–2009 were 19.7 and 18.9 per 1000 persons, per one-year follow-up, respectively (Table 2). Thus, the overall incidence of HF remained similar over the past two decades. In Poisson models adjusting for age, sex, and cohort, where the first decade = referent, the incidence rate ratio of overall HF was 0.94 (95% CI 0.86–1.02, p=0.13), indicating no significant change in HF incidence between decades, consistent with the rates of standardized HF incidence. In similar Poisson models of HF subtypes, the incidence rate ratio of HFREF was 0.80 (95% CI 0.69–0.93, p=0.0029) and that of HFPEF was 1.53 (95% CI 1.30–1.79, p<0.0001). These results are also consistent with those of standardized HF incidence rates, suggesting the decline in HFREF incidence observed from the first to second decade is significant when additionally accounting for cohort, and confirming the significant rise in HFPEF incidence over this time.
Table 2
Incidence of HF in FHS and CHS participants from 1990–2009.
| 1990–1999 | 2000–2009 | p-value | |
|---|---|---|---|
| Number at risk | 8762 | 6455 | |
| Person-years follow-up | 70548 | 45155 | |
| Age at start of window, y | 73±8 | 74±9 | |
| Women, n (%) | 5128 (59%) | 3954 (61%) | |
| All HF* | |||
| HF events, n | 1367 | 1157 | |
| Std HF incidence per 1000 | 19.7 (18.4, 21.0) | 18.9 (17.7, 20.1) | 0.37 |
| HFREF | |||
| HFREF events, n | 491 | 353 | |
| Std HF incidence per 1000 | 6.6 (5.9, 7.3) | 6.2 (5.4, 6.9) | 0.40 |
| HFPEF | |||
| HFPEF events, n | 309 | 431 | |
| Std HF incidence per 1000 | 4.7 (4.2, 5.2) | 6.8 (6.1, 7.5) | <0.001 |
Participants were ≥60 years of age at the start of each decade. Std HF incidence= reported as n (95% confidence interval), standardized to age- and sex-specific 2010 (ages 60–95) US population rates, per 1 year follow up.
Trends in HF and HF Subtype Incidence by Sex
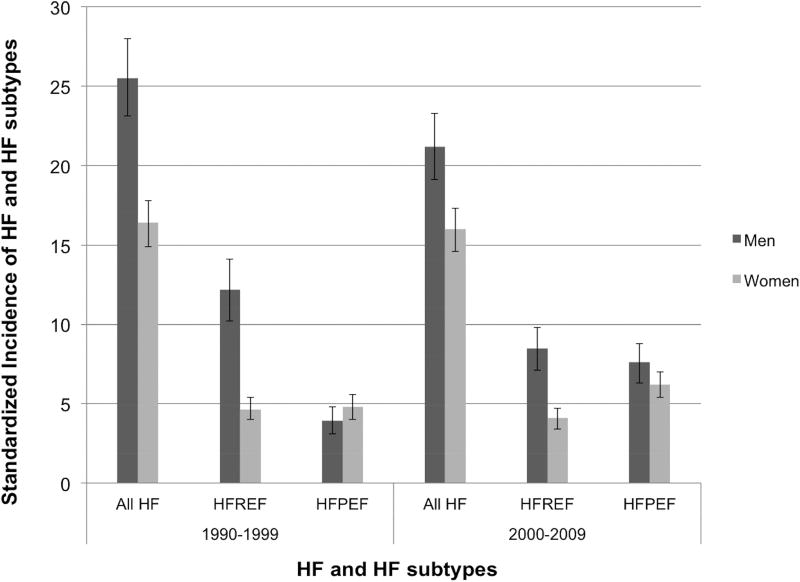
HF incidence from 1990–2009 is presented by sex in the Figure and Supplemental Table 2. The incidences of all HF and HFREF were greater in men than women in both decades (all p<0.001). The incidence of HFPEF was similar between men and women in both decades (p=0.16 and p=0.08, respectively). The incidence of overall HF decreased by nearly 17% between decades in men (25.5 to 21.2 per 1000 persons, p=0.01), but remained relatively constant in women (16.4 and 16.0 per 1000 persons, p=0.71). Between the two decades, the decline in the incidence of HFREF was largely driven by men, in whom HFREF incidence declined by 30% (12.2 to 8.5 per 1000 persons, p<0.001), as women showed a non-significant decrease (4.6 to 4.1 per 1000 persons, p=0.23). The incidence of HFPEF increased in both sexes over 1990–2009, nearly doubling in men (3.9 to 7.6 per 1000 persons, p<0.001) but still a substantial (29%) increase in women (4.8 to 6.2 per 1000 persons, p=0.02).
Risk of Mortality in HFREF and HFPEF over 1990–2009
Participants were followed for mortality up to 5 years (2.75±2.03 years), with 2,306 and 2,093 person-years of follow-up for HFREF and HFPEF, respectively. Of 2,524 individuals with HF, 1,701 participants died within 5 years of its onset. Among HFREF and HFPEF groups during follow-up, 557 of 844 (66.0%) and 474 of 740 (64.1%) died, respectively. Comparing the second to first decade, mortality was unchanged for all HF (HR 0.94, 95% CI 0.85–1.03, p=0.26), and both HFREF and HFPEF groups (p=0.70 and p=0.84, respectively, Table 3). Mortality was also similar between HFREF and HFPEF within each decade (p=0.14 and p=0.54). There was no significant effect modification by cohort in the relations of HF subtypes with mortality both within and across decades.
Table 3
Risk of mortality from HF in FHS and CHS combined from 1990–2009—excluding individuals with undetermined LVEF
| 1990–1999 HR (95% CI) | P value | 2000–2009 HR (95% CI) | P value | p-value for cohort interaction | |
|---|---|---|---|---|---|
| Hazards for mortality between decades | |||||
| HFREF (n=844) | Referent | - | 0.97 (0.81, 1.15) | 0.70 | 0.52 |
| HFPEF (n=740) | Referent | - | 0.98 (0.81, 1.19) | 0.84 | 0.11 |
| Hazards for mortality comparing HF subtypes | |||||
| HFREF (n=844) | Referent | - | Referent | - | |
| HFPEF (n=740) | 0.87 (0.72, 1.05) | 0.14 | 0.95 (0.80, 1.13) | 0.54 | 0.52/0.20 |
Analyses adjusted for age, sex.
HF = heart failure, HR = hazard ratio, CI = confidence interval.
HFREF and HFPEF= HF with reduced (<50%) and preserved (≥50%) left ventricular ejection fraction.
There were 894 (53%) CVD deaths and 807 (47%) non-CVD deaths in individuals with HF. Between onset of HF and death, the prevalence of interim MI was 15% and unchanged between 1990–2009. The prevalence of SCD was low in both 1990–1999 and 2000–2009 (3% and 0.8%, respectively). More individuals with HFREF died of CVD than non-CVD causes (63% vs. 37%, respectively), whereas we observed the reverse for HFPEF (45% vs. 55%, respectively), p<0.0001. The risk for CVD mortality between decades was similar for HFREF or HFPEF (p=0.10 and 0.50, respectively, Supplemental Table 3). Compared with HFREF, individuals with HFPEF had a lower risk of CVD-death (p<0.01 for both decades). Non-CVD mortality risk was similar between decades for both HF subtypes (p=0.13 and 0.75, Supplemental Table 4). Individuals with HFPEF had a greater risk of non-CVD mortality than those with HFREF in 1990–1999 (p=0.021). There were no cohort interactions in the associations of HF subtypes with CVD or non-CVD mortality within or between decades. Table 4 summarizes our study results and direction of change of HF metrics between decades.
Table 4
Summary of Changes in HF Epidemiology between 1990–2009
| 2000–2009 (vs. 1990–1999) | |
|---|---|
|
| |
| Incidence | |
| All HF | ← → |
| HFREF | ↓ |
| HFPEF | ↑ |
|
| |
| CVD and non-CVD Mortality* | |
| All HF | ← → |
| HFREF | ← → |
| HFPEF | ← → |
|
| |
| Prevalent use of cardiovascular meds in HF | |
| Aspirin | ↑ |
| Beta blockers | ↑ |
| Angiotensin-converting enzyme inhibitors | ← → |
| Lipid-lowering medications | ↑ |
| Diuretics | ↓ |
| Digoxin | ↓ |
DISCUSSION
In our large cohorts spanning multiple community samples, HF incidence has remained relatively constant over the past 20 years. Our findings suggest a decline in the incidence of HFREF, particularly in men, and rise in the incidence of HFPEF in both sexes, but more marked in men. Whereas female predominance has been reported in HFPEF, our results suggest a balance shift. We observed no significant changes in the incidence of HF and HFREF in women or mortality associated with HFREF and HFPEF, either between decades or between these subtypes.
Incidence of Overall HF, HFREF, and HFPEF from 1990–2009 in the Community
Trends in HF incidence have been mixed in past decades, with a decline suggested in Olmsted County.(2,14,19–24) A study of three Danish registries during this time period are also consistent with a mixed picture, with a decline in HF incidence in older individuals but rise in HF incidence in younger individuals.(25) A recent U.K. report suggests a decline in HF incidence over the past decade.(26) Our findings suggest that contemporary HF incidence over the past two decades is relatively unchanged. Differences in the absolute incidence of HF in our study may relate to methodologic and population sample differences, with our inclusion of adults ≥60 years (those at risk for HF) and our cohorts being relatively fixed, rather than dynamic study samples. Our study used standardized criteria for inpatient and outpatient HF constant over the time in the cohort studies. Studies defining HF by hospitalization billing codes(21,23,24,27) may be subject to misclassification bias, and may underestimate HF events compared to physician-adjudicated data(11) and HF initially diagnosed in an outpatient setting.(20,24,28) Additionally, our greater geographic and/or racial-ethnic diversity may have contributed to these findings, highlighting the role for broader population samples to investigate disease trends. Examination of HF incidence by ethnic/racial groups is an area for future investigation.
Temporal trends and sex-differences in the incidence of HFREF vs. HFPEF, which have differential risk factors, have not been well described in broad population samples in the contemporary era. Our observed temporal decrease in HFREF incidence in men, contrasted with an increase in HFPEF incidence in both sexes, suggests a shift in the epidemiology of HF. Our findings are consistent with improvements in primary and secondary prevention and treatment of coronary artery disease, with a decline in ST-elevation myocardial infarctions, and increase in and prolonged survival of individuals with non-ST segment infarctions.(29,30) Sex differences in HFREF incidence after myocardial infarction may be explained by differences in and responses to treatment (invasive vs. conservative) strategies.(31) Our observed rise in HFPEF incidence in men is consistent with national inpatient data showing a rising prevalence of men with HFPEF over time.(32)
Risk of Mortality in HF 1990–2009
Despite major advances in HFREF therapeutics in the contemporary era, we observed similar mortality rates in HFREF and HFPEF, consistent with reports of HF mortality prior to 2000.(3,33) The risk of death from CVD- and non-CVD causes was not significantly improved, and the prevalence of MI and of sudden cardiac death were similar. That mortality was not improved over 1990 to 2009 is noteworthy, considering the increased use of evidence-based CVD and HF medications between decades in our cohort. However, our data also suggests that an equal to greater proportion of individuals with HF were not taking these medicines. Further, ACE inhibitors were administered in the minority of participants with HF, and prevalent use of this medication class was similar between decades. Our findings that blood pressure remained similar and suboptimal in both HFREF and HFPEF groups during both decades enforces the suggestion that individuals with HF were inadequately treated with guideline-directed medical therapy. In addition to the limited use of optimal medical therapy in the community, the lack of improvement in HF-associated mortality may reflect other factors, including non-cardiovascular morbidities not addressed by appropriate therapies, and/or differences in “real world” vs. trial HF patient characteristics, follow-up, and/or treatment. Notably, in HFPEF, non-CVD morbidity is significant and therapies prolonging survival remain elusive. In total, our results emphasize the need to improve prevention and treatment strategies for HF, particularly addressing HFREF in women and HFPEF in both men and women.
Strengths and Limitations
Strengths of our study include the large, broad sample of five U.S. communities and meticulous participant surveillance and adjudication outcome standards. HF adjudication in both cohorts have shown similar outcomes.(13) We examined all-cause, CVD, and non-CVD mortality associated with HF and HF subtypes and the prevalence of interim MI and sudden death occurring between HF and mortality, but we did not have validated data on laboratory chemistries across the cohorts and time, or adequate statistical power to examine cause-specific mortality. However, because HF is associated with substantial comorbidities, all-cause mortality may be a preferred analytic outcome.
Additional limitations merit consideration. While greater recognition of the entity of HFPEF in the 2000s may have contributed to the observed rise in HFPEF incidence, adjudicated cardiovascular outcomes in FHS use standard criteria (regardless of LVEF) that have remained constant over time. Additionally, CHS investigators reported normal LVEF in the majority of participants with HF in 1994–95, demonstrating that HFPEF was a recognized condition in the first decade.(34) Our observed differential increase in HFPEF in men and women is also consistent with a lack of diagnostic bias, which would be expected to affect both sexes equally. We noted that at least half of our participants with HFREF had LVEF <35%, indicating lack of bias towards a healthier group. Though LVEF assessment was obtained within a year of HF diagnosis in FHS, limiting the time between LVEF and HF to 30 days would have reduced the sample size by 32%. Individuals with unavailable LVEF contributed to analyses for overall HF incidence and mortality, but could not be analyzed in HF subgroups. However, the observed constant incidence of overall HF is consistent with a decline in HFREF and rise in HFPEF incidence. Moreover, differences in HF incidence and mortality by race and ethnicity have been reported.(35–38) Future studies with a greater diversity may be able to better examine for racial/ethnic differences in contemporary incidence of HF and its subtypes. A final consideration in our evaluations of HF and HF subtype incidence and mortality over time may be introduction of error through multiple testing. While Bonferroni correction of p values would have been overly strict, further validation of our findings in other cohorts may reinforce our results.
CONCLUSIONS
From 1990–2009 in our large community-based sample, we observed a relatively constant incidence of HF with differential trends by HF subtypes and between sexes. The incidence of HFREF declined in men and that of HFPEF rose in both men and women. HF and its subtypes remain conditions with high mortality. Our findings underscore the need to investigate and implement HF preventative strategies to reduce its incidence and mortality.
Acknowledgments
Funding: This study was supported by grants from the NIH N01-HC-25195, and HHSN268201500001I to the Framingham Heart Study/ Boston University School of Medicine and the CHS is supported by contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. CWT was supported by K23 HL118529. JEH was supported by K23 HL116780.
List of Abbreviations
| CVD | Cardiovascular disease |
| HF | Heart failure |
| HFPEF | Heart failure with preserved ejection fraction |
| HFREF | Heart failure with reduced ejection fraction |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.jchf.2018.03.006
Article citations
Nutritional Interventions in Older, Frail Persons with Heart Failure-A Systematic Narrative Review.
JAR Life, 13:99-107, 05 Nov 2024
Cited by: 0 articles | PMID: 39525678
Review
Changing the paradigm in heart failure: shifting from treatment to prevention.
Heart Fail Rev, 23 Oct 2024
Cited by: 0 articles | PMID: 39441333
Review
Impact and consequences of the error of estimated GFR in patients with heart failure.
Sci Rep, 14(1):25840, 28 Oct 2024
Cited by: 0 articles | PMID: 39468066 | PMCID: PMC11519478
CMR to characterize myocardial structure and function in heart failure with preserved left ventricular ejection fraction.
Eur Heart J Cardiovasc Imaging, 25(11):1491-1504, 01 Oct 2024
Cited by: 1 article | PMID: 39205602 | PMCID: PMC11522877
Review Free full text in Europe PMC
Clinical Update in Heart Failure with Preserved Ejection Fraction.
Curr Heart Fail Rep, 21(5):461-484, 03 Sep 2024
Cited by: 0 articles | PMID: 39225910
Review
Go to all (201) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Sex and Race Differences in Lifetime Risk of Heart Failure With Preserved Ejection Fraction and Heart Failure With Reduced Ejection Fraction.
Circulation, 137(17):1814-1823, 19 Jan 2018
Cited by: 76 articles | PMID: 29352072 | PMCID: PMC6417883
Risk Factors and Outcomes Associated With Heart Failure With Preserved and Reduced Ejection Fraction in People With Chronic Kidney Disease.
Circ Heart Fail, 17(5):e011173, 14 May 2024
Cited by: 0 articles | PMID: 38742428
Causes and Temporal Patterns of 30-Day Readmission Among Older Adults Hospitalized With Heart Failure With Preserved or Reduced Ejection Fraction.
J Am Heart Assoc, 7(9):e007785, 23 Apr 2018
Cited by: 21 articles | PMID: 29686028 | PMCID: PMC6015286
Epidemiology of heart failure with preserved ejection fraction.
Nat Rev Cardiol, 14(10):591-602, 11 May 2017
Cited by: 601 articles | PMID: 28492288
Review
Funding
Funders who supported this work.
NHLBI NIH HHS (12)
Grant ID: N01HC85081
Grant ID: N01HC85086
Grant ID: N01HC85079
Grant ID: U01 HL130114
Grant ID: U01 HL080295
Grant ID: K23 HL116780
Grant ID: N01HC55222
Grant ID: N01HC85080
Grant ID: N01HC85082
Grant ID: N01HC25195
Grant ID: K23 HL118529
Grant ID: N01HC85083
NIA NIH HHS (1)
Grant ID: R01 AG023629